Abstract
The effects of adhesive layer thickness and drug loading on estradiol crystallization were studied in a drug-in-adhesive patch. Patches containing different estradiol loadings (1.1% and 1.6% w/w) in different thicknesses (45, 60, and 90 μm) were prepared by coating of a homogenous mixture of adhesive solution and the drug on a siliconized release liner by a film applicator. After drying, the film was laminated on a Poly(ethylene terephthalate) backing layer and cut into appropriate size. Release tests were performed using thermostated Chien-type diffusion cells. Cross-section of the patches was observed by optical microscopy. Scanning electron microscopy was done for surface analysis of the patches after drug release test. Crystal formation was not expected in the adhesive layer based on the linear free-energy relationship formalisms however; crystalline regions were observed in different locations through the thickness of the patches. These regions were significantly more discontinuous in 45 μm samples which elucidated the effective role of adhesive layer thickness in drug crystallization. Extensive crystallization observed for thicker patches was attributed to the strong crosslinking capability of estradiol hemihydrate. Drug release study confirmed some of the crystallization results. No significant increase was observed in the burst release with increasing in thickness from 45 to 60 μm which can be attributed to the severe increase in the crystallization extent. Also, formation of a crystalline layer near the releasing surface and more discontinuous pattern of the crystals in some samples was confirmed by investigation of the drug release curves.
Key words: acrylic adhesive, coating, crystallization, estradiol, transdermal drug delivery systems
INTRODUCTION
Pressure sensitive adhesives (PSAs) are commonly used in a variety of applications including transdermal drug delivery systems (TDDSs) (1,2). The important advantages of TDDSs are the avoidance of first-pass metabolism, ease of application and withdrawal (in the case of side effects), and better patient compliance (3). The efficacy of estrogen replacement therapy is well established in the treatment of postmenopausal complications in women. Administration of 17-β estradiol (Fig. 1) sex steroid is the first choice in this regard which also protects against increasing risk of osteoporosis or cardiovascular disease (4). Currently, most of the commercially available estradiol patches are drug-in-adhesive matrix-type in which the adhesive is sandwiched between a backing layer and a release liner film. Drug-in-adhesive systems exhibit efficient utilization of size because they combine the functions of skin adhesion and controlled drug delivery simultaneously in a single layer (3).
Fig. 1.
Chemical structure of estradiol
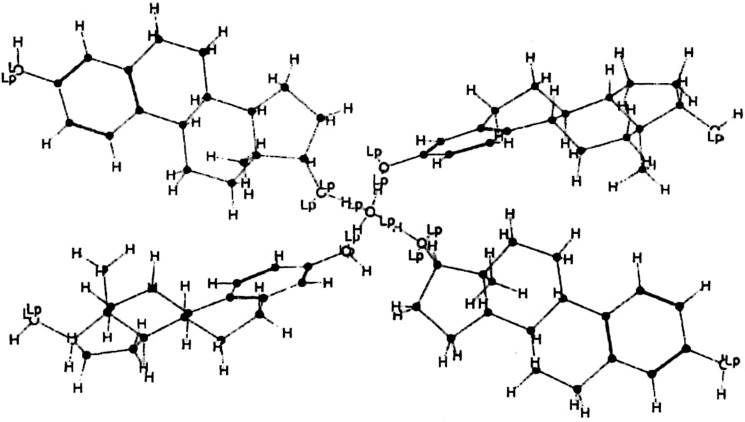
Increasing estradiol concentration in the initial adhesive solution or dried adhesive layer causes increased flux of the drug through human skin (5,6). However, crystallization of the initially dissolved drugs may also be occurred during the storage period of a supersaturated system which is not a rare case. Nucleation is often regarded as a decisive step in crystallization process and the number of molecules necessary to achieve a nucleating cluster is inversely proportional to the degree of supersaturation (7). Estradiol has a pronounced tendency for crystallization (8) and its crystallization in acrylic adhesives has been reported both after evaporation of the adhesive solvents during the drying stage of process (6) or storage period (8–10). It should be noted that the most characteristic property of estradiol is its tendency to adopt the hemihydrated form (Fig. 2), which it precipitates in this state, not only from partially aqueous solutions, but also from ethyl acetate, chloroform, absolute ethanol, and other anhydrous solvents (11). However, the effect of adhesive layer thickness on the crystallization phenomenon has not been investigated yet.
Fig. 2.
Structural model of estradiol hemihydrate (Adopted from ref. (7))
Here, the effect of adhesive layer thickness and drug loading on the estradiol crystallization in a PSA based on an acrylate–vinyl acetate copolymer will be discussed. Some of the results related to estradiol crystallization were confirmed by investigation of drug release curves.
MATERIALS AND METHODS
17-β Estradiol (United States Pharmacopoeia (USP) XXIII grade) was supplied by Medichem (Guangdong, China). Duro-Tak 87–2196 i.e., a self-curing medical grade adhesive; was purchased from National Starch and Chemical Co. (Manchester, UK). It was based on acrylate–vinyl acetate dissolved (45% solid content) in a mixture of organic solvents. Poly(ethylene terephthalate; PET) and one-side siliconized PET films were both kind gifts from Daroupat Shargh Co. (Tehran, Iran) and Perlen Converting AG (Perlen, Switzerland); respectively. High-performance liquid chromatography (HPLC) grade acetonitrile and USP grade propylene glycol (PG) were both from Merck Chemicals (Darmstadt, Germany).
Fabrication Process
The acrylic adhesive solution with a predetermined solid content (prior to each batch) was mixed with the drug in different weight ratios to satisfy final loading of 1.1% or 1.6% w/w of estradiol in the finally dried thin film. Mixing was performed in a rotary mixer until a clear solution was obtained. The mixed solutions were cast on one-side siliconized PET films, as release liners, by a film applicator (BYK-Gardner, Wesel, Germany) with 5.1 cm application width and adjustable in ±5 μm thickness intervals. The adhesive-coated films were left at room temperature for about 10 min and then oven-dried at 65 °C for at least 20 min to remove any residual organic solvents. The dried films were laminated on PET backing layers using a 4.5-lbs roller. The patches were cut into pieces with surface area of about 9 cm2.
Drug Release Studies
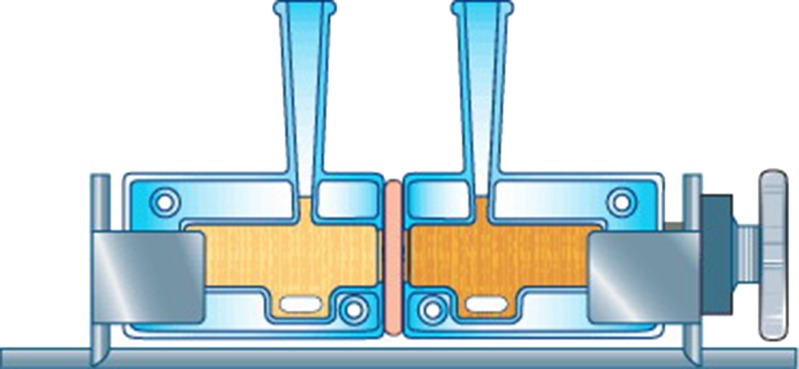
A set of side-by-side Chien-type diffusion cells (Permegear Co., Hellertown, USA) comprising a donor and receptor compartment equipped with magnetic-stirrer bar and jacket for water circulation was used in the release experiments (Fig. 3). After removal of the release liner, each of the patches was mounted between two half-cells with the drug-releasing surface facing the receptor half-cell which contained 3 mL of PG solution (40% v/v in distilled water) as the receptor solution so each compartment was used as an independent receptor compartment with a transdermal patch adhered on the orifice as the donor. All the studies were carried out at 37 °C. At predetermined time intervals, 1 mL aliquot was withdrawn and replaced with the same volume of the fresh release medium. A sample aliquot collection times was 0.5, 1, 2, 4, 23, 27, 30, 49, 51, and 54 h.
Fig. 3.
Chien-type diffusion cell
Analytical Procedure
HPLC technique was used in determination of drug concentration in the withdrawn aliquots. The equipment was consisted of a M930D pump (YoungLin Instruments, Anyang, South Korea) equipped with a variable wavelength UV-visible detector and a Rheodyne injector (Oak Harbor, USA) with 20-μL loop. Acetonitrile and water (45:55) was used as the mobile phase at a flow rate of 1 mL/min. The drug was resolved using a μ-Bondapak C18 column with dimensions of 4.6 × 250 mm (Waters Corp., USA), and detected at a wavelength of 270 nm. Analytical procedure was fully validated according to the guideline of International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (12). Validation parameters for estradiol analysis by HPLC method are tabulated in Table I.
Table I.
Validation Parameters for Estradiol Analysis by HPLC
| Validation parameter | Value |
|---|---|
| Regression equation (n = 7) |
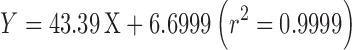
|
| Linear range (μg.mL−1) | 0.250–8.00 |
| Limit of detectiona (μg.mL−1) | 0.08 |
| Limit of quantitationb (μg.mL−1) | 0.24 |
| Precision (n = 7) | |
| Repeatability | |
| SD of intra-assay | 0.136–2.42 |
| RSD of intra-assay | 0.408–1.14 |
| Intermediate precision | |
| SD of inter days | 0.375–5.78 |
| RSD of inter days | 0.578–3.21 |
| Accuracy (n = 7) | |
| RE%c | 0.156–8.87 |
| Recovery% | 91.1–101.3 |
aSignal to noise ratio (SNR) > 3
bSignal to noise ratio (SNR) > 10
cRelative error percentage
Microscopic Observations
Optical microscopy through the thickness of the adhesive layer was done by a Zeiss-Neophot 32 microscope (Oberkochen, Germany) equipped with a Vickers microhardness tester. Small pieces of the patches were cut and adhered from their cross-section side on the glass lamellas by a double-coated PSA.
The patches were separated from diffusion chambers (after 9 h drug release) and dried thoroughly. SEM analysis was performed on the surface of these dried patches using a Cambridge S360 Scanning Electron Microscope (Cambridge, UK) equipped with an image analyzing system (AnalySIS, SIS Software GmbH, Muenster, Germany).
Statistical Analysis
Statistical analysis was performed using MiniTab software (Release 11.12, Minitab Inc., State College, PA, USA). Data were reported as mean ± standard deviation at significance level of p < 0.05. Outliers were rejected during data processing using the T procedure (13). Differences between groups were analyzed using one-way analyses of variance and considered statistically significant when the p value was less than 0.05.
RESULTS
Crystallization
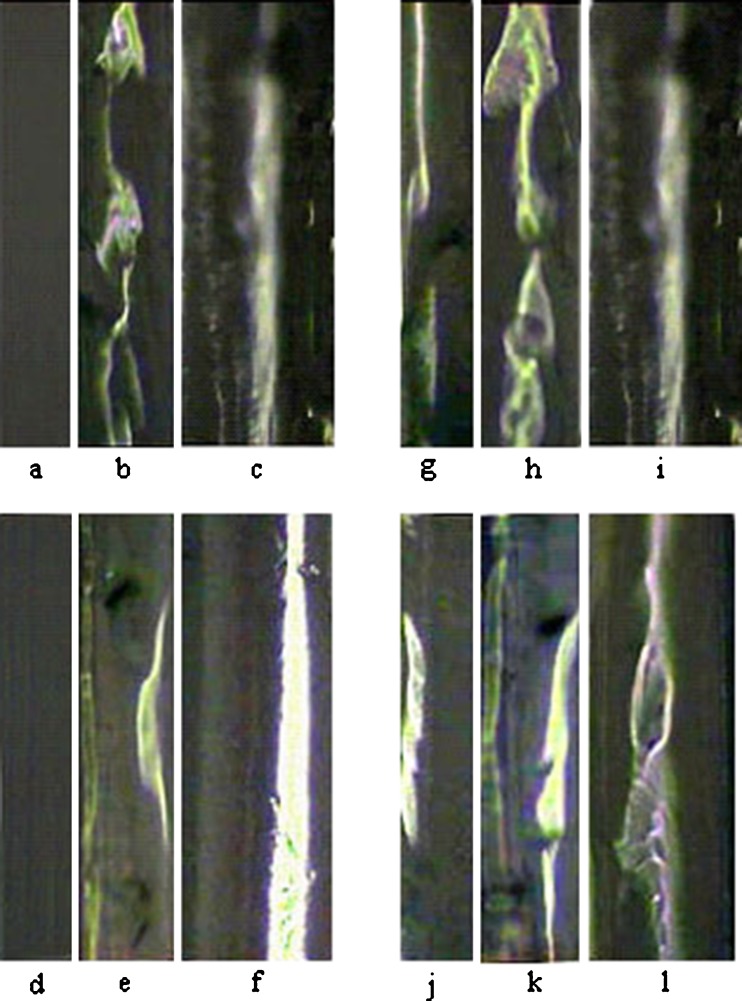
Distribution pattern for estradiol crystals throughout the polymeric adhesive matrix cross-section was checked out microscopically. Optical micrographs of adhesive layer cross-section for patches containing 1.1% and 1.6% (w/w) of estradiol in different thicknesses are shown in Fig. 4. Two micrographs from different samples of each patch cross-section are shown except for one of the samples (i.e., 1.1% w/w drug loading and of 90 μm thickness) which was examined just once. Some inclusions could be observed throughout the adhesive layer thickness about 2 or 6 days after preparation of the patches. The micrographs depicted in Fig. 4 are the results of optical microscopy after 6 days.
Fig. 4.
Optical micrographs of two different samples from adhesive thickness of patches containing 1.1% (w/w) of estradiol with thickness of 45 μm (a, g), 60 μm (b, h) and 1.6% (w/w) of estradiol with thickness of 45 μm (d, j), 60 μm (e, k) and 90 μm (f, l). Only the positions c and i show one sample related to the patch with drug loading of 1.1% (w/w) and thickness of 90 μm
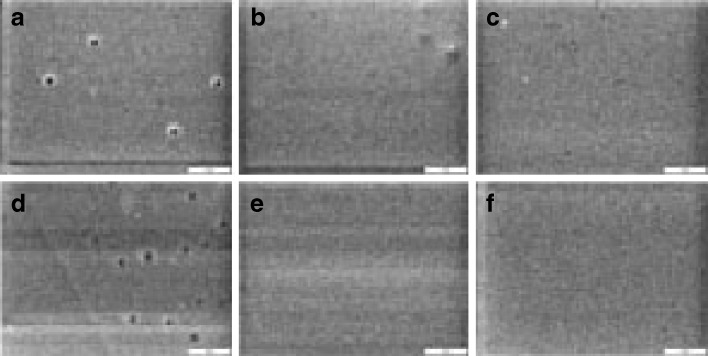
Relatively large surface area of the inclusions especially for the thicker patches can be observed in Fig. 4. Surface morphology of the different samples, after performing of drug release experiment for about 9 h and drying them, was studied by SEM (Fig. 5). There are some pores on the surface of 45 μm patches in different loadings which can be attributed to the more discontinuous crystalline regions formed near the releasing surface of 45 μm patches.
Fig. 5.
Scanning electron micrographs of samples surface after drug release for patches containing 1.1% (w/w) of estradiol in thickness of a 45, b 60, c 90 μm and 1.6% (w/w) in thickness of d 45, e 60 and f 90 μm
Variankaval et al. observed that estradiol crystals were formed near the center of the patch thickness (9). Simulation results supported that crystal growth is limited to the middle third of the thickness (14). However, the crystallization position and its continuity were not similar in the different thicknesses according to our findings (Fig. 1). The crystalline regions were observed near the middle of adhesive layer thickness in the most samples including patches of 60 μm (drug loading = 1.1% w/w) and 90 μm (drug loading = 1.1% and 1.6% w/w) thickness. However, these regions were observed both near the releasing surface (left side) and near the opposite side (backing layer side) for 60 μm patches containing 1.6% (w/w) of estradiol.
Release
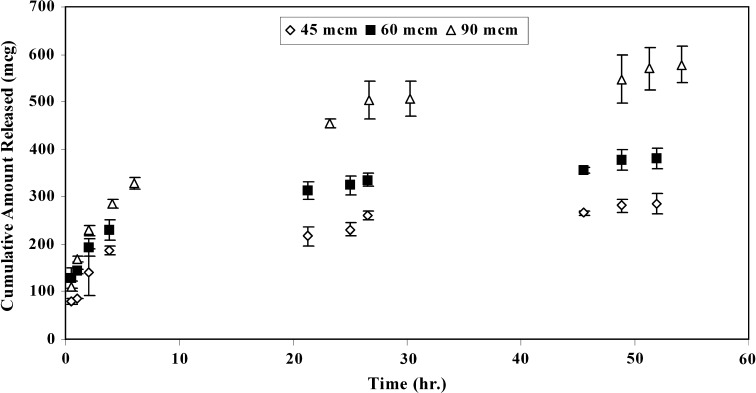
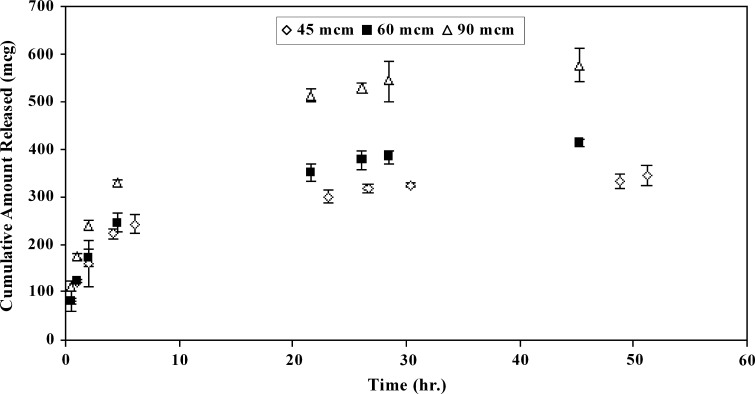
A biphasic release profile was observed for different samples; comprising an initial burst followed by a constant and steady release as shown in Figs. 6 and 7. The released amount of the active agent at the late times (i.e., at constant release phase after ≈ 20 h) was increased with increasing in thickness. There was a linear relationship between the cumulative amount released per surface area and square-root-of-time at the late times for all samples (results not shown) which elucidates that a Higuchi model governs estradiol release from matrix.
Fig. 6.
Release profiles for samples containing 1.1% (w/w) of estradiol in different thicknesses
Fig. 7.
Release profiles for samples containing 1.6% (w/w) of estradiol in different thicknesses
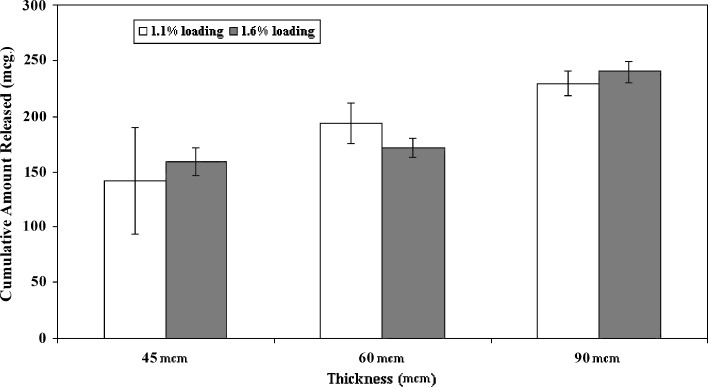
The amounts of estradiol released at initial burst phase were increased with increasing in the sample thickness from 45 to 90 or 60 to 90 μm at different loadings as shown in Fig. 8. However, no significant increase (p ≤ 0.172 for 1.1% loading and p ≤ 0.23 for 1.6% loading) in the amount of drug released at burst phase was observed with increasing of thickness from 45 to 60 μm. It was also shown that changing drug loading from 1.1% to 1.6% (w/w) had no statistically significant (p ≤ 0.57, 0.14, and 0.268 for 45, 60, and 90 μm samples, respectively) effect on the burst amount in all thicknesses. Precise consideration of the burst release results elucidates that the amount released initially (after 0.5 h) for 60 μm patches with estradiol loading of 1.1% w/w (130.18 μg) was largely higher than what obtained for 1.6% w/w patches (79.75 μg). There was no significant difference between the amount released initially (after 0.5 h) from 60 and 90 μm patches with the same content of estradiol (1.1% w/w). At the late times, the release rate was increased for all of the patches with increasing of the thickness and drug loading (Table II).
Fig. 8.
Comparison of burst release for initial 2 h of samples different in thickness (45, 60, and 90 μm) and loading (1.1 and 1.6% w/w)
Table II.
Estradiol Release Rates for Samples with Different Loadings and Thicknesses at Late Times
| Drug loading (% (w/w)) | 1.1 | 1.6 | ||||
|---|---|---|---|---|---|---|
| Thickness (μm) | 45 | 60 | 90 | 45 | 60 | 90 |
| Release rate (μg/cm2h1/2) ± SD | 38.75 ± 1.44 | 49.21 ± 5.14 | 89.25 ± 8.66 | 48.11 ± 5.23 | 74.3 ± 4.90 | 109.43 ± 10.60 |
DISCUSSION
Estradiol has a pronounced tendency to crystallize (8) in polymeric matrices in the absence of anti-nucleating agents. The amorphous form of estradiol is highly unstable and rapidly crystallizes if left exposed to air (9). Estradiol should be cooled rapidly from high temperatures to form amorphous state and immediately used to minimize crystallization (15). In this research, the crystalline state of estradiol was mixed with an acrylic adhesive solution and no specific condition was adopted to turn the drug into its amorphous state during the process of patch fabrication. It is also noteworthy that no other component rather than the drug, amorphous acrylic polymer, and solvents were present in the system. Then, it can be concluded that inclusions observed through the thickness of the adhesive layer absolutely contain estradiol crystals.
The solvent system of the used adhesive was comprised of isopropanol (44% w/w, bp = 82 °C), heptane (34% w/w, bp = 98 °C), ethyl acetate (15% w/w, bp = 77 °C), toluene (7% w/w, bp = 110–111 °C) and 2,4-pentanedione (1% w/w, bp = 140.4 °C). The prepared formulations were dried at room temperature and consecutively at 65 °C in a forced air convection oven until achieving constant weight as described in the section under experimentals. Estradiol solubility in this specific adhesive matrix can be estimated using the linear free-energy relationship formalisms previously developed by Foreman et al. as:
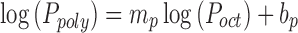 |
1 |
where; Ppoly and Poct are polymer/water and octanol/water partition coefficients of the drug, respectively which can be found in the literature. mp and bp are constants usually determined by regression analysis. Estradiol solubility in Duro-Tak 87–2196 can be calculated based on this empirical model to be nearly 3.04% w/w assuming 4.01 for Log10 of the estradiol partition coefficient between octanol and water and 3.9 mg/L for its saturated solubility in water (16). No crystallization was expected based on these preliminary data hence, the observed crystallization for different samples (Fig. 4) can be attributed to the drying procedure. During the drying process, first, a dried superficial layer is formed at ambient temperature which is more viscous and inhibits solvent molecules from free diffusion to the matrix exterior. Then, estradiol molecules dissolved in the solvent system; accumulate under the superficial layer leading to formation of regions or domains with higher estradiol concentrations that will be supersaturated during the drying process. Then, spontaneous crystallization just below the initially dried superficial adhesive layer occurs. As the value for surface to volume ratio for 45 μm samples is 33% and 50% more than the similar value for 60 and 90 μm samples, respectively, it can be postulated that in 45 μm thickness samples a rapid drying process will be occurred leading to lower crystalline percent with more discontinuity.
Acrylate–vinyl acetate copolymers with large side-groups are amorphous polymers which negates any chance for their crystallization. Then, the relatively large surface area of the inclusions observed (especially for the thicker samples) may be attributed to the polymer inclusion into estradiol crystalline structure or an interaction between estradiol and the adhesive. However, inclusion of the polymer into the crystalline structure of estradiol is not probable which can be confirmed using the findings of Latsch also Iervolino (10,17). Latsch et al., investigating of estradiol hemihydrate crystallization by X-ray powder diffraction, could not detect any evidence that the amorphous acrylate–vinyl acetate copolymer matrix had an effect on the crystal structure (10). Also, according to the findings reported by Iervolino et al., the polymer molecules cannot become included into the Ibuprofen crystals due to their incompatibility in size (17). Evidences on the occurrence of transesterification reaction between estradiol and acrylic adhesive have been investigated (15). Also, hydrogen bonding between hydroxyl groups of estradiol and carbonyl groups of the acrylate–vinyl acetate copolymer is possible. Hydrogen bonds have been shown to drive molecular associations that lead to crystalline or non-crystalline solids due to their strength and directionality (18). Hydrogen bonding can change the morphology of drug crystals if the degree of polymer adsorption at different crystal sides is different (19). It can be concluded that the large surface area of the inclusions through the adhesive layer thickness may be related to a transesterification or hydrogen bonding between the drug and adhesive polymer.
There are four different crystal forms of estradiol, two of which are solvated and the other two are anhydrous (20). It may seem that formation of the solvated forms of estradiol including estradiol hemihydrate occurs in aqueous solutions. However, the most characteristic property of estradiol is its tendency to adopt the hemihydrated form, which it precipitates in this state, not only from partially aqueous solutions, but also from ethyl acetate, chloroform, absolute ethanol, and other anhydrous solvents (11). It has been demonstrated that water is selectively incorporated into the lattice when estradiol crystallizes from solution and the presence of water molecules is a crucial requirement for the crystallographic stability of estradiol (21). Different studies on the crystallization of estradiol (8,20) and estradiol hemihydrate (10) have proved formation of estradiol hemihydrate in acrylic adhesives. Estradiol (Fig. 1) has two hydroxyl groups capable of forming hydrogen bonds with acrylate–vinyl acetate copolymer. However, the inclusions observed by ordinary microscopy in Fig. 4 can be more related to the transesterification reaction or hydrogen bonds formation between estradiol hemihydrate and adhesive polymer.
Estradiol hemihydrate can exist as a tetra-functional agent (Fig. 2) which acts as a strong physical or chemical cross-linker in both cases of hydrogen bonding or transesterification interactions, respectively. Investigation of all micrographs (for samples different in loading and thickness) shows that the ratio of crystalline regions to the total surface area of adhesive layer is increased with increasing in the adhesive layer thickness. Also, crystallization is increased with increasing of estradiol content. The increasing of crystalline regions proportion with thickness can be attributed to a longer nucleation time available for thicker samples. Drying of the thicker samples is completed in a longer period of time which leads to the formation of more supersaturated regions in the adhesive layer due to solvent migration. Referring to Zang et al. report on the simulation of crystal growth in an adhesive matrix (14), the crystals are smaller at lower temperatures. As surface evaporation of solvents leads to a severe drop in surface temperature, the temperature is lower just beneath the superficial layer in thicker samples. Then, It is expected that in thicker samples smaller crystals with more interaction surface with the adhesive to be formed.
Some of the crystallization results which can be confirmed by study of the release curves:
No significant increase (p ≤ 0.172 for 1.1% loading and p ≤ 0.23 for 1.6% loading) in the burst release was observed with increasing of thickness from 45 to 60 μm which can be related to a severe increase in the area of crystalline regions. It should also be noted that the presence of crystalline regions decrease the drug concentration in the dissolved state throughout the matrix.
It was also shown that changing drug loading from 1.1% to 1.6% (w/w) had no statistically significant (p ≤ 0.57, 0.14, and 0.268 for 45, 60, and 90 μm samples, respectively) effect on the amount of drug released at burst phase in all thicknesses which can be attributed to no severe changes in crystallization regions.
More precise consideration of the burst release results elucidates that the amount of drug released initially (after 0.5 h) for 60 μm samples at 1.1% (w/w) of estradiol loading was largely higher than what obtained for 1.6% (w/w) samples i.e., 130.18 vs. 79.75 μg which can be attributed to positioning of estradiol crystalline region near the releasing surface of the 1.6% (w/w) samples.
There was no significant difference between the amount of estradiol released initially (after 0.5 h) from 60 and 90 μm samples with the same content of estradiol (1.1% w/w). This can be mainly due to more discontinuity in the crystalline regions in the 60 μm samples which leads to higher surface area of the crystalline regions and more drug migration toward the surface. At the late times, release rate was increased for all of the samples with increasing of the thickness and drug loading (Table II) that can be attributed to the disappearance of the observed crystalline regions.
CONCLUSIONS
This study showed that on the basis of theoretical prediction of drug solubility in the adhesive polymer, it cannot be concluded that the product will be really devoid of crystals. A major increase in the crystallization degree and continuity was observed beyond the thickness of 45 μm. It can be concluded that there is a critical point of thickness about this and less amount of crystallization inhibitor is required if the adhesive thickness is about 45 μm. No significant increase was observed in the burst release with increasing in thickness from 45 to 60 μm which can be assigned to the severe increase in the area of crystalline regions. Also, formation of a crystalline layer near the releasing surface and more discontinuous pattern of the crystals in some samples was confirmed by investigation of the drug release curves.
Acknowledgment
The authors express their sincere thanks to Ms. Mivehchi for her kind assistance in editing the manuscript. We also express sincere gratitude to Mr. Hamid Mahdavi for technical assistance and also Permegear Co. (Hellertown, USA) for granting us copyright of Fig. 3.
References
- 1.Tan HS, Pfister WR. Pressure-sensitive adhesives for transdermal drug delivery systems. Pharm Sci Technol Today. 1999;2:60–69. doi: 10.1016/S1461-5347(99)00119-4. [DOI] [PubMed] [Google Scholar]
- 2.Venkatraman S, Gale R. Skin adhesives and skin adhesion 1. Transdermal drug delivery systems. Biomaterials. 1998;19:1119–1136. doi: 10.1016/S0142-9612(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 3.Wick SM. Developing a drug-in-adhesive design for transdermal drug delivery. Adhes Age. 1995;38:18–24. [Google Scholar]
- 4.Marty JP. Menorest: technical development and pharmacokinetic profile. Eur J Obstet Gynecol Reprod Biol. 1996;64:S29–S33. doi: 10.1016/0301-2115(95)02354-2. [DOI] [PubMed] [Google Scholar]
- 5.Megrab NA, Williams AC, Barry BW. Oestradiol permeation through human skin and silastic membrane: effects of propylene glycol and supersaturation. J Control Release. 1995;36:277–294. doi: 10.1016/0168-3659(95)00062-D. [DOI] [Google Scholar]
- 6.Yu J, Chien T, Chien YW. Transdermal dual-controlled delivery of testosterone and estradiol: (I) impact of system design. Drug Dev Ind Pharm. 1991;17:1883–1904. doi: 10.3109/03639049109048057. [DOI] [Google Scholar]
- 7.Rodriguez-Hornedo N, Murphy D. Significance of controlling crystallization mechanisms and kinetics in pharmaceutical systems. J Pharm Sci. 1999;88:651–660. doi: 10.1021/js980490h. [DOI] [PubMed] [Google Scholar]
- 8.Lipp R, Müller-Fahrnow A. Use of x-ray crystallography for the characterization of single crystals grown in steroid containing transdermal drug delivery systems. Eur J Pharm Biopharm. 1999;47:133–138. doi: 10.1016/S0939-6411(98)00055-1. [DOI] [PubMed] [Google Scholar]
- 9.Variankaval NE, Jacob KI, Dinh SM. Crystallization of beta-estradiol in an acrylic transdermal drug delivery system. J Biomed Mater Res. 1999;44:397–406. doi: 10.1002/(SICI)1097-4636(19990315)44:4<397::AID-JBM5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Latsch S, Selzer T, Fink L, Kreuter J. Crystallization of estradiol containing TDDS determined by isothermal microcalorimetry, X-ray diffraction, and optical microscopy. Eur J Pharm Biopharm. 2003;56:43–52. doi: 10.1016/S0939-6411(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 11.Salole EG. The physicochemical properties of oestradiol. J Pharm Biomed Anal. 1987;5:635–642. doi: 10.1016/0731-7085(87)80076-6. [DOI] [PubMed] [Google Scholar]
- 12.International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. ICH harmonized tripartite guideline: validation of analytical procedures: text and methodology Q2(R1), Nov. 2005.
- 13.Bolton S, Bon C. Pharmaceutical statistics: practical and clinical applications. New York: Marcel Dekker; 2004. pp. 289–310. [Google Scholar]
- 14.Zeng J, Jacob KI, Tikare V. Numerical simulations of crystal growth in a transdermal drug delivery system. J Cryst Growth. 2004;262:602–611. doi: 10.1016/j.jcrysgro.2003.06.002. [DOI] [Google Scholar]
- 15.Zeng J. Constrained crystallization and depletion in the polymer medium for transdermal drug delivery system. Ph.D. thesis, Georgia Institute of Technology, 2004.
- 16.Foreman P, Hansen A, Silverberg E, Manegold T, Yang H, Li J, et al. Predicting drug solubility in transdermal adhesives. 37th AAPS Annual Pharmaceutical Technologies Conference; 2002 Jan 13–18; New York, USA.
- 17.Iervolino M, Cappello B, Raghavan SL, Hadgraft J. Membrane penetration enhancement of ibuprofen using supersaturation. Int J Pharm. 2000;198:229–238. doi: 10.1016/S0378-5173(00)00346-X. [DOI] [PubMed] [Google Scholar]
- 18.Seefeldt K, Miller J, Alvarez-Nunez F, Rodriguez-Hornedo N. Crystallization pathways and kinetics of carbamazepine-nicotinamide cocrystals from the amorphous state by in situ thermomicroscopy, spectroscopy and calorimetry studies. J Pharm Sci. 2007;96:1147–1158. doi: 10.1002/jps.20945. [DOI] [PubMed] [Google Scholar]
- 19.Raghavan SL, Trividic A, Davis AF, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001;212:213–221. doi: 10.1016/S0378-5173(00)00610-4. [DOI] [PubMed] [Google Scholar]
- 20.Variankaval NE, Jacob KI, Dinh SM. Polymorphism of 17-beta estradiol in a transdermal drug delivery system. J Mater Sci Mater Med. 2002;13:271–280. doi: 10.1023/A:1014058817352. [DOI] [PubMed] [Google Scholar]
- 21.Salole EG. Estradiol. In: Florey K, editor. Analytical profiles of drug substances, vol 15. New York: Academic; 1986. pp. 283–318. [Google Scholar]