Abstract
l-ascorbic acid has been widely used in cosmetic and dermatological products because of its ability to scavenge free radicals and destroy oxidizing agents. However, it is chemically unstable and can easily be oxidized. The current cosmetic facial masks available in the market are pre-moistened, which means that the aqueous fluid content of the mask may oxidize some of the unstable active ingredients such as ascorbic acid. This work presents an anti-wrinkle nanofiber face mask containing ascorbic acid, retinoic acid, gold nanoparticles, and collagen. This novel face mask will only be wetted when applied to the skin, thus enhancing product stability. Once moistened, the content of the mask will gradually dissolve and release the active ingredients and ensure maximum skin penetration. The high surface area-to-volume ratio of the nanofiber mask will ensure maximum contact with the skin surface and help to enhance the skin permeation to restore its healthy appearance. Electrospun fiber mats may provide an attractive alternative to the commercial facial cotton masks.
KEY WORDS: ascorbic acid, cis-retinoic acid, collagen, gold nanoparticle, topical facial mask
INTRODUCTION
Many marketing strategies include the incorporation of antioxidants and other skin nutrients into cosmetic products. Collagen is important for the strength and function of the skin and is an important factor in skin rejuvenation and wrinkle reversal effect. The amount of collagen in the skin tends to decline with age; therefore, it is widely used as a moisturizer in cosmetic creams and products. Vitamin C (l-ascorbic acid) has been widely used in cosmetic and dermatological products because of its photoprotective effect and the ability to scavenge free radicals and destroy oxidizing agents. It can also induce collagen synthesis and suppress the pigmentation of the skin while reducing signs of photoaging. However, it is chemically unstable, and it can easily be oxidized; therefore, more stable derivatives of ascorbic acid such as ascorbyl palmitate, ascorbyl tetraisopalmitate, and magnesium ascorbyl phosphate are widely used in pharmaceutical industry (1,2). These derivatives can easily be converted to the active compound, ascorbic acid, after ingestion. However, topical applications of these derivatives are not able to efficiently increase the skin levels of this antioxidant (3). A formulation strategy to improve the stability of ascorbic acid is to deliver using emulsions. The oil phase may partially protect the vitamin from oxidative degradation caused in aqueous solutions (4–6).
Retinoic acid enhances the repair of ultraviolet (UV)-damaged skin and can reduce wrinkles caused by photoaging. It can also be used in the treatment of acne (7–11). Due to its lipophilic structure, it is practically insoluble in aqueous solution, which decreases its bioavailability (12–14).
Gold nanoparticles have been studied as potential vaccine carriers and in transdermal delivery (15–18). Gold facial masks are now being used in beauty clinics and saloons. They help to improve blood circulation, skin elasticity, and can rejuvenate the skin and reduce the formation of wrinkles. Skin permeation studies demonstrate that spherical gold nanoparticles are not inherently toxic to human skin cells (15).
Cyclodextrins (CDs) are a group of cyclic oligosaccharides. They are able to form inclusion complexes with lipophilic guest molecules which have been shown to improve aqueous solubility, dissolution rate, and bioavailability of such drugs (19,20). Due to the solubility, hygroscopicity, and toxicity concerns of CDs, they were modified to randomly methylated β-CD (RM β-CDs). Methylated β-CD has been assessed in cultures of human skin fibroblasts without reported toxicity (21).
Polyvinyl alcohol (PVA) is a biodegradable, hydrophilic polymer with distinct properties such as high degree of swelling, inherent non-toxicity, and good biocompatibility (22,23).
Electrospinning is a remarkably simple and effective method for fabricating polymeric nanoscale fibers with high surface area-to-volume ratio and porosity. The collected nonwoven fabric may be applied to different areas of research such as drug delivery (24,25).
Conventional beauty face masks available in the market are cotton masks that are pre-moistened with skin nutrients. The aqueous phase of the pre-moistened mask can increase the degradation rate of the unstable ingredients such as ascorbic acid.
The objective of this work is to develop a novel polymeric nanofiber face mask (from PVA and RM β-CD) that can accommodate several skin nutrients such as ascorbic acid, retinoic acid, gold, and collagen. These face masks were developed and characterized using field emission scanning electron microscope and X-ray elemental analysis. Skin permeation of ascorbic acid and retinoic acid from these nanofiber face masks was tested using human epidermis.
METHOD
Materials and Methods
l-ascorbic acid, chloroauric acid, PVA, trisodium citrate, and collagen were obtained from Sigma, Singapore. 13-cis-Retinoic acid was obtained from Toronto Research Chemicals. RM β-CD (degree of substitution of about 1.8) was a gift from Wacker (Burghausen, Germany).
Electrospinning
Gold nanoparticles were prepared by trisodium citrate reduction of HAuCl4 in PVA ethanol solution (30% v/v) (26,27). Briefly, 2 ml of 2% w/v trisodium citrate was added to the PVA solution mixture and stirred at 95–100°C, and then 0.7 ml 1% w/v chloroauric acid aqueous solution was added to this mixture where the color of the solution changed to blood red. The mixture was cooled to room temperature while stirring using a magnetic stirrer at 700 rpm. RM β-CD, ascorbic acid, retinoic acid, or collagen was dissolved in the mixture prior electrospinning. The face mask is produced from electrospinning the aqueous phase containing the polymer and the active ingredients shown in Table I. The electrostatic spinner used for the experiments was equipped with an adjustable DC power supply (RP50-1.25 R/230 DDPM, Gamma high voltage Research, USA) and a syringe pump (KD-100, KD Scientific, Inc., USA) on which a 3-ml syringe was connected to a blunt 27-G stainless steel needle. The applied voltage was set at 25 kV. A constant flow rate of 2 ml/h was applied to all formulations. The depositions were performed at room temperature. The nonwoven electrostatically spun fabric was removed from the collector and was dried under vacuum for a week at room temperature to remove water prior to usage.
Table I.
The Compositions of the Face Mask Formulations
| Formulations | Ingredient | |||
|---|---|---|---|---|
| Ascorbic acid (1% w/v) | cis-Retinoic acid (0.1% w/v) | Collagen (0.01% w/v) | Gold (0.1% w/v) | |
| A | √ | – | – | – |
| B | √ | – | – | √ |
| C | – | √ | – | – |
| D | √ | √ | √ | – |
| E | √ | √ | √ | √ |
All formulations contain 10% w/v polyvinyl alcohol and 20% w/v randomly methylated β-cyclodextrin
Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-Ray Spectroscopy (EDS) Analysis
The surface topography of the electrospun fibers was assessed using a field emission scanning electron microscopy (FESEM; Jeol JSM-6701F, Japan). Electrospun fibers were placed at the center of an aluminum stud using carbon tape. A very thin layer of platinum was applied to the fibers by a sputtering unit (Jeol JFC-1600 auto fine coater, Japan). Coated fibers were placed in the microscope chamber at high vacuum. Surface morphological features were obtained under 5 kV accelerating voltage. The diameter distribution of the electrospun fibers was derived from a random sample of at least 20 fibers.
Energy dispersive X-ray spectroscopy (EDS) measurements were carried out by means of a FESEM equipped with an energy dispersive X-ray source to identify the presence of gold in the fibers. After coating with platinum, samples were analyzed at 15 kV voltage. The area to be analyzed was selected, and the electron beam scanned and identified the intensity of characteristic X-ray energies of specific elements.
Fourier Transform Infrared Measurements (FTIR)
Fourier transform infrared (FTIR) spectra of the polymeric nanofiber mats were taken with a Perkin Elmer (Spectrum 100) in the wavelength region 500–4,000 cm−1 at room temperature. Samples were placed on KBr holder and mounted in the enclosed sample chamber, away from moisture to get their spectra.
UV–Vis Spectroscopy
UV absorption measurements were carried out at room temperature on a UV–Vis spectrophotometer (U-1800 spectrophotometer, Hitachi, Japan) at 261 and 349 nm for ascorbic acid and retinoic acid, respectively. Standard solutions of ascorbic acid and retinoic acid (0.05 to 2.00 μg/ml) were prepared in water and water ethanol (60% v/v) solutions, and the R2 value of the calibration curves for the standard solutions were 0.9994 and 0.9992, respectively.
Skin Preparation and Permeation Studies
Abdominal skin from an adult was obtained with patient consent and ethics approval post-plastic surgery. Subcutaneous fat was carefully separated from the epidermis after immersing the whole skin in distilled water at 60 ± 5°C for 2 min. Samples were stored at −80°C until use. Prior to permeation studies, the skin samples with stratum corneum (SC) side facing upwards were equilibrated for 2 h in 0.9% w/v sodium chloride solutions containing 1% v/v antibacterial antimycotic solution (28).
Permeation studies were performed using a flow-through diffusion cell apparatus. The donor compartment was filled with 25 mg of vitamin-loaded mat and hydrated with 300 μl of water. The skin permeation studies were only performed from formulations containing single ingredient (A, B, and C), and the results were compared to cotton masks that contained the same concentration of the active ingredient. This was done in order to prevent the UV interference from multi-ingredients. However, it is assumed that presence of all the active ingredients in one formulation should not have a significant impact on the release pattern of the molecules. Loading efficiencies of the fiber masks were calculated by fully dissolving a certain amount of mask in 20% v/v ethanol and measuring its concentration with UV spectrophotometer.
The receptor compartment was phosphate buffer saline with pH adjusted to 5.5. Control samples were cotton sheet pre-moistened with vitamin solutions. The exposed surface area of the skin for the permeation of the drug was 0.785 cm2. Samples from the receptor compartment were collected at predetermined time points over a 4-h period, and the amounts of ascorbic acid and retinoic acid permeated were analyzed by UV spectrometer. Cell temperature was kept at 37 ± 0.5°C throughout the experiment.
Skin Histology
Skin samples used in diffusion studies were processed for light microscopy. Samples were soaked overnight in 85 ml of 80% ethanol, 10 ml formaldehyde, and 5 ml acetic acid (29). After a series of dehydration, samples were embedded in paraffin, and semi-thin sections were stained with hematoxylin and eosin prior being examined with a light microscope (Leica EC 3, USA).
Statistical Analysis
Results were expressed as the mean ± SD of at least three experiments. Analysis of variance was carried out (Graph Pad Prism, version 2.0) followed by the Tukey post hoc and Student's t test to determine the differences between treatment groups. A value of p < 0.05 was considered statistically significant.
RESULTS
Fiber Morphology and EDS Analysis
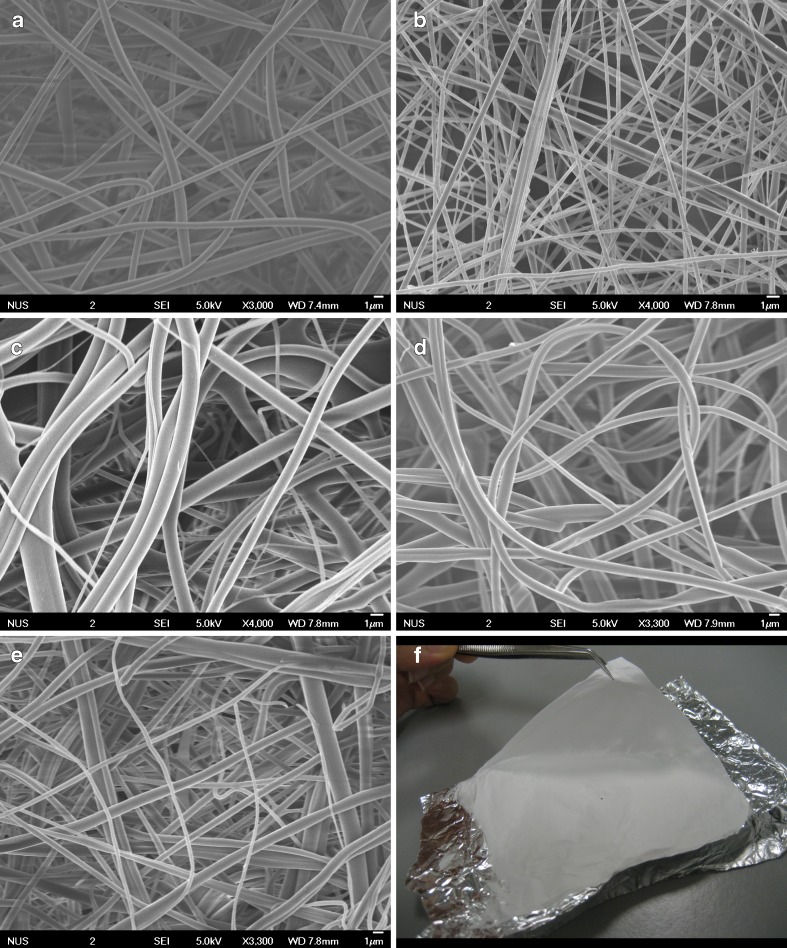
Continuous fibers without beads or sputtering of the solution were obtained with diameter ranging from ~100 nm to 2 μm (Fig. 1). There was a decrease in fiber diameter of electrospun solutions containing gold. This may be due to the increase in the charge density of the solution due to the presence of gold (30). Figure 2 illustrates the spectra of gold present on the fibers.
Fig. 1.
Field emission scanning electron microscopy images of nanofibers formulation and a macrographic image of a typical nanofiber mat
Fig. 2.
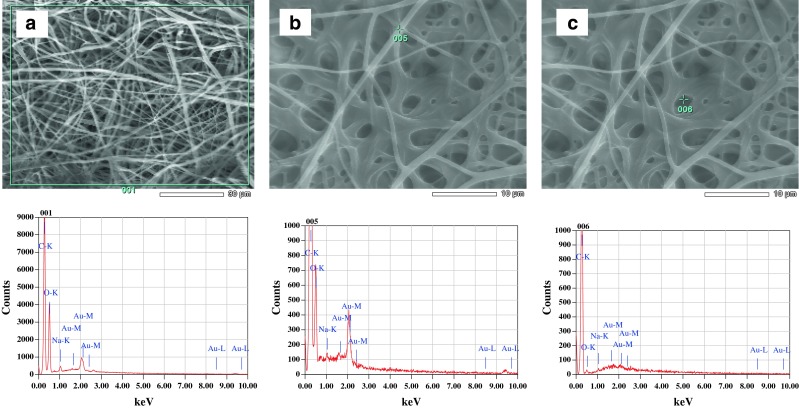
X-ray energy spectrum of gold-loaded nanofiber sheet, demonstrating the presence of the mineral-specific signals. a Area analysis and b, c spot analysis of the nanofiber surface. C and O signal is due to the backbone of the polymers, and Na signal is due to the addition of sodium citrate used in the reduction of gold. Au signals represent the presence of gold on the fiber structure
FTIR Studies
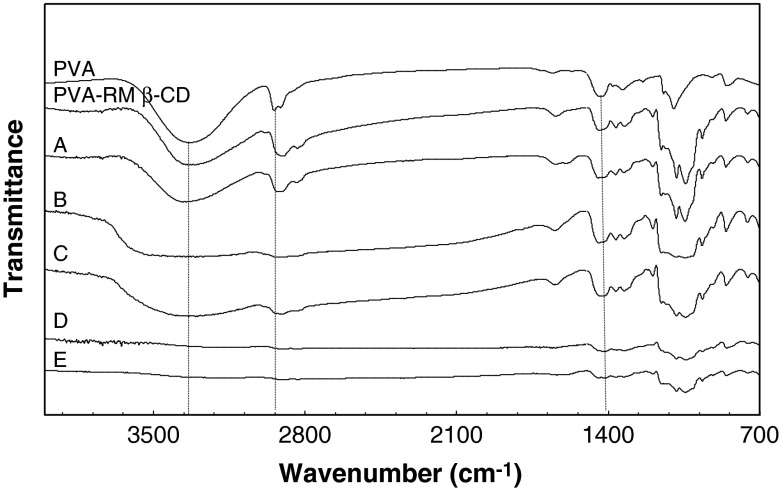
Figure 3 indicates FTIR analysis of PVA, PVA–RM β-CD, and PVA–RM β-CD loaded with the active ingredients. For pure PVA, a broad band around 3,369 cm−1 is attributed to the O–H stretching vibration of the hydroxyl group. The vibrational bands at 2,932 and 1,455 cm−1 represent the –CH stretching. The sharp peak band at 1,090 cm−1 corresponds to C–O–C symmetrical stretching present on the PVA backbone (31–33). With the addition of RM-β-CD, the band corresponding to O–H stretch of PVA showed a decrease in intensity. This suggested that RM-β-CD formed hydrogen bond with PVA, which could explain the enhanced solubility of the fibers when RM-β-CD was added to the PVA. There was a decrease in the intensity of the O–H group when collagen was added to the solution. The addition of gold to PVA/RM-β-CD fiber caused a shift of O–H band to a higher frequency of 3,389 cm−1. This suggests that PVA stabilized the gold particles (34).
Fig. 3.
Fourier transform infrared analysis of nanofiber mats containing pure polyvinyl alcohol (PVA), PVA and randomly methylated β-cyclodextrin, and formulations A–E as listed in Table I
Ascorbic acid can rapidly decompose in aqueous solutions and produce many degradation products. Studies show that CD has minor effect on the degradation of ascorbic acid, and the inclusion of ascorbic acid into the CD cavity is negligible (35). Stability of collagen has been studied, and results show that blending collagen with PVA increases the thermal and photochemical stability of collagen (36). PVA acts as a good stabilizer to protect gold nanoparticles (34). The stability of retinoic acid from nanofiber has been tested, and it was found that the retinoic acid was stable in the medium (25).
Skin Permeation
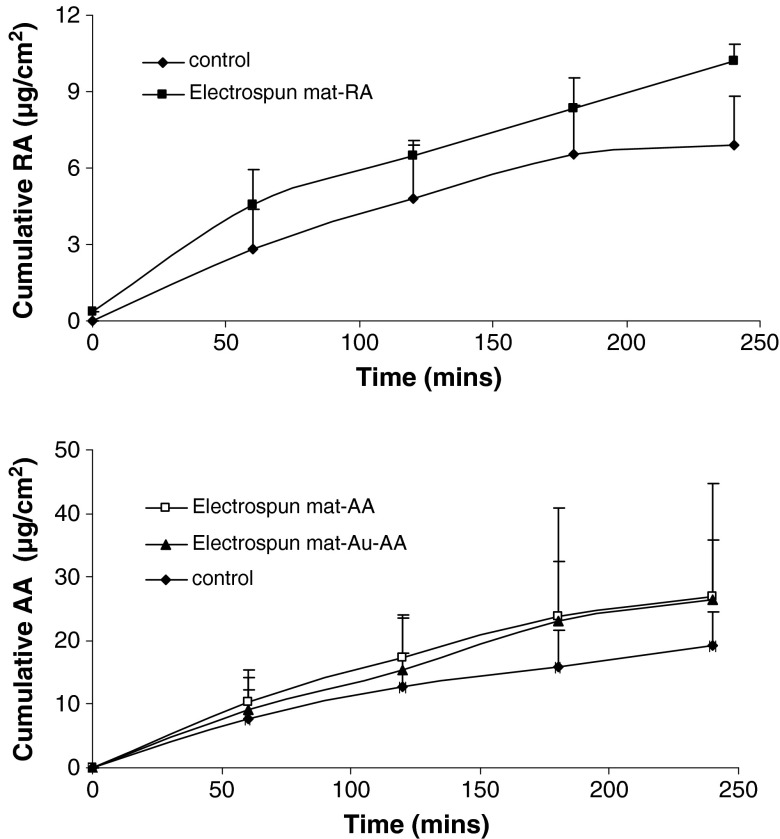
Results of the skin permeation are shown in Fig. 4. It can be seen that nanofiber-loaded vitamins slightly increased the skin permeation of ascorbic acid and retinoic acid when compared to the vitamin-loaded cotton face mask (p > 0.05).
Fig. 4.
Permeation profile of ascorbic acid and retinoic acid across human epidermis (n = 3)
The addition of a penetration enhancer to the nanofiber formula may result in significantly higher skin permeation rate.
Gold did not cause any significant change in the skin permeation of ascorbic acid (p > 0.05). This effect might be due to the limited concentration of gold present in the formulations.
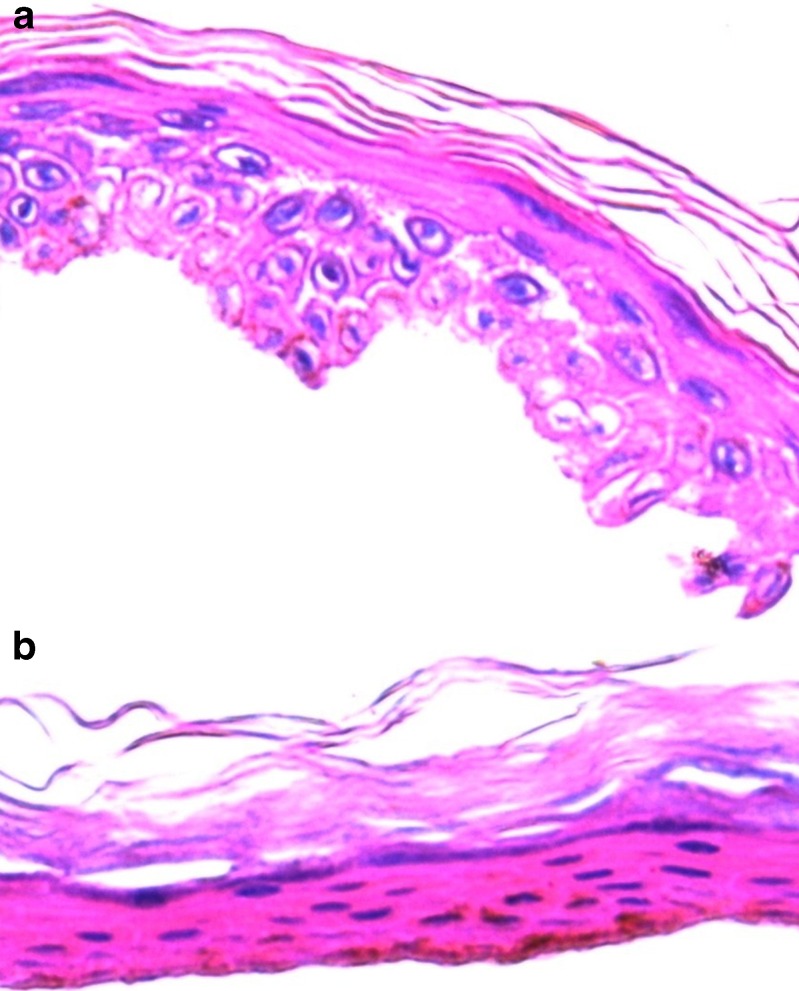
Microscopic appearance of the skin treated with the gold-loaded nanofiber mask is shown in Fig. 5. In the control, skin samples treated with nanofiber mask containing PVA and RM β-CD, a clearly defined SC, could be seen (Fig. 5a), but after treatment with gold-loaded nanofiber, slight detachment of the SC layer occurred. The SC layer was fragmented, and enlargement of inter-keratinocyte spaces was observed, while the other epidermis layers were more compact. There is a need to further study the effect of gold and gold nanoparticles on the skin surface and to investigate its toxicological effects.
Fig. 5.
Morphology of human epidermis: histological skin examinations were performed in vitro 24 h after skin permeation studies (×400). a Skin samples treated with nanofiber mask containing polyvinyl alcohol (PVA) and randomly methylated β-cyclodextrin (RM β-CD). b Skin samples treated with gold-loaded nanofiber containing PVA and RM β-CD. The nucleated cells of the epidermis have been stained blue; unsaturated lipids, including fatty acids and esters, have been stained red
These novel facial masks have high surface area-to-volume ratio that can increase the contact area of the mask with the skin surface. Compared to conventional pre-moistened cotton masks, these nanofiber masks have a dry nature that increases the stability of the nutrients and minimizes the oxidation of the antioxidants. Incorporation of RM β-CD to the PVA solution decreased the degradation rate of the resulting fiber mat.
Selection of the type of CD and its addition to the polymeric solution prior to electrospinning can increase the disintegration rate of the nanofibers and help it dissolve on the skin surface within a shorter period of time. PVA can produce fibers that have a disintegration time of a few weeks (23). Addition of RM β-CD to PVA solution can produce fibers that dissolve within minutes upon contact with an aqueous medium. This may be due to the –OH bonding between PVA and RM β-CD as observed on the FTIR studies mentioned in the earlier section (37). In other words, RM β-CD can decrease the dissolution time of PVA fibers. This rapid dissolution of the face mask will provide fast release of the active ingredients that are incorporated within the polymeric fiber mat. Therefore, once the dry face mask is placed on the skin surface and wetted with water, it will dissolve in less than 15 min and release its entire active components thus ensuring maximum skin penetration of the active molecules.
Angberg et al evaluated degradation reaction of ascorbic acid in aqueous solution. It was found that oxidation rate of Ascorbic Acid was dependent on the availability of oxygen and its dissolution in the head space of the vial. Therefore, the amount of oxidation was higher at the beginning when oxygen content was high and directly available in the solution, and the percentage decrease in ascorbic acid was greater in the first 5 h (38). Although in our work the experiment was carried out for 4 h, however, as with commercial facial masks, the nanofiber mat formulation is intended to be placed on the skin surface for no more than 15 min, which will decrease the oxidation rate of ascorbic acid.
These properties of the electrospun mat make it an attractive alternative facial mask.
CONCLUSION
An innovative nanofiber facial mask composed of ascorbic acid, collagen, retinoic acid, and gold nanoparticle was developed. The face mask is dry in nature and will only be moistened with water prior to application. The wetted mask will then gradually dissolve and release the ingredients condensed on it. The dry nature of the face mask increases the stability of the antioxidants and its shelf life when compared to pre-moistened cotton masks available in the market. Electrospun fiber mats may provide an attractive alternative to the commercial facial cotton masks.
References
- 1.Segall AI, Moyano MA. Stability of vitamin C derivatives in topical formulations containing lipoic acid, vitamins A and E. Int J Cosmet Sci. 2008;30:453–8. doi: 10.1111/j.1468-2494.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar LR, Campo PMBGM. Photostability and efficacy studies of topical formulations containing UV-filters combination and vitamins A C and E. Int J Pharm. 2007;343:181–9. doi: 10.1016/j.ijpharm.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 3.Pinnell SR, Madey DL. Topical vitamin C in skin care. Aesthet Surg J. 1998;18:468–70. doi: 10.1016/S1090-820X(98)70085-8. [DOI] [PubMed] [Google Scholar]
- 4.Farahmand S, Tajerzadeh H, Farboud ES. Formulation and evaluation of a vitamin C multiple emulsion. Pharm Dev Technol. 2006;11:255–61. doi: 10.1080/10837450500464172. [DOI] [PubMed] [Google Scholar]
- 5.Rozman B, Gašperlin M. Stability of vitamins C and E in topical microemulsions for combined antioxidant therapy. Drug Deliv. 2007;14:235–45. doi: 10.1080/10717540601067786. [DOI] [PubMed] [Google Scholar]
- 6.Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–85. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Watson REB, Long SP, Bowden JJ, Bastrilles JY, Barton SP, Griffiths CEM. Repair of photoaged dermal matrix by topical application of a cosmetic ‘antiageing’ product. Br J Dermatol. 2008;158:472–7. doi: 10.1111/j.1365-2133.2007.08364.x. [DOI] [PubMed] [Google Scholar]
- 8.Cao C, Wan S, Jiang Q, Amaral A, Lu S, Hu G, et al. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J Cell Physiol. 2008;215:506–16. doi: 10.1002/jcp.21336. [DOI] [PubMed] [Google Scholar]
- 9.Kang S, Voorhees JJ. Photoaging therapy with topical tretinoin, an evidence based analysis. J Am Acad Dermatol. 1998;39:S55–61. doi: 10.1016/S0190-9622(98)70446-3. [DOI] [PubMed] [Google Scholar]
- 10.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 11.Thielitz A, Abdel-Naser MB, Fluhr JW, Zouboulis CC, Gollnick H. Topical retinoids in acne: An evidence-based overview. J Dtsch Dermatol Ges. 2008;6:1023–31. doi: 10.1111/j.1610-0387.2008.06741.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin HS, Chean CS, Ng YY, Chan SY, Ho PC. 2-Hydroxypropyl-b-cyclodextrin increases aqueous solubility and photostability of all-trans-retinoic acid. J Clin Pharm Ther. 2000;25:265–9. doi: 10.1046/j.1365-2710.2000.00285.x. [DOI] [PubMed] [Google Scholar]
- 13.Montassier P, Duchene D, Poelman MC. Inclusion complexes of tretinoin with cyclodextrins. Int J Pharm. 1997;153:199–209. doi: 10.1016/S0378-5173(97)00104-X. [DOI] [Google Scholar]
- 14.Hu LD, Tang X, Cui FD. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J Pharm Pharmocol. 2004;56:1527–35. doi: 10.1211/0022357044959. [DOI] [PubMed] [Google Scholar]
- 15.Sonavane G, Tomoda K, Sano A, Ohshima H, Terada H, Makino K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids Surf B Biointerfaces. 2008;65:1–10. doi: 10.1016/j.colsurfb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Menon GK, Brandsma JL, Schwartz PM. Particle-mediated gene delivery and human skin: Ultrastructural observations on stratum corneum barrier structures. Skin Pharmacol Physiol. 2007;20:141–7. doi: 10.1159/000098165. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland WJ, Arbuthnott EAH, Bellhouse BJ, Cornhill JF, Austyn JM, Kendall MAF, et al. Multiphoton high-resolution 3d imaging of langerhans cells and keratinocytes in the mouse skin model adopted for epidermal powdered immunization. J Invest Dermatol. 2006;126:1541–8. doi: 10.1038/sj.jid.5700290. [DOI] [PubMed] [Google Scholar]
- 18.Dean HJ, Haynes J, Schmaljohn C. The role of particle-mediated DNA vaccines in biodefense preparedness. Adv Drug Deliv Rev. 2005;57:1315–42. doi: 10.1016/j.addr.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Mannila J, Järvinen T, Järvinen K, Tarvainen M, Jarho P. Effects of RM-β-CD on sublingual bioavailability of Δ9-tetrahydrocannabinol in rabbits. Eur J Pharm Sci. 2005;26:71–7. doi: 10.1016/j.ejps.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 20.de Araujo DR, Tsuneda SS, Cereda CMS, Carvalho FDGF, Preté PSC, Fernandes SA, et al. Development and pharmacological evaluation of ropivacine-2- hydroxypropyl-b-cyclodextrin inclusion complex. Eur J Pharm Sci. 2008;33:60–71. doi: 10.1016/j.ejps.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Pfitzner I, Francz PI, Biesalski UK. Carotenoid: methyl-b3-cyclodextrin formulations: An improved method for supplementation of cultured cells. Biochim Biophys Acta. 2000;1474:163–8. doi: 10.1016/s0304-4165(00)00014-3. [DOI] [PubMed] [Google Scholar]
- 22.Taepaiboon P, Rungsardthong U, Supaphol P. Drug-loaded electrospun mats of poly (vinyl alcohol) fibres and their release characteristics of four model drugs. Nanotechnology. 2006;17:2317–29. doi: 10.1088/0957-4484/17/9/041. [DOI] [Google Scholar]
- 23.Kenawy E-R, Abdel-Hay FI, El-Newehy MH, Wnek GE. Controlled release of ketoprofen from electrospun poly(vinyl alcohol) nanofibers. Mater Sci Eng A. 2007;459:390–6. doi: 10.1016/j.msea.2007.01.039. [DOI] [Google Scholar]
- 24.Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, et al. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of water-insoluble, nonbiodegradable polymer. J Control Release. 2003;92:349–60. doi: 10.1016/S0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 25.Taepaiboon P, Rungsardthong U, Supaphol P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents for vitamin A acid and vitamin E. Eur J Pharm Biopharm. 2007;67:387–97. doi: 10.1016/j.ejpb.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Bai J, Li Y, Yang S, Du J, Wang S, Zheng J, et al. A simple and effective route for the preparation of poly (vinylalcohol) (PVA) nanofibers containing gold nanoparticles by electrospinning method. Solid State Commun. 2007;141:292–5. doi: 10.1016/j.ssc.2006.10.024. [DOI] [Google Scholar]
- 27.Wang Y, Li Y, Sun G, Zhang G, Liu H, Du J, et al. Fabrication of Au/PVP nanofiber composites by electrospinning. J Appl Polym Sci. 2007;105:3618–22. doi: 10.1002/app.25003. [DOI] [Google Scholar]
- 28.Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:702–5. doi: 10.1001/archderm.1963.01590240026005. [DOI] [PubMed] [Google Scholar]
- 29.Gaspar LR, Campos PM. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int J Pharm. 2006;307:123–8. doi: 10.1016/j.ijpharm.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Bai J, Yang Q, Li M, Wang S, Zhang C, Li Y. Preparation of composite nanofibers containing gold nanoparticles by using poly (N-vinylpyrrolidone) and β- cyclodextrin. Mater Chem Phys. 2008;111:205–8. doi: 10.1016/j.matchemphys.2008.04.027. [DOI] [Google Scholar]
- 31.Şanlı O, Ay N, Işıklan N. Release characteristics of diclofenac sodium from poly (vinyl alcohol)/sodium alginate and poly (vinyl alcohol)-grafted-poly (acrylamide)/sodium alginate blend beads. Eur J Pharm Biopharm. 2007;65:204–14. doi: 10.1016/j.ejpb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Hong J, Hong CK, Shim SE. Synthesis of polystyrene microspheres by dispersion polymerization using poly (vinyl alcohol) as a steric stabilizer in aqueous alcohol media. Colloids Surf A Physicochem Eng Asp. 2007;302:225–33. doi: 10.1016/j.colsurfa.2007.02.027. [DOI] [Google Scholar]
- 33.Arndt KF, Richter A, Ludwig S, Zimmermann J, Kressler J, Kuckling D, et al. Poly (vinyl alcohol)/poly (acrylic acid) hydrogels: FT-IR spectroscopic characterization of crosslinking reaction and work at transition point. Acta Polym. 1999;50:383–90. doi: 10.1002/(SICI)1521-4044(19991201)50:11/12<383::AID-APOL383>3.0.CO;2-Z. [DOI] [Google Scholar]
- 34.Khanna PK, Gokhale R, Subbarao VVVS, Kasi Vishwanath A, Das BK, Satyanarayana CVV. A simple and effective route for the preparation of poly (vinylalcohol) (PVA) nanofibers containing gold nanoparticles by electrospinning method. Mater Chem Phys. 2005;92:229–33. doi: 10.1016/j.matchemphys.2005.01.016. [DOI] [Google Scholar]
- 35.Garnero C, Longhi M. Study of ascorbic acid interaction with hydroxypropyl-β-cyclodextrin and triethanolamin, separately and in combination. J Pharm Biomed Anal. 2007;45:536–45. doi: 10.1016/j.jpba.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Sionkowska A, Skopiska J, Wishiewski M. Photochemical stability of collagen/poly (vinyl alcohol) blends. Polymer Degrad Stability. 2004;83:117–25. doi: 10.1016/S0141-3910(03)00232-5. [DOI] [Google Scholar]
- 37.Fathi-Azarbayjani A, Chan SY. Single and multi-layered nanofibers for rapid and controlled drug delivery. Chem Pharm Bull. 2010;58:143–6. doi: 10.1248/cpb.58.143. [DOI] [PubMed] [Google Scholar]
- 38.Angberg M, Nyström C, Castensson S. Evaluation of heat-conduction microcalorimetry in pharmaceutical stability studies VII. Oxidation of ascorbic acid in aqueous solution. Int J Pharm. 1993;90:19–33. doi: 10.1016/0378-5173(93)90292-N. [DOI] [PubMed] [Google Scholar]