Abstract
The purpose of this study was to design and investigate the transdermal controlled release cubic phase gels containing capsaicin using glycerol monooleate (MO), propylene glycol (1,2-propanediol, PG), and water. Three types of cubic phase gels were designed based on the ternary phase diagram of the MO–PG–water system, and their internal structures were confirmed by polarizing light microscopy (PLM) and small-angle X-ray scattering (SAXS). Release results showed the cubic phase gels could provide a sustained system for capsaicin, while the initial water content in the gels was the major factor affecting the release rate. Release kinetics was determined to fit Higuchi’s square-root equation indicating that the release was under diffusion control. The calculated diffusion exponent showed the release from cubic phase gels was anomalous transport. The unique structure of the cubic phases, capsaicin distributed in the lipid bilayers, and cubic phase gel swelling contributed to the release mechanism. The cubic phase gel may be an interesting application for transdermal delivery system of capsaicin in alleviating the post-incision pain.
KEY WORDS: capsaicin, cubic phase gel, kinetics
INTRODUCTION
Capsaicin is a naturally occurring alkaloid extracted from fruit of the capsicum plant family. It exhibits broad bioactivity including the treatment of psoriasis, pruritus, apocrine chromhidrosis, and contact allergy (1). It has been employed topically to treat various diseases such as rheumatoid arthritis, osteoarthritis, and diabetic neuropathy (2), and to relieve post-operative pain (3–6). The high degree of first-pass metabolism of intragastric capsaicin and the short half-life of capsaicin by intravenous administration (7.06 min) (7,8) made topical application of capsaicin advantageous. Not only could topical application provide patient convenience and improve patient compliance, it could also circumvent the hepatic metabolism and achieve better bioavailability. In addition, it may be particularly suitable to short half-life drugs since transdermal absorption tends to be controlled and prolongs effects (9).However, satisfactory pain relief for post-operation and post-incision is still challenging clinicians which may be due to a low level of capsaicin reaching the target site over time. Furthermore, the replacement of post-operation or post-incision wound dressing is very painful; capsaicin formulation with long-lasting analgesic effects conforming a low replacing-frequency would be optimal. Since cubic phase gel carrier systems can enhance skin penetration (10–13) and slowly release the loaded drug (14,15), the potential of cubic phase gel carrier for a topical application of capsaicin was investigated in this study.
Cubic phase was spontaneously formed when more than 20% water (weight in ratio) was mixed with glycerol monooleate (MO) at room temperature (16). The cubic phase gel was transparent but stiff and viscous which limited its potential use as the delivery system (17). However, lower viscous cubic phase gel could be obtained by adding organic solvents (e.g., propylene glycol (PG), ethanol, polyethylene glycol, and N-methyl-2-pyrrolidone) (18,19). In this study, the MO–PG–water was selected to investigate the cubic phase system based on the following two reasons: first, PG could decrease the cubic phase gel viscosity and second, PG was a well-known skin penetration enhancer and used as a common additive in topical formulations.
Structure of the lipid cubic phase is unique and consists of a curved bi-continuous lipid bilayer extending in three dimensions, separating two congruent networks of water channels (17,20). As the thickness of the lipid bilayer is approximately 3.5 nm and the water channel diameter is roughly 5 nm (21), the cubic phase can be regarded as a soft nanostructural material (11). The cubic phase is isotropic and thermodynamically stable. It can dissolve hydrophilic, amphiphilic, and hydrophobic substances (17). As a promising delivery system for various actives ranging from low-molecular-weight drugs to proteins, peptides, amino acids, and nucleic acids, the properties of the cubic phase received considerable attention (17).
The cubic phase gel had also been used as a local delivery system in the prophylaxis and for treatment of post-surgical wound infections. Local anesthetics such as bupivacaine was formulated in the cubic phase gel and applied at the wound site to provide sustained release of the drug (22). To the best of our knowledge, there is no published study to date reporting the influence of cubic phase gel properties on the release of entrapped capsaicin for the topical application. It is pertinent to understand the release mechanisms of entrapped actives when cubic phase gel is used as the basis of the sustained release formulation. Therefore, the aim of this study was to investigate how the composition of the cubic phase gels influenced the release of the entrapped capsaicin.
MATERIALS AND METHODS
Materials
MO (DIMODAN®MO/D KOSHER, Material number 116703) was kindly provided by Danisco Cultor (Brabrand, Denmark) and used as received. Capsaicin was purchased from Wuhan Hengshuo Technology Development Co., Ltd. (Hubei, China) with a purity of 95%. PG (1,2-propanediol) was purchased from Tianjin Fuyu Chemical Co., Ltd. (Tianjin, China). Water was doubly distilled in a PURELAB Option-R 7/15 water purification system (ELGA Lab Water) until the resistivity was 18.2 MΩ. All other reagents were of analytical grade and used as received.
Preparation of MO–PG–Water Systems
Ternary phase diagrams were generated to identify the compositions of different liquid crystalline phases. Ternary phase diagrams of MO–PG–water systems were prepared by first mixing PG with the molten MO (45°C) in ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1 (w:w) to a total of 20 g. A specific amount of each mixture was transferred into a 10-ml glass vial and mixed with water in ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1 (w:w) to a total weight of 2 g (18). The preparations were then homogeneously vortex-mixed for 3 min and then stored for at least 1 week prior to mesophase identification.
Preparation of Cubic Phase Gels Containing Capsaicin
Based on the ternary phase diagrams, cubic phase gels composed of MO–PG–water as shown in Table I were selected for drug release studies. Capsaicin (a hydrophobic drug that could be dissolved in MO) was weighed into the MO and heated to melt at 45°C. After the mixture was homogeneously vortexed, PG and water were added according to the ratios and then homogeneously vortexed. The obtained cubic phase gels were stored for at least 1 week prior to phase identification and release experiments (12,15).
Table I.
Composition and Phase Structure of Cubic Phase Gels Chosen for In Vitro Capsaicin Release Studies
| Sample | Composition MO:PG:water (w:w:w) | Capsaicin concentration (mg/g) | Space group of the internal structure | Lattice parameter(Å) |
|---|---|---|---|---|
| F1 | 63:7:30 | 2.5 | Pn3m | 90 |
| F2 | 70:0:30 | 2.5 | Pn3m | 82 |
| F3 | 85:0:15 | 2.5 | Ia3d | 107 |
Structure Determination
The liquid crystalline structure was identified from its optical pattern with polarized light and from the characteristic periodicity ratios in the small-angle X-ray diffraction pattern at room temperature. Optical observations were made with a PLM using a BM-59XCC (China). The liquid crystalline phases were identified by images and classified according to the visualized textures of liquid crystalline described by literatures (15,23). SAXS measurements were performed using a Kratky compact camera (HMBG, Austria) with Ni and W filtered CuKα radiation (wavelength λ = 0.15418) generated by a PW3830 X-ray generator (5 kV × 40 mA). Scattering intensities were plotted versus reciprocal spacing (q = 4πsinθ/λ), where θ was the scattering angle.
Release of Capsaicin from Cubic Phase Gels
Cylindrical (10.0 mm in diameter and 4.0 mm in thickness) sample of the cubic phase gel was placed on a platform composed of supporter. The platform was transposed to a 50-ml tube with screw caps in which 30 ml of isotonic phosphate buffered solution (PBS) was filled. The tube was whirled at 100 rpm on a shaker at temperature 37°C. Aliquots of 1 ml was taken periodically and replaced with the same volume of PBS. The concentration of the released capsaicin was determined by HPLC on the basis of the standard curve.
Evaluation of the Release Mechanism
The mechanism of capsaicin release from the cubic phases was determined by fitting the release rate data into the following equations: Zero-order model equation,
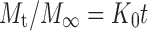 |
1 |
Higuchi’s square-root equation,
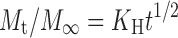 |
2 |
Ritger–Peppas’ empirical equation,
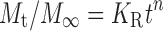 |
3 |
where Mt/M∞ is the fraction of drug released at time t; the amount of drug released at any given time (Mt) was obtained from the calibration curve, and the maximum amount of drug available for release (M∞) was determined by gravimetric measurements. K0, KH, and KR are release rate constants for Eqs. 1, 2, and 3, respectively. In Eq. 3, n is the diffusion exponent, indicating the release mechanism for matrices of varying shape and swelling or non-swelling systems. For moderately swelling system (equilibrium swelling ratio not greater than 1.33, equivalent to 25.0% increase in volume) of cylinder shape, a value of 0.45 for n indicates Fickian diffusion, where drug is released by the molecular diffusion through the system. A value between 0.45 and 0.89 for n is indicative of anomalous transport in which Fickian transport and matrix relaxation occur simultaneously. A value of 0.89 for n indicates case-II relaxational mechanism associated with the stresses and state transitions that occur in the swelling (24–29).
RESULTS AND DISCUSSION
Phase Diagram and PLM Images
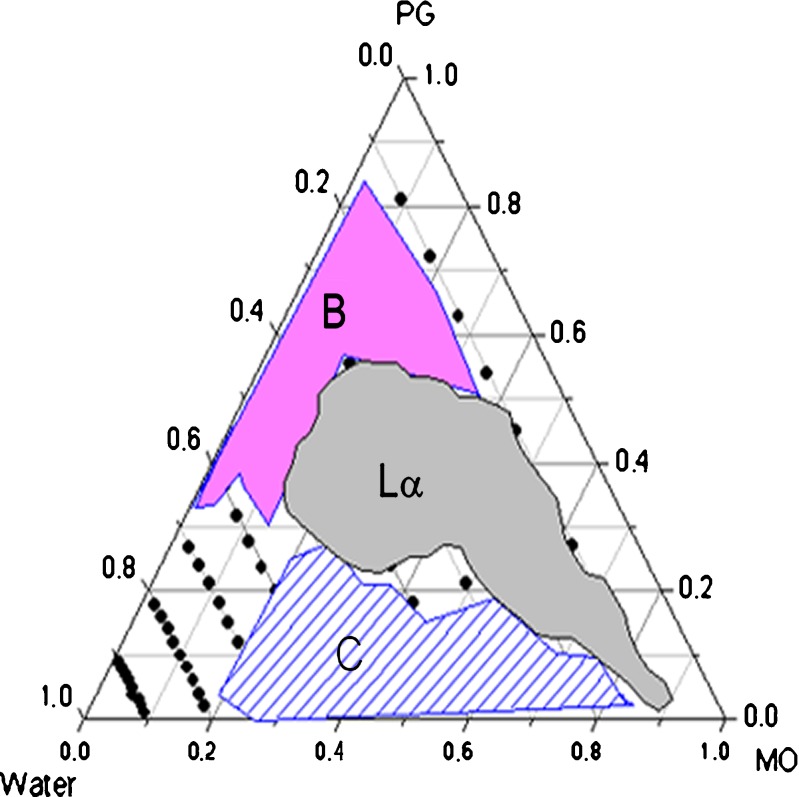
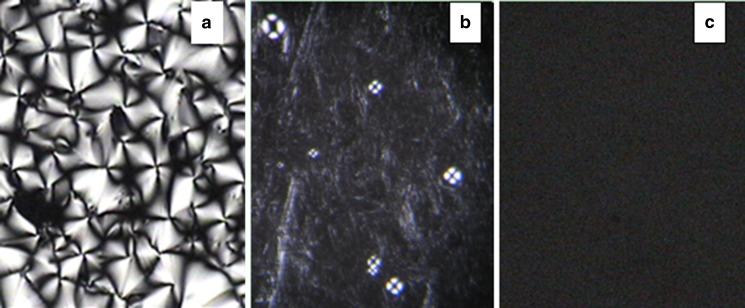
Ternary phase diagram of MO–PG–water was constructed as shown in Fig. 1. The region marked as shadow B is a two-phase region, Lα represents lamellar phase, and the cubic phase region is marked as C. The lamellar phase samples showed a low viscosity with medium flow and were optically anisotropic with the presence of oily streak textures and Maltese crosses as illustrated by PLM images in Fig. 2a, b. The cubic phase samples were stiff and did not flow, and these transparent and optically isotropic gels only left a dark background under PLM (Fig. 2c).
Fig. 1.
Ternary phase diagram for the MO–PG–water system at room temperature. The dots represent samples prepared. B two-phase region, Lα lamellar phase, C cubic phase
Fig. 2.
Polarized photomicrographs of Lamellar phase (a, b) and cubic phase (c) at room temperature (magnification ×400). a MO–PG–water (90–0–10). b MO–PG–water (21–49–30). c MO-PG-water(63-7-30)
Three samples, labeled as F1, F2, and F3, were selected to study drug release profiles. The contents of each component are listed in Table I.
SAXS
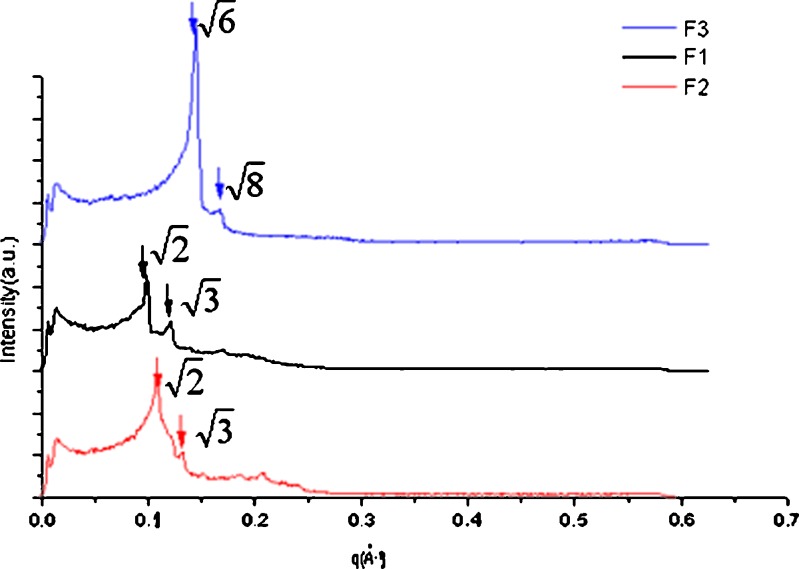
SAXS experiments were carried out to investigate the internal structure of the cubic phase gels. From the scattering spectra in Fig. 3, the values of the scattering vector q corresponding to the scattering peaks for three samples F1, F2, and F3 appeared in the ratio of 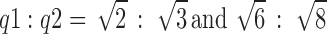 . The scattering vector ratio is a characteristic of cubic periodicity indicative of a diamond (Pn3m space group) or a gyroid (Ia3d space group) (30). Figure 4 shows the plot of q for the observed reflections as a function of Miller indexes
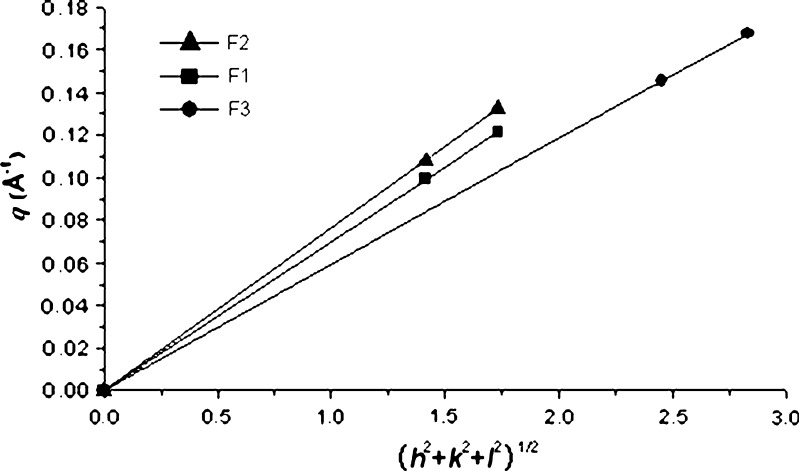
. The scattering vector ratio is a characteristic of cubic periodicity indicative of a diamond (Pn3m space group) or a gyroid (Ia3d space group) (30). Figure 4 shows the plot of q for the observed reflections as a function of Miller indexes 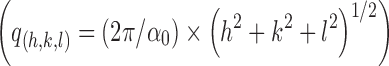 , where h, k, and l are Miller indices. The plot generated a straight line passing through the origin for each of the three samples, which is the expected behavior for the cubic phase (31–33). The lattice parameters (α0) of the cubic phase samples were calculated from the linear slope (2π/α0) in Fig. 4, and the values were listed in Table I.
, where h, k, and l are Miller indices. The plot generated a straight line passing through the origin for each of the three samples, which is the expected behavior for the cubic phase (31–33). The lattice parameters (α0) of the cubic phase samples were calculated from the linear slope (2π/α0) in Fig. 4, and the values were listed in Table I.
Fig. 3.
SAXS diffraction patterns obtained from the three chosen cubic phase samples
Fig. 4.
Plots of the reciprocal d-spacings (q) as a function of the Miller indices, (h 2 + k 2 + l 2)1/2, from the observed reflections in the SAXS diffraction patterns for the cubic phase gels
In Vitro Release Study
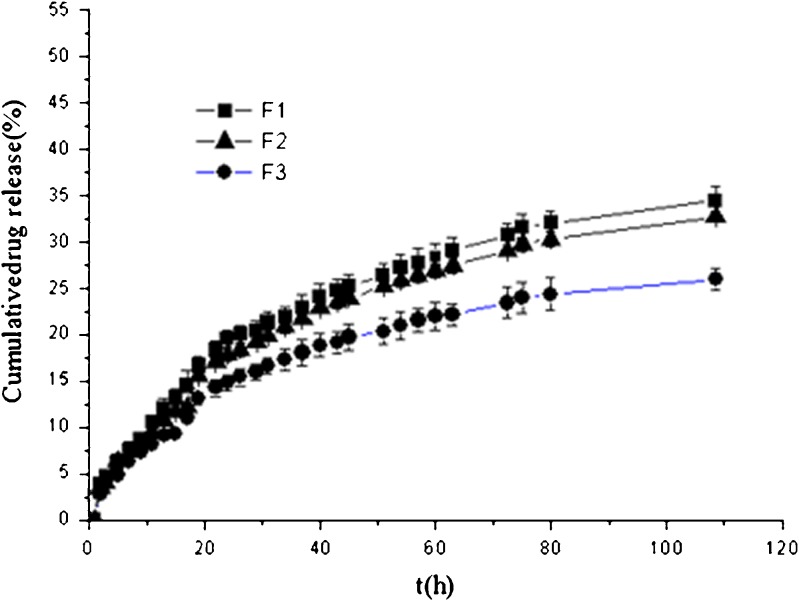
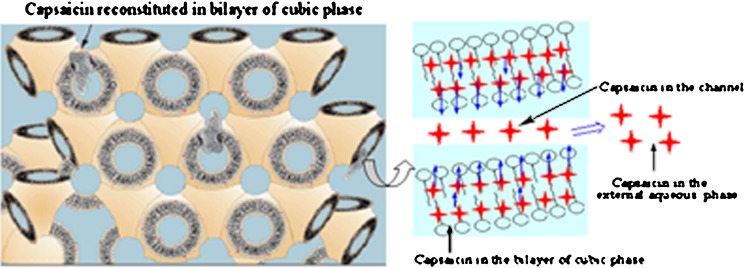
Figure 5 plots the percentage of capsaicin released as a function of time from the cubic phase gels. After 108 h, around 34% of capsaicin was released from sample F1 which showed the fastest release rate, followed by 33% and 26% for sample F2 and F3, respectively. Therefore, all three cubic phase gels could provide a sustained release system for capsaicin, and these findings could be attributed to the unique structure of the cubic phase. Capsaicin, a hydrophobic drug that can be dissolved in MO, is mostly distributed in the lipid bilayers as illustrated in Fig. 6. The permeation of capsaicin from the MO bilayers to the water channels is a rate-limited process. Table II summarizes the results of fitting the release data for the cubic phase gels into the different release mechanism models. The linear relationship was found between the capsaicin release and the square root of time (r > 0.99) which indicated that the release kinetics can be explained by Higuchi’s equation. Namely, the capsaicin release was under diffusion control (34). Meanwhile, the matrix swelling may have further increased the diffusion path of capsaicin thereby retarding its release (26). F1 and F2 samples were almost fully hydrated with 30% initial water content; it was expected that the influence of the swelling was less for F1 and F2 compared with F3. This was confirmed by the release profiles in Fig. 5 as capsaicin release rate was faster from F1 and F2 than from F3. The diffusion exponent (n) calculated from Eq. 3 further confirmed the swelling influence on the drug release. The calculated n values are greater than 0.5 (Table II) which indicates that the release mechanism for all cubic phase gels is anomalous transport. Capsaicin release was dependent on two simultaneous processes, water migrating into the cubic phase gel and drug diffusing through continuously swelling gels. Therefore, the capsaicin characteristic, the matrix swelling, and the tortuosity of the water channels of the cubic phases contribute together to the sustained release.
Fig. 5.
Cumulative capsaicin released from cubic phase gels versus time
Fig. 6.
Scheme of cubic phases’ structure illustrating the mechanism for capsaicin release from the cubic phases (Adapted with modifications from Refs (14, 20))
Table II.
Fitting of Drug Release Data for Cubic Phase Gels into Various Models
| Sample | Ritger–Peppas’ empirical equation | Higuchi’s square-root equation | Zero-order model equation | ||||
|---|---|---|---|---|---|---|---|
| K R (% h −n) | n | r d | K H (% h −0.5) | r f | K0 (% h −1) | r h | |
| F1 | 2.586 | 0.592 | 0.992 | 4.007 | 0.994 | 0.327 | 0.934 |
| F2 | 2.349 | 0.602 | 0.992 | 3.831 | 0.994 | 0.314 | 0.938 |
| F3 | 2.108 | 0.578 | 0.991 | 3.051 | 0.992 | 0.247 | 0.924 |
r d , r f , r h correlation coefficients for Ritger–Peppas’ empirical equation, Higuchi’s square-root equation, and zero-order model equation, respectively.
CONCLUSIONS
The prepared cubic phase gels could provide sustained systems for capsaicin, and the release rate was dependent upon the swelling of the matrix and the tortuosity of the water channels. The release mechanism from the cubic phase gels was proved to be anomalous transport. The sustained drug release providing a low replacing-frequency, together with the thermodynamically stable system, make the cubic phase gels interesting for topical delivery of capsaicin in alleviating the post-incision pain.
ACKNOWLEDGEMENTS
The authors are grateful to Danisco Company, Denmark, for the generous gift samples of MO. We thank the National Natural Science Foundation of China for the financial support (No 81001643/H2806). This work was also supported in part by the Ministry of Science and Technology of Dongguan (NO 2008108101064).
REFERENCES
- 1.Magnusson BM, Koskinen LOD. In vitro percutaneous penetration of topically applied capsaicin in relation to in vivo sensation responses. Int J Pharm. 2000;195(1–2):55–62. doi: 10.1016/S0378-5173(99)00337-3. [DOI] [PubMed] [Google Scholar]
- 2.Fusco B, Giacovazzo M. Peppers and pain: the promise of capsaicin. Drugs (Basel) 1997;53(6):909–914. doi: 10.2165/00003495-199753060-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hayman M, Kam PCA. Capsaicin: a review of its pharmacology and clinical applications. Curr Anaesth Crit Care. 2008;19(5–6):338–343. [Google Scholar]
- 4.Hamalainen MM, Subieta A, Arpey C, Brennan TJ. Differential effect of capsaicin treatment on pain-related behaviors after plantar incision. J Pain. 2009;10(6):637–645. doi: 10.1016/j.jpain.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang S, Wu C, Banik RK, Brennan TJ. Effect of capsaicin treatment on nociceptors in rat glabrous skin one day after plantar incision. Pain. 2010;148(1):128–140. doi: 10.1016/j.pain.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pospisilova E, Palecek J. Post-operative pain behavior in rats is reduced after single high-concentration capsaicin application. Pain. 2006;125(3):233–243. doi: 10.1016/j.pain.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Donnerer J, Amann R, Schuligoi R, Lembeck F. Absorption and metabolism of capsaicinoids following intragastric administration in rats. Naunyn-Schmiedebergs Arch Pharmakol. 1990;342(3):357–361. doi: 10.1007/BF00169449. [DOI] [PubMed] [Google Scholar]
- 8.Kawada T, Watanabe T, Katsura K, Takami H, Iwai K. Formation and metabolism of pungent principle of Capsicum fruits. XV. Microdetermination of capsaicin by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1985;329(1):99–105. doi: 10.1016/S0021-9673(01)81899-9. [DOI] [PubMed] [Google Scholar]
- 9.Fang JY, Wu PC, Huang YB, Tsai YH. in vivo percutaneous absorption of capsaicin, nonivamide and sodium nonivamide acetate from ointment bases: pharmacokinetic analysis in rabbits. Int J Pharm. 1996;128(1–2):169–177. doi: 10.1016/0378-5173(95)04274-1. [DOI] [Google Scholar]
- 10.Geraghty P, Attwood D, Collett J, Dandiker Y. The in vitro release of some antimuscarinic drugs from monoolein/water lyotropic liquid crystalline gels. Pharm Res. 1996;13(8):1265–1271. doi: 10.1023/A:1016036908947. [DOI] [PubMed] [Google Scholar]
- 11.Bender J, Ericson M, Merclin N, Iani V, Rosén A, Engström S, et al. Lipid cubic phases for improved topical drug delivery in photodynamic therapy. J Control Release. 2005;106(3):350–360. doi: 10.1016/j.jconrel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Lopes LB, Lopes JLC, Oliveira DCR, Thomazini JA, Garcia MTJ, Fantini MCA, et al. Liquid crystalline phases of monoolein and water for topical delivery of cyclosporin A: characterization and study of in vitro and in vivo delivery. Eur J Pharm Biopharm. 2006;63(2):146–155. doi: 10.1016/j.ejpb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Clogston J, Craciun G, Hart D, Caffrey M. Controlling release from the lipidic cubic phase by selective alkylation. J Control Release. 2005;102(2):441–461. doi: 10.1016/j.jconrel.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Fa N, Babak VG, Stebe MJ. The release of caffeine from hydrogenated and fluorinated gel emulsions and cubic phases. Colloids Surf A Physicochem Eng Asp. 2004;243(1–3):117–125. doi: 10.1016/j.colsurfa.2004.05.014. [DOI] [Google Scholar]
- 15.Rizwan SB, Hanley T, Boyd BJ, Rades T, Hook S. Liquid crystalline systems of phytantriol and glyceryl monooleate containing a hydrophilic protein: characterisation, swelling and release kinetics. J Pharm Sci-Us. 2009;98(11):4191–4204. doi: 10.1002/jps.21724. [DOI] [PubMed] [Google Scholar]
- 16.Larsson K. Cubic lipid-water phases: structures and biomembrane aspects. J Phys Chem. 1989;93(21):7304–7314. doi: 10.1021/j100358a010. [DOI] [Google Scholar]
- 17.Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Deliv Rev. 2001;47(2–3):229–250. doi: 10.1016/S0169-409X(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 18.Chang C, Bodmeier R. Low viscosity monoglyceride-based drug delivery systems transforming into a highly viscous cubic phase. Int J Pharm. 1998;173(1–2):51–60. doi: 10.1016/S0378-5173(98)00180-X. [DOI] [Google Scholar]
- 19.Wadsten-Hindrichsen P, Bender J, Unga J, Engström S. Aqueous self-assembly of phytantriol in ternary systems: effect of monoolein, distearoylphosphatidylglycerol and three water-miscible solvents. J Colloid Interface Sci. 2007;315(2):701–713. doi: 10.1016/j.jcis.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Caffrey M. A lipid’s eye view of membrane protein crystallization in mesophases. Curr Opin Struct Biol. 2000;10(4):486–497. doi: 10.1016/S0959-440X(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 21.Hyde S, Andersson S, Ericsson B, Larsson K. A cubic structure consisting of a lipid bilayer forming an infinite periodic minimum surface of the gyroid type in the glycerolmonooleat-water system. Z Kristallogr. 1984;168(1–4):213–219. doi: 10.1524/zkri.1984.168.1-4.213. [DOI] [Google Scholar]
- 22.Park E, Maniar M, Shah J. Biodegradable polyanhydride devices of cefazolin sodium, bupivacaine, and taxol for local drug delivery: preparation, and kinetics and mechanism of in vitro release. J Control Release. 1998;52(1–2):179–189. doi: 10.1016/S0168-3659(97)00223-X. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Kellaway I. In vitro peptide release from liquid crystalline buccal delivery systems. Int J Pharm. 2000;195(1–2):29–33. doi: 10.1016/S0378-5173(99)00356-7. [DOI] [PubMed] [Google Scholar]
- 24.Ritger P, Peppas N. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5(1):37–42. doi: 10.1016/0168-3659(87)90035-6. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Shen Y, Li W, Hao X. A common profile for polymer-based controlled releases and its logical interpretation to general release process. J Pharm Pharm Sci. 2006;9(2):238–244. [PubMed] [Google Scholar]
- 26.Lara M, Bentley M, Collett J. In vitro drug release mechanism and drug loading studies of cubic phase gels. Int J Pharm. 2005;293(1–2):241–250. doi: 10.1016/j.ijpharm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Ravi PR, Kotreka UK, Saha RN. Controlled release matrix tablets of zidovudine: effect of formulation variables on the in vitro drug release kinetics. AAPS PharmSciTech. 2008;9(1):302–313. doi: 10.1208/s12249-007-9030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anumolu S, Singh Y, Gao D, Stein S, Sinko P. Design and evaluation of novel fast forming pilocarpine-loaded ocular hydrogels for sustained pharmacological response. J Control Release. 2009;137(2):152–159. doi: 10.1016/j.jconrel.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Ruvalcaba A, Sanchez-Diaz JC, Becerra F, Cruz-Barba LE, Gonzalez-Alvarez A. Swelling characterization and drug delivery kinetics of polyacrylamide-co-itaconic acid/chitosan hydrogels. Express Polym Lett. 2009;3(1):25–32. doi: 10.3144/expresspolymlett.2009.5. [DOI] [Google Scholar]
- 30.Rizwan S, Dong Y, Boyd B, Rades T, Hook S. Characterisation of bicontinuous cubic liquid crystalline systems of phytantriol and water using cryo field emission scanning electron microscopy (cryo FESEM) Micron. 2007;38(5):478–485. doi: 10.1016/j.micron.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Alexandridis P, Olsson U, Lindman B. A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil) Langmuir. 1998;14(10):2627–2638. doi: 10.1021/la971117c. [DOI] [Google Scholar]
- 32.Polyzos A, Alderton M, Dawson R, Hartley P. Biofunctionalized surfactant mesophases as polyvalent inhibitors of cholera toxin. Bioconjugate Chem. 2007;18(5):1442–1449. doi: 10.1021/bc0700640. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Zheng L, Inoue T. Effect of sucrose on the structure of a cubic phase formed from a monoolein/water mixture. J Colloid Interface Sci. 2005;288(2):638–641. doi: 10.1016/j.jcis.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka N, Imai K, Okimoto K, Ueda S, Tokunaga Y, Ohike A, et al. Development of novel sustained-release system, disintegration-controlled matrix tablet (DCMT) with solid dispersion granules of nilvadipine. J Control Release. 2005;108(2–3):386–395. doi: 10.1016/j.jconrel.2005.08.024. [DOI] [PubMed] [Google Scholar]