Abstract
Levothyroxine is a narrow therapeutic index, and to avoid adverse effect associated with under or excessive dosage, the dose response is carefully titrated. The tablets are marketed with a score providing an option to split. However, there are no systematic studies evaluating the effect of splitting on dose accuracy, and current study was undertaken to evaluate effects of splitting and potential causes for uniformity failures by measuring assay and content uniformity in whole and split tablets. Stability was evaluated by assaying drug for a period of 8 weeks. Effect of formulation factors on splittability was evaluated by a systematic investigation of formulation factors by preparing levothyroxine tablets in house by varying the type of excipients (binder, diluent, disintegrant, glidant) or by varying the processing factors (granulating liquid, mixing type, compression pressure). The tablets were analyzed using novel analytical tool such as near infrared chemical imaging to visualize the distribution of levothyroxine. Assay was not significantly different for whole versus split tablets irrespective of method of splitting (hand or splitter), and splitting also had no measurable impact on the stability. Split tablets either by hand or splitter showed higher rate of content uniformity failures as compared to whole tablets. Tablet splitter produced more fragmentation and, hence, more content uniformity and friability failures. Chemical imaging data revealed that the distribution of levothyroxine was heterogeneous and was dependent on type of binder and the process used in the manufacture of tablets. Splitting such tablets could prove detrimental if sub- or super-potency becomes an issue.
Key words: content uniformity, hand splitting, levothyroxine sodium, narrow therapeutic index, splitter, tablet splitting
INTRODUCTION
Tablet splitting is a practice often recommended by healthcare providers and implemented by healthcare systems aimed at reducing the cost of prescriptions. It also allows the practitioner to administer lower dose to better titrate the dose and facilitate administration of large tablets to patients that may find difficulty swallowing as the whole tablets, e.g., scored Augmentin XR ER Tablets are available for greater convenience to adult patients who have difficulty swallowing. The scored tablet is not intended to reduce the dosage of medication taken.
Tablets are either hand-splitted or use of a variety of tablet splitters has been frequently cited for scored or unscored tablets (1–3). It is an easy-to-use device in which tablets are cut into halves and is reported to have higher patients adherence due to ease of splitting as well as cost benefits (1,2). For instance, Fawell and colleagues (2) in a study of 1,617 patients who were divided into groups that split and did not split tablets found that tablet splitting did not hinder compliance as measured by tablet counting. Moreover, patients reported no concerns with tablet splitting, and only 4% thought that it affected their willingness to take medication. Also, it is reported that splitting may not be detrimental to drugs with a high therapeutic index such as psychotropics since their clinical actions depend primarily on relatively long-term alterations in neurotransmitter production and receptor sensitivity. Small variations in doses are generally not critical to effectiveness (4). Similar results were found for other classes of drugs (5,6).
In spite of all the above advantages of tablet splitting, critics have identified a number of potential concerns related to this practice. Some of the concerns relate to weight variation, uneven drug content, and drug stability in the half tablets. These quality concerns can potentially impact the product performance and are especially true for drugs with narrow therapeutic index, drugs with closely spaced multiple strengths and drugs with nonlinear pharmacokinetics. Several studies have indicated that tablet splitting may result in high incidences of weight variation that may or may not be of clinical consequence depending on the drug being split (6–13). McDevitt et al. (12) found that 41% of split tablets deviated from the ideal weight by more than 10% and that 12% of pills deviated by more than 20%. Such differences can be critical with certain heart or thyroid medications with a narrow therapeutic index. In such case, splitting could bring about subpotency or superpotency issues which might cause harm to the patients.
Levothyroxine tablet is cited as one of the drug products that could be safely split to cut the cost (14). However, it is a drug recognized to have a narrow toxic to therapeutic ratio with significant clinical consequences of excessive or inadequate treatment. There are 12 available tablet strengths of levothyroxine that vary by as little as 9% in drug content, reflecting the close titration that is required for optimal patient management. Thirteen million Americans are on levothyroxine products and those especially susceptible to incorrect titration include the elderly, pregnant women, their developing fetuses, and those with thyroid cancer (15).
Levothyroxine is an example of a narrow therapeutic index drug as well as critical dose drug (16). Narrow therapeutic index drugs are defined as less than a 2-fold difference in median lethal dose (LD50) and median effective dose (ED50) or less than 2-fold difference in the minimum toxic concentration and minimum effective concentration in the blood. Critical dose drugs are defined as the drugs in which comparatively small differences in dose or concentration may lead to serious therapeutic failures and/or serious adverse drug reactions. Levothyroxine has a complex stability profile and is sensitive to light, temperature, moisture, pH, and oxidation (17–22). Tablets split in advance and returned into the bottle may be subject to stability problems: increased friability and fragmentation, hygroscopic adsorption of water, and altered shelf-life due to, for example, a break in the tablet’s protective coating (12). Splitting a tablet might compound the problem of stability and thus ultimately affect the dose uniformity.
In recognition of the problems of dose consistency, two advisory committee meetings were held in US Food and Drug Administration to discuss the potency and stability issues of levothyroxine sodium drug products. The American Thyroid Association, the Endocrine Society, and the American Association of Clinical Endocrinologists indicated a lack of consistency of clinical performance. As a consequence of those meetings and recommendations, the potency limits were tightened from 90–110% to 95–105% to improve the uniformity of Levothyroxine inter-batch and for a closer dose titration (23).
The present research was aimed at evaluating effect of splitting on dose uniformity of levothyroxine sodium and product stability. Weight variation or assay has been used to determine dose uniformity of split tablets; however, we hypothesize that these might not be the best practice and that content uniformity might be a better measure of dose uniformity of split tablets of narrow therapeutic drugs. Also in the present research, we used novel technique such as near infrared chemical imaging to view chemical composition on the surface of tablets as it has the potential to predict spatial identification of species in a sample. This nondestructive technique combines traditional near infrared spectroscopy with microscopic and macroscopic imaging to produce an integrated near infrared system.
MATERIALS AND METHODS
Chemicals
Levothyroxine sodium tablets were purchased from a local CVS caremark pharmacy (Silver Spring, MD): L-1, L-2, L-3, L-4, and L-5. HPLC ready deionized 18 MΩ water was obtained, in-house, from a Milli-Q Gradient A-10 water purification system, Millipore, (Bedford, MA). l-Thyroxine sodium (T4) was obtained from KVPharmaceutical (St. Louis, MO). 3,3′,5-Triiodo-l-thyronine (T3), 3,5-diiodo-l-thyronine (T2), 3,5-diiodo-l-tyrosine (DIT), 3-iodo-l-tyrosine (MIT), l-thyronine (T0), l-tyrosine (Tyr), 3,3′,5-tri-iodo-l-thyroacetic acid, and 3,3′,5,5′-tetra-iodo-l-thyroacetic acid, Inertsil 5 μm column, 250 × 4.6 mm, and security guard cartridge were purchased from Sigma (St. Louis, MO). Theophylline reagents, Methanol, 0.01 M NaOH, 0.1% trifluoroacetic acid (TFA), acetonitrile, and fisherbrand low adhesion specialty tips (21-381-83) were purchased from Fisher Sci (Suwanee, GA).
For dissolution studies: Acrodisc CR 25-mm syringe filters were purchased from the Pall Corp. (Ann Arbor, MI). Potassium phosphate monobasic, phosphoric acid, and hydrochloric acid were purchased from Fisher Scientific (Fairlawn, NJ). Sodium lauryl sulfate was purchased from Sigma (St. Louis, MO).
For preparation of tablets: lactose monohydrate (Kerry BioScience, Chicago, IL), talc (Spectrum Chemicals, Gardena, CA), colloidal silicon dioxide (Aerosil, Evonik Degussa, Orange, CA), microcrystalline cellulose (FMC Biopolymer, Philadelphia, PA), sodium starch glycolate (Explotab, Patterson, NY), hydroxypropylmethyl cellulose (HPMC), corn starch, and mannitol (Sigma, St. Louis, MO) were used as received.
Marketed Tablets
Levothyroxine tablets from each manufacturer were split into two parts in two different ways—using hand-splitting and using a tablet-splitter (Apothecary Products, Inc., Burnsville, MN). Thirty-four intact tablets and 68 split halves of each product were randomly dispensed into individual amber pharmacy container for each time point and closed using a child-resistant cap. Since manufacturing dates were unknown for the marketed samples, the stability study was conducted 5 months before their expiry date which was considered week 0 in our study. Tablets were stored in a controlled condition (temperature between 20°C and 22°C) prior to start of the stability studies. Conducting stability studies 5 months prior to expiry can be used to compare the stability profiles head-to-head since the manufacturing dates for all different marketed tablets might be different. Samples were stored in monitored environmental chambers (Hotpack, Philadelphia, PA or Electrotech, Glenside, PA) under long-term storage conditions (25°C/60% relative humidity (RH)) for 8 weeks. On the appropriate time points (0, 4, and 8 weeks), the closed pharmacy vials were removed from the incubators and allowed to equilibrate at room conditions for at least 1 h. Samples were then analyzed for potency. At 0 week time point, the samples were also analyzed for weight variation, content uniformity (CU), and dissolution. Also near infrared (NIR) chemical imaging was used to visualize the contents of the surface of the whole tablets.
Preparation of Tablets
A fractional factorial screening design of experiments was employed to create 12 different formulations under a Plackett Burman design. The diluent (~82.6%) was either lactose monohydrate or mannitol; the disintegrant (10% w/w) was either Na starch glycolate or Na carboxymethylcellulose, binder (5% w/w) was either starch or HPMC, and glidant (2% w/w) was either talc or silicon dioxide (SiO2). Levothyroxine (200 μg/tablet) was mixed as either dry or was sprayed as a solution in the granulating liquid (10 mM NaOH solution or methanol). All the ingredients, except glidant, were mixed and granulated using granulating liquid. The granules were passed through sieve # 16, dried in an oven at 40°C until loss on drying value of <2% was obtained. The dried granules were again sieved through sieve #20, and glidant was added to the granules and mixed in a V blender for 15 min at 15 rpm. The granules were compressed on a rotary tablet press (Globepharma, NJ) at compression pressure of either 1,000 or 2,000 lbs. The in-house-manufactured tablets were evaluated for content uniformity for split portions to evaluate main factors responsible for the failure to meet specification limits. Also, tablet surfaces were viewed with NIR chemical imaging to visualize distribution of various components.
Potency and CU
Although various HPLC methods of potency and content uniformity determination exist in USP, it does not separate all the eight potential degradation impurities. Therefore, we utilized previously published method for the determination of levothyroxine from the tablet samples (24). The method was applicable for all the tablets included in the study. The calibration samples for potency and CU were prepared in 0.01 M methanolic NaOH. Levothyroxine Na and all the impurities (T3, T2, T0, MIT, DIT, T3AA, T4AA, and Tyr) were added into the calibration standards. An internal standard, theophylline, was also added to all the above diluted calibration ranges. The standards were then transferred to an automatic injector for HPLC analysis.
Potency determination for tablet samples was performed on each stability time point. Twenty whole tablets or split portions were transferred to a mortar and ground with a pestle to a fine, homogenous powder. Powder obtained from tablet grinding was aliquot into labeled vials for subsequent potency experiments.
CU determination for tablet samples was performed by grinding 10 whole tablets or split portions individually followed by quantitatively transferring them into individual volumetric flasks for subsequent CU experiments.
HPLC method used for all the samples included a reversed phase Inertsil ODS 2 column (250 × 4.6 mm, 5 μm, 150 A) with Inertsil ODS Security Guard cartridge (4.0 × 3.0 mm, 10 μm). A gradient elution included 0.1% TFA (A) and Acetonitrile (B) from 92% to 8% A in 25 min, at 8% B and 92% A from 25–30 min, from 8% to 92% A from 30–35 min. The flow rate was 0.8 mL/min, the column temperature was 25°C, and the injection volume was 50 μL. The UV detection wavelength was set at 215, 223, 228, 232, and 240. However, all the calculations were performed at 223 nm. The amount of levothyroxine in the test samples was calculated, as quantity and percent remaining, from the measured peak area response for the test samples (ru) and compared to peak area response (rs) for the standard levothyroxine solution using the following equation
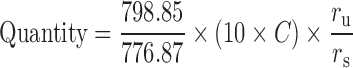 |
1 |
C is the concentration in micrograms per milliliter of the USP levothyroxine reference standard and 798.85 and 776.87 are the molecular weights of levothyroxine sodium and levothyroxine.
Friability
A friability apparatus (USP, Globepharma, NJ) was used at 100 drops in 4 min. An exception was made to the sample size from USP method since USP recommends 6.5 g of tablet weight. However, in the study, 30 whole tablets (approx weight of 3.8 g) and 60 split halves for half tablets (both hand and splitter cut) were used. The tablets were weighed and placed in the drum and were rotated at 25 rpm for 4 min. At the end of the test, the tablets were weighed, the weight loss was determined, and the percent friability was calculated.
Dissolution
A calibrated dissolution apparatus (USP II, (Varian, Palo Alto, CA)) was used with paddles at 50 and 75 rpm depending on the drug product tested. The bath temperature was maintained at 37 ± 1°C. Dissolution medium consisted of degassed 0.01% HCl solution with 0.2% sodium lauryl sulfate. Six tablets per drug product were evaluated, and dissolution samples were collected at 15 and 45 min. At each time point, a 2-mL sample was removed from each vessel using a glass syringe and filtered through an acrodisc filter (0.45 μm, 25 mm) into labeled glass tubes. One milliliter was removed and transferred to the HPLC vial. Next, 1 mL of 0.01 M phosphate buffer (pH = 3.0)/methanol solution (45:55) was added to the HPLC vial, vortexed, and analyzed by HPLC.
A single method of HPLC analysis for dissolution samples was used for all the study tablets of levothyroxine sodium (marketed and in-house), although different methods exist in USP. The previously published method was developed, validated, and found to be suitable for the analysis of the samples (25). Briefly, a Nova-pak C18 column (3.9 × 150 mm, 4 μm, Waters) fitted with a Nova-pak guard column (3.9 × 20 mm, Waters). The flow rate was 1.0 mL/min. The elution was achieved by gradient elution: 0.01 M phosphate buffer (pH = 3.0) (A) and methanol (B) from 45% to 20% A in 7 min, at 20% A from 7 to 12 min, from 20% to 45% A from 12 to 16 min, and at 45% A to 20 min as equilibration time. The column temperature was 28°C, and the injection volume was 800 μL. The UV detection wavelength was 225 nm.
The% dissolved was calculated from the following equation:
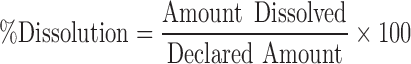 |
2 |
NIR Chemical Imaging
Sapphire® chemical imaging system (Malvern, Westborough, MA) with SapphireGo® data acquisition system (version 1.4) equipped with diffuse reflectance detector was used to collect NIR spectra from 1,400 to 2,350 nm in 10 nm increments. Each tablet was scanned eight times and averaged on the imager. Background signal was removed initially by subtracting a dark cube without any sample. Spectralon-99% was used as a reflectance standard which is nominally 99% reflectant over the 350–2500 nm range. Each sample spectra is then recorded as a ratio of the specimen reflectance and the source light reflected from a Spectralon 99%. The pure component spectra were used to create a spectral library against which each tablets’ spectra were analyzed.
Preprocessing and data transformation were carried out on ISys® chemical imaging software (Version 5.0, Malvern). All the reflectance spectra were converted to absorbance spectra, and the background was masked by applying sample statistics showing two distinct regions for tablet and background. The masked data for pure components were truncated to 50 × 50 pixels where each pixel represents 40 × 40 μm. The images were analyzed with and without data transformation. For the transformation, normalization (mean center and scale to unit variance by spectrum) was applied. A PLS-2 model was developed based on the spectral library of pure components and was applied to each tablet. Untransformed images and spectral data were examined to understand physical characteristics on the tablet surface.
Statistical Analysis
Statistical analysis was performed using a single factor ANOVA or unpaired Student’s t test assuming equal variance. A p value of <0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
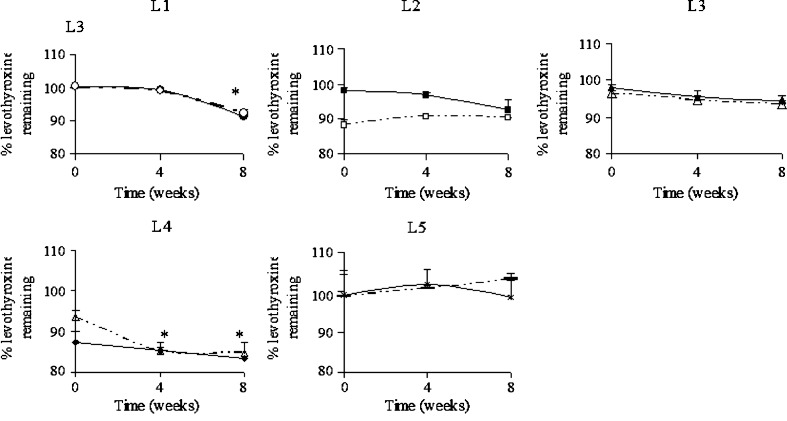
The current study was undertaken to evaluate the effects of splitting and the potential causes for uniformity failures. Five marketed drug products which included several brand and generic levothyorixne tablets were evaluated for effect of splitting on dose uniformity by performing both assay and content uniformity using the methods described above. The tablets were split either by hand or tablet splitter. The assay for all the five marketed tablets for whole as well as split portions are shown in Fig. 1a–e. The time zero refers to the day of 5 months before expiry of these tablets since manufacturing dates were unknown. This approach makes it possible to compare various formulations from different manufacturers to see whether the assay values were within the range. A specification range of 90–110% was followed since the tablets were purchased before Oct 2009, after which, the assay specifications was tightened by USP to be within 95–105% (23). Except for L-4, all the other whole tablets were well within the range. Since we found that L-4 was still within the expiry but failed to meet assay specifications, another lot of product was bought and assayed to confirm that the extraction method used was able to extract the drug. For the new lot, the potency was found to be within the specification range. Thus, the extraction procedure was adequate and sub-potency of L-4 product might be due to rapid degradation of levothyroxine from the product even though it was within the expiry. In practice, when such failures are observed, the sponsors are required to reduce the expiration dating and recall the lots that failed to meet the quality standards from the market. Potency and stability issues have been prevalent for levothyroxine products, and a better reformulation should prevent future problems. The formulations were undergoing transition to reformulated products so as to meet new USP potency requirements; hence, it was decided to continue with old lot of L-4 product.
Fig. 1.
Assay of whole (solid line) and splitter cut tablets (dashed line) of marketed levothyroxine sodium tablets L-1 to L-5 (*p < 0.05)
Effect of splitting was evaluated by splitting the tablets in to halves either by hand or by using a tablet splitter. Splitting was done by one analyst to avoid inter-person variability. However, in actual use, it will be done by different individuals and, therefore, might be variable since accuracy of tablet splitting also depends on one’s technique or device (26). Moreover, patients or their caregivers must have good vision, manual dexterity, and the mental capacity to accurately split a tablet. It was found that splitting with hand was challenging since the diameter of tablets varied from 5 to 8 mm for round- or oblong-shaped tablets, respectively. It has been cited that splitting a smaller tablet is generally more challenging than a larger size tablet. Spang (27) states that scored tablets should be at least of 8 mm diameter to be well manageable. This is in line with the finding of Muller and Kublik (28) who found in four commercial formulations that the smallest tablet, with 7 mm diameter, was the most difficult to break. Although most of the tablets were about 8 mm in diameter, it was found to be difficult to split them uniformly. Although an increasing attention is paid to breaking ease by the researchers, no formal regulatory requirements exist for this quality issue of scored tablets. It is known that shape also plays an important role in uniform splitting. In case of levothyroxine, except L-1, all the other marketed products were oblong-shaped. Round tablets being smallest in size (5 mm diameter) were found to be most challenging in terms of hand-splitting. All these tablets were scored; therefore, differences due to score line might not be a factor although depth of score line is known to affect the splittability of a tablet (29).
Assay was performed on all the split portions for all the marketed formulations (Fig. 1a–e). At time zero, which refers to the 5 months before expiry, it was found that most of the split portions passed the assay splitted by splitter and hand, respectively. Split portions if kept for later use might undergo degradation if the active pharmaceutical ingredient (API) is sensitive to external factors such as oxygen, light, or moisture. Therefore, stability studies were carried out for split tablets which were stored at 25°C/60% RH and were compared with the stability of whole tablets also stored under the long-term stability condition. Results are presented in Fig. 1a–e.
It was seen that L-1 formulations showed significantly lower amount of levothyroxine sodium at 8 weeks as compared to earlier time points for whole as well as splitter cut tablets (p < 0.05; Fig. 1a). The assay values were well within the range of 90–110% at the end of 8 weeks. There was no significant difference between whole and split portions stability as seen from Fig. 1a.
For formulations L2, L-3, and L-5, there were no signs of instability as seen from Fig. 1b, c, and e, respectively. For L-2, although splitter cut portions seem to have higher assay values at 4 and 8 weeks as compared to time 0, they were not statistically significant (p > 0.05) since they were within assay variability (Fig. 1b), Although assay values for formulation L-3 at times 4 and 8 weeks were lower than week 0 for all the tablets, the results were not statistically different (p > 0.05; Fig. 1c). For formulation L-5, whole as well as split portions showed no statistically significant differences for stability time points as compared to time 0 for whole and split portions (p > 0.05).
For formulation L-4, assay values for all the tablets showed signs of instability at 8 weeks as the values were significantly different as compared to time 0 (p < 0.05; Fig. 1d). The splitter cut portion showed slightly higher rate of instability from 0 to 4 weeks as compared to whole tablets. Increase in the exposed surface area might play a role in accelerating the reaction and thus causing instability. However, it was not observed in other tablet formulations. It is known that certain excipients help to prevent degradation of active pharmaceutical ingredients. It was observed that L-4, although within expiry, had lower levothyroxine assay values which were outside of 90–110%. Lack of complete extraction was ruled out by conducting assay on different lot of L-4 for which the assay was found within the range.
Thus, it was observed from assay results that the split portions (hand or splitter cut) in general were not significantly different as compared to whole tablets stability profile except of L-4 which showed slightly accelerated rate initially.
Since assay is a composite test, content uniformity was also performed for all the split portions and was compared with whole tablets (Table I). Since content uniformity failed for L-4 whole tablets, split portions also failed to have levothyroxine content within the specifications. A higher fragmentation was observed for tablets cut with the splitter as compared to those cut with the hand (Fig. 2), and this might be the reason for the higher failures observed for the splitter cut portions. Dissolution on whole and split tablets was performed, and the results were in line with content uniformity, i.e., for split portions, some of the tablets failed to meet the USP specification as compared to whole tablets (Table II). It can be noted that the dissolution failures were less pronounced as compared to CU since the specifications are more relaxed for dissolution as compared to CU specifications. Therefore, dissolution might not be the best quality-control test for immediate release split tablets. However, this cannot be generalized, and a case-by-case approach is needed to confirm. It should be noted that dissolution test could prove to be very valuable for extended release tablets when they are splittable.
Table I.
Content Uniformity of Split Tablets of Levothyroxine Sodium Marketed Products
| Tablet | L-1 | L-2 | L-3 | L-4 | L-5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole | Hand | Splitter | Whole | Hand | Splitter | Whole | Hand | Splitter | Whole | Hand | Splitter | Whole | Hand | Splitter | |
| 1 | 103.2 | 110.0 | 111.9 | 106.5 | 100.4 | 101.4 | 100.0 | 100.2 | 111.1 | 77.5 | 89.6 | 91.4 | 98.1 | 84.5 | 88.2 |
| 2 | 102.7 | 97.5 | 89.9 | 101.1 | 102.4 | 84.2 | 97.3 | 101.8 | 80.2 | 79.8 | 83.1 | 78.4 | 103.6 | 118.9 | 99.5 |
| 3 | 102.7 | 95.8 | 111.5 | 101.7 | 105.2 | 98.9 | 97.8 | 92.8 | 109.1 | 81.5 | 90.7 | 82.5 | 98.7 | 104.1 | 93.3 |
| 4 | 102.6 | 118.4 | 90.9 | 98.3 | 97.4 | 85.7 | 97.3 | 110.2 | 90.8 | 79.3 | 80.2 | 81.9 | 101.2 | 88.9 | 101.9 |
| 5 | 102.4 | 115.9 | 118.0 | 100.0 | 99.6 | 102.0 | 99.2 | 96.2 | 110.1 | 77.9 | 82.1 | 81.2 | 102.0 | 99.0 | 100.8 |
| 6 | 100.6 | 91.0 | 92.7 | 97.4 | 100.3 | 86.5 | 99.2 | 104.0 | 82.2 | 81.5 | 92.9 | 87.2 | 102.7 | 100.3 | 92.5 |
| 7 | 104.0 | 97.3 | 114.3 | 99.2 | 95.7 | 93.3 | 99.6 | 105.2 | 106.5 | 81.7 | – | 88.0 | 101.6 | 93.0 | 104.3 |
| 8 | 106.2 | 110.1 | 95.1 | 99.1 | 112.0 | 93.7 | 100.5 | 95.9 | 95.5 | 78.4 | 83.5 | 80.5 | 99.8 | 110.3 | 96.8 |
| 9 | 103.2 | 99.4 | 119.4 | 100.0 | 94.8 | 101.4 | 99.9 | 100.2 | 106.0 | 83.6 | 79.3 | 76.7 | 102.7 | 102.3 | 100.1 |
| 10 | 102.3 | 108.0 | 89.9 | 100.3 | 98.4 | 97.9 | 100.7 | 101.8 | 87.2 | 87.1 | 88.4 | 74.4 | 101.1 | 92.0 | 94.5 |
| Mean | 103.0 | 104.3 | 103.4 | 100.4 | 100.6 | 94.5 | 99.1 | 100.8 | 97.9 | 80.8 | 85.5 | 82.2 | 101.2 | 99.3 | 97.2 |
| Stdev | 1.4 | 9.3 | 12.6 | 2.5 | 5.0 | 6.9 | 1.3 | 5.0 | 12.1 | 2.9 | 4.7 | 5.3 | 1.8 | 10.3 | 5.0 |
| % CV | 1.4 | 8.9 | 12.2 | 2.5 | 5.0 | 7.3 | 1.3 | 5.0 | 12.4 | 3.6 | 5.4 | 6.5 | 1.8 | 10.4 | 5.2 |
The assay values presented as italic bold red fonts represent the failure to meet specification of 90.0% to 110.0% of assay value. All the whole tablets except L-4 met the specification, however, failures were observed for both hand- and splitter- split portions for all the marketed tablets
Fig. 2.
Marketed levothyroxine tablets (L-3) cut with hand and tablet splitter. Tablet splitter produced more fragments for all the tablets (only figure for L-3 is shown here)
Table II.
Dissolution Results of Whole Versus Split Marketed Levothyroxine Tablets
| Dissolution time point | L-1 | L-2 | L-3 | L-4 | L-5 | |
|---|---|---|---|---|---|---|
| Whole | 15 min | 58.62 ± 4.09 | 84.19 ± 3.76 | 95.52 ± 1.83 | 76.39 ± 2.09 | 57.19 ± 2.77 |
| 45 min | 82.41 ± 13.5 | 86.22 ± 2.92 | 77.11 ± 2.15 | 80.80 ± 1.85 | 63.09 ± 4.21 | |
| Split | 15 min | 100.75 ± 26.03 | 80.60 ± 5.57 | 97.97 ± 10.59 | 84.70 ± 4.62 | 61.54 ± 9.06 |
| 45 min | 121.52 ± 21.6 | 81.18 ± 4.11 | 86.18 ± 7.13 | 88.26 ± 7.71 | 70.15 ± 9.41 | |
| USP specifications | 45 min | NLT 80% | NLT 80% | NLT 70% | NLT 70% | NLT 70% |
It might be difficult to position the blade directly on the score line and get a clean cut for some tablets (11,30). There have been concerns that splitter does not produce equal halves, and weight variation in split portions have been observed for many formulations. For very narrow therapeutic window, it might be important to accurately dose the patient. Levothyroxine is a narrow therapeutic index drug with clinical efficacy dependent even on slight variation in its dose. For this reason, very recently, USP monograph was updated to reflect potency limits from 90–110% to 95–105% (23) in order to ensure that the tablets can adequately provide therapeutic concentrations needed for the patients. Caution should be exercised when splitting tablets for drugs with short half-lives or low therapeutic indices because even slight variability may affect clinical outcomes. Although no regulatory requirements exist in USA for split tablets, A Ph.Eur. draft monograph for the mass uniformity of subdivided tablets required compliance of the subdivided tablets to the Ph.Eur. test for content uniformity (31). This requirement was restricted to tablets with active substances having a critical dosing. This draft monograph was not adopted. In 2001, the Ph.Eur. adopted a change in the monograph on Tablets, including under Production a paragraph requiring subdivided parts of scored tablets to comply either with the Ph.Eur. test for uniformity of content or uniformity of mass. This requirement is not restricted to substances with a critical dosing. The adoption of this requirement is a milestone: for the first time, a pharmacopoeial requirement on score lines is set. Also, recently, a stimuli article was published by USP Pharmaceopeial Forum suggesting to adopt EP standards for the subdivision characteristics of the scored tablets (32). This requirement limits deviation to ±15% of average mass of the subdivided parts. The second standard recommended includes limit on loss of mass upon subdivision which should be ≤3%. However, it does not propose any limits on content uniformity and assay values which could be critical for narrow therapeutic index drug, such as levothyroxine.
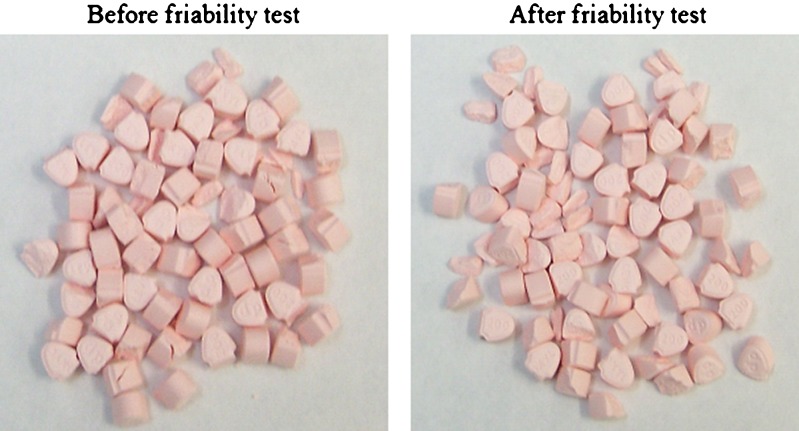
Friabiliy results are presented in Table III for whole, hand-split, and splitter-split marketed tablets. In general, the tablets were more prone to friability failures for half tablets as compared to the whole tablets. The splitter cut tablets had higher weight losses for L2–L5 tablets as compared to hand-split tablets. Weight loss was more prominent for L-3 splitter-cut tablets as a number of small fragments were generated from the fractured split tablets which were excluded from the weighing purpose (Fig. 3). This is striking because the tablets may be cut and shipped sometimes from mail order pharmacies. Since friability tests are not routinely performed and evaluated for split tablets, it might pose a greater concern for shipped split tablets.
Table III.
Friability (% Weight Loss) Results for Marketed Levothyroxine Tablets for Whole, Hand-Split, and Splitter-Split Tablets
| Whole | Hand-split | Splitter-split | |
|---|---|---|---|
| L-1 | 0.21 | 0.47 | 0.40 |
| L-2 | 0.05 | 0.49 | 1.02 |
| L-3 | 0.09 | 0.16 | 3.24 |
| L-4 | 0.09 | 0.25 | 0.30 |
| L-5 | 0.19 | 0.69 | 0.87 |
Fig. 3.
Levothyroxine tablets (L-3) before and after friability test on splitter cut tablets
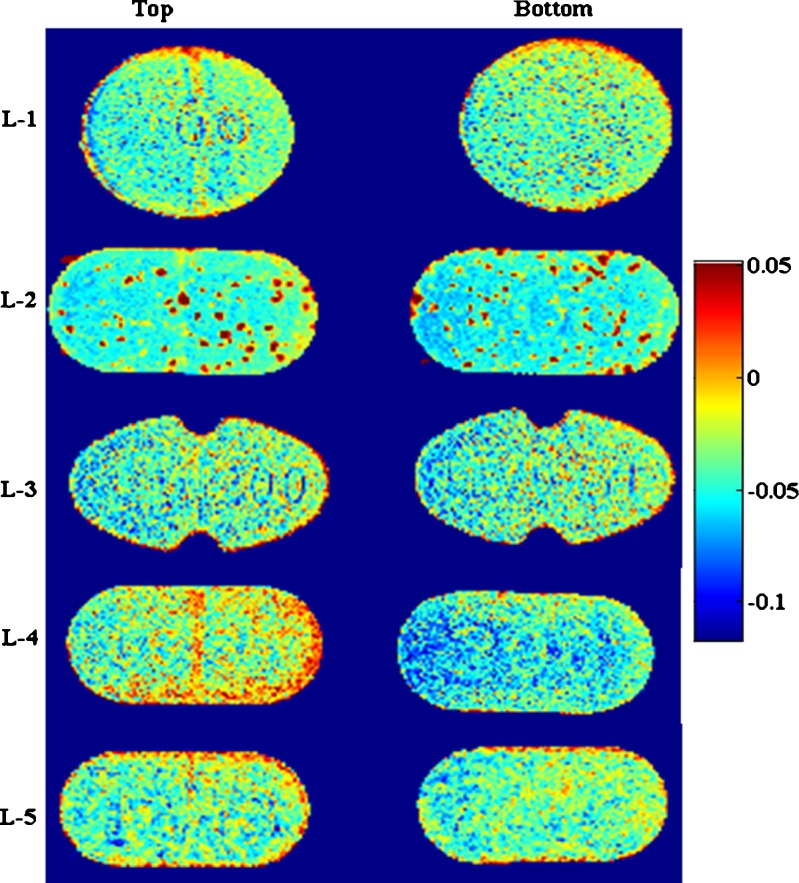
Near infrared chemical imaging was used to view chemical composition on the surface of tablets. It has the potential to predict spatial identification of species in a sample. A confounding factor in NIR analysis (traditional or imaging) is that physical properties of the tablets such as density, hardness, and particle size have an impact on the spectrum, typically a linear baseline shift (33,34). This impact was minimized by chemometrics preprocessing step including multiplicative signal correction (normalization). The images are shown in Fig. 4. The images shown are with respect to factor index of levothyroxine sodium. The color index indicates that the region rich in levothyroxine concentration is red whereas regions with minimum levothyroxine are blue in color. It was observed that L-2 and L-4 tablets’ surfaces showed heterogeneity in the distribution of API as compared to other marketed tablets. A more careful evaluation at the composition of tablets from the package insert revealed that these formulations might be compressed directly without any granulation step since there was no binder present in both these formulations. API% is very low in the tablet (200 mcg in 120 mg tablet ~0.167%); hence, if the API is mixed directly with other ingredients without formation of granules, it seems likely that the drug will not be homogeneously mixed with the other ingredients. This might have implications on split tablets since splitting them into halves equally by weight might still not contain the same amount of API in the split portion due to unequal distribution. Therefore, during formulation development, this needs to be addressed by performing systematic study as presented here to avoid sub- or super-potency issues for these types of drugs.
Fig. 4.
NIR chemical images of marketed levothyroxine sodium tablets (L-1 to L-5) with factor index of levothyroxine sodium
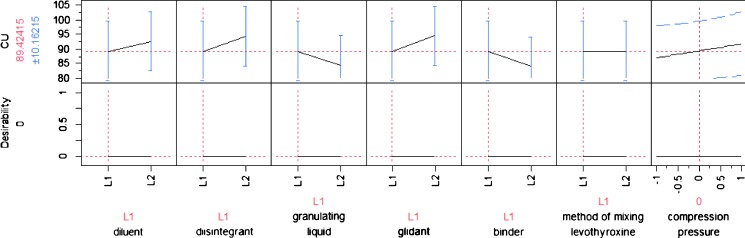
In order to understand the relationship between the dividing properties of scored tablets and the characteristics of the powder mixtures from which the tablets have been compressed, a screening design of experiments was employed. Formulation and processing factors were varied in a 12-run Plackett–Burman screening design as outlined in methods section. Assay and content uniformity results were analyzed by statistical software JMP (Version 7.0) which revealed that binder was found to be one of the main factors affecting splitting (Fig. 5). Other two factors affecting assay and content uniformity were glidant and disintegrant although they were not statistically significant. The effect of factors and the p values are listed in Table IV. A prediction profiler was plotted to understand effects of each of the factors on the CU of split tablets of in-house tablets, and the results are presented in Fig. 6. It was seen that although statistically insignificant, there was a trend towards better CU with starch as a binder, colloidal SiO2 as a glidant and sodium carboxymethyl cellulose as a disintegrant, mannitol as a diluent, water as a granulating liquid. Method of mixing and compression pressure or hardness of the tablets had minimal effects on CU of split tablets. It is cited in the literature that a more uniform and denser packing resulted in a better uniformity of mass of the subdivided tablet parts. From a theoretical point of view, filler and binder excipients with only a limited elastic recovery after compaction are more suitable for breaking tablets (35). The two binders used in the current study were HPMC and starch. It was observed that for better content uniformity, starch was giving a higher content uniformity results as compared to when HPMC was used as a binder. Thus, formulation development with proper excipient might be able to produce tablets with better splittability.
Fig. 5.
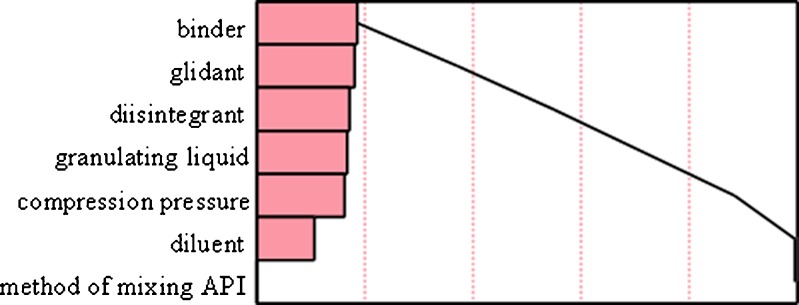
Pareto chart showing main effects of various factors on content uniformity of levothyroxine sodium tablets manufactured in-house
Table IV.
Estimates and P Values of Factors on Content Uniformity of Levothyroxine Sodium Split Tablets Manufactured In-House
| Factor | Estimate | p Value |
|---|---|---|
| Binder | +2.70 | 0.12 |
| Glidant | −2.61 | 0.13 |
| Disintegrant | −2.50 | 0.14 |
| Granulating liquid | +2.44 | 0.15 |
| Compression pressure | +2.37 | 0.16 |
| Diluent | −1.58 | 0.31 |
| Method of mixing API | +0.02 | 0.99 |
Fig. 6.
Prediction profiler for content uniformity for levothyroxine sodium split tablets manufactured in-house
CONCLUSIONS
Effect of hand and tablet splitter on levothyroxine dose uniformity was evaluated by performing chemical analysis such as assay, content uniformity, and friability. Assay was used to evaluate stability of whole and split tablets which showed no significant differences for their stability profiles. Content uniformity was found to be a measure for dose uniformity of split tablets. Among five marketed levothyroxine sodium tablets, it was found that split tablets either by hand or splitter showed higher rate of failures as compared to whole tablets. Tablet splitter produced more fragmentation; hence, more tablets failed content uniformity and friability if splitted with the splitter. However, it cannot be generalized as it depends on the type of splitter and dexterity of individuals. Also, it was found that with the tablets which were directly compressed without any granulation step, the distribution of levothyroxine sodium was not homogeneous on the surface of the tablets. Splitting such tablets could prove detrimental if sub- or super-potency becomes an issue for narrow therapeutic drugs. In-house formulation efforts revealed that type of binder plays an important role in splittability of the tablet to provide more consistent dose uniformity for levothyroxine sodium tablets.
Acknowledgements
The study was partially funded as a critical path project and also by the Office of Generic Drugs, FDA. Authors also wish to thank Christopher Ellison and Abhay Gupta for their technical help.
DISCLAIMER
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy.
References
- 1.Carr-Lopez M, Mallett M, Morse T. The tablet splitting: barrier to compliance or cost-saving instrument? Am J Health-Syst Pharm. 1995;53:2707–2708. doi: 10.1093/ajhp/52.23.2707. [DOI] [PubMed] [Google Scholar]
- 2.Fawell NG, Cookson TL, Scranton SS. Relationship between tablet splitting and compliance, drug acquisition cost, and patient acceptance. Am J Health-Syst Pharm. 1999;56:2542–2545. doi: 10.1093/ajhp/56.24.2542. [DOI] [PubMed] [Google Scholar]
- 3.Boggie DT, Delattre ML, Schaefer MG, Morreale AP, Plowman BK. Accuracy of splitting unscored valdecoxib tablets. Am J Health-Syst Pharm. 2004;61:1482–1483. doi: 10.1093/ajhp/61.14.1482. [DOI] [PubMed] [Google Scholar]
- 4.Cohen C, Cohen S. Potential savings from splitting newer antidepressant medications. CNS Drugs. 2002;16:353–358. doi: 10.2165/00023210-200216050-00007. [DOI] [PubMed] [Google Scholar]
- 5.Parra D, Beckey NP, Raval HS, Schnacky KR, Calabrese V, Coakley RW, Goodhope RC. Effect of splitting simvastatin tablets for control of low-density lipoprotein cholesterol. Am J Cardiol. 2005;95:1481–1483. doi: 10.1016/j.amjcard.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Stafford RS, Radley DC. The potential of pill splitting to achieve cost savings. Am J managed Care. 2002;8:706–712. [PubMed] [Google Scholar]
- 7.Rosenberg JM, Nathan JP, Plakogiannis F. Weight variability of pharmacistdispensed split tablets. J Am Pharm Assoc. 2002;42:200–205. doi: 10.1331/108658002763508498. [DOI] [PubMed] [Google Scholar]
- 8.Teng J, Song CK, Willliams RL, Polli JE. Lack of medication dose uniformity in commonly split tablets. J Am Pharm Assoc. 2002;42:195–199. doi: 10.1331/108658002763508489. [DOI] [PubMed] [Google Scholar]
- 9.Stimpel M, Kuffer B, Groth H, Vetter W. Breaking tablets in half. Lancet. 1984;1:1299. doi: 10.1016/S0140-6736(84)92481-4. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P, Gupta K. Broken tablets: does the sum of the parts equal the whole? Am J Hosp Pharm. 1988;45:1498. [PubMed] [Google Scholar]
- 11.Sedrati M, Arnaud P, Fontan JE, Brion F. Splitting tablets in half. Am J Hosp Pharm. 1994;51:548–552. [PubMed] [Google Scholar]
- 12.McDevitt JT, Gurst AH, Chen Y. Accuracy of tablet splitting. Pharmacotherapy. 1998;18:193–197. [PubMed] [Google Scholar]
- 13.Peek BT, Al-Achi A, Coombs SJ. Accuracy of tablet splitting by elderly patients. Research letter JAMA. 2002;288:451–452. doi: 10.1001/jama.288.4.451. [DOI] [PubMed] [Google Scholar]
- 14.Shopper’s guide to prescription drugs number 1: pill splitting. Consumer Reports Best Buy Drugs. 2006. http://www.consumerreports.org/health/resources/pdf/best-buy-drugs/money-saving-guides/english/PillSplitting-FINAL.pdf. Accessed 25 Mar 2010.
- 15.AllThyroid.org. Thyroid patient advocacy group warns thyroid patients of potential problems with thyroid drug resulting from FDA approval of generic versions. http://www.allthyroid.org/news/latest/04_07_13_patientadvocacy.html.
- 16.Frueh L. Interchangeability of critical dose drugs: Clinical perspective. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AdvisoryCommitteeforPharmaceuticalScienceandClinicalPharmacology/UCM209319.pdf. Accessed Aug 2010.
- 17.Post A, Warren R. Sodium levothyroxine. In: Florey K, editor. Analytical Profiles of Drug Substances. New York: Academic; 1976. pp. 226–281. [Google Scholar]
- 18.Won CM. Kinetics of degradation of levothyroxine in aqueous solution and in solid state. Pharm Res. 1992;9:131–137. doi: 10.1023/A:1018952415732. [DOI] [PubMed] [Google Scholar]
- 19.Kazemifard AG, Moore DE, Aghazadeh A. Identification and quantitation of sodium-thyroxine and its degradation products by LC using electrochemical and MS detection. J Pharm Biomed Anal. 2001;25:697–711. doi: 10.1016/S0731-7085(01)00370-3. [DOI] [PubMed] [Google Scholar]
- 20.Patel H, Stalcup A, Dansereau R, Sakr A. The effect of excipients on the stability of levothyroxine sodium pentahydrate tablets. Int J Pharm. 2003;264:35–43. doi: 10.1016/S0378-5173(03)00387-9. [DOI] [PubMed] [Google Scholar]
- 21.Wortsman J, Papadimitriou DC, Borges M, Defesche CL. Thermal inactivation of L-thyroxin. Clin Chem. 1989;35:90–92. [PubMed] [Google Scholar]
- 22.Jarrod C, Shah RB, Gupta A, Sayeed V, Habib MJ, Khan MA. Influence of formulation and processing factors on stability of levothyroxine sodium pentahydrate. AAPS PharmSciTech. 2010;11(2):818–825. doi: 10.1208/s12249-010-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.USP. USP announces a revised monograph for levothyroxine sodium tablets. http://www.usp.org/USPNF/notices/iraLevothyroxine.html. Accessed March 25, 2010.
- 24.Shah RB, Bryant A, Collier J, Habib MJ, Khan MA. Stability indicating validated HPLC method for quantification of levothyroxine with eight degradation peaks in the presence of excipients. Int J Pharm. 2008;360:77–82. doi: 10.1016/j.ijpharm.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Jarrod C, Shah RB, Bryant AR, Habib MJ, Khan MA, Faustino P. Development and application of a validated HPLC method for the analysis of dissolution samples of levothyroxine sodium drug products. J Pharm Biomed Anal. 2010 (in press). [DOI] [PMC free article] [PubMed]
- 26.Sales MM. Tablet splitting: topics in Patients safety. TIPS NCPS VA National Center for Patients Safety. 2006;6:1–5. [Google Scholar]
- 27.van Santen E, Barends DM, Frijlink HW. Breaking of scored tablets: a review. Eur J Pharm Biopharm. 2002;53:139–145. doi: 10.1016/S0939-6411(01)00228-4. [DOI] [PubMed] [Google Scholar]
- 28.Muller BW, Kublik H. Dosiergenauigkeit bei Tabletten mit Bruchrille? Dtsch Apoth Ztg. 1993;133:15–17. [Google Scholar]
- 29.Gupta P, Gupta K. Broken tablets: does the sum of the parts equal the whole? Am J Hosp Pharm. 1998;45:1498. [PubMed] [Google Scholar]
- 30.McNeil Consumer & Specialty Pharmaceuticals. Study raises concerns about tablet splitting. http://www.scienceblog.com/community/older/2004/10/200410126.shtml. Accessed March 25, 2010.
- 31.Anonymous. Note on the general method. 2.9. Test for the subdivision of tablets, Pharmeuropa. 2000;12:300.
- 32.Green G, Berg C, Polli J, Berends DM. Pharmacopeial standards for the subdivision characteristics of scored tablets. USP Pharmacopeial Forum. 2009;35(6):1598–1611. [Google Scholar]
- 33.Kirsch JD, Drennen JK. Nondestructive tablet hardness testing by near-infrared spectroscopy: a new and robust spectral best-fit algorithm. J Pharm Biomed Anal. 1999;19:351–362. doi: 10.1016/S0731-7085(98)00132-0. [DOI] [PubMed] [Google Scholar]
- 34.Shah RB, Tawakkul MA, Khan MA. Process analytical technology: chemometric analysis of Raman and near Infra-red spectroscopic data for predicting physical properties of extended release matrix tablets. J Pharm Sci. 2007;96:1356–1365. doi: 10.1002/jps.20931. [DOI] [PubMed] [Google Scholar]
- 35.Ito A, Dobashi Y, Sugihara M. The relationship between dividing properties of scored tablets and dynamic characteristics of various mixed powders. Chem Pharm Bull. 1993;41:590–594. [Google Scholar]