Abstract
The aim of this work was to evaluate the in vitro performance of a nebulized nanoemulsion formulation which had been optimised previously. To do so, a transparent nanoemulsion preparation containing 1.5 mg/ml of budesonide was prepared and diluted to achieve concentrations of 250 and 500 μg/ml budesonide. The in vitro characteristics of the diluted nanoemulsions were then compared with the commercially available suspension of budesonide (Pulmicort Respules®) when nebulized using a jet and a vibrating mesh nebulizer. A smaller MMAD with improved aerosol output was observed in the nanoemulsion preparations compared with the corresponding suspension formulations indicating an improved in vitro performance for the nanoemulsion-based preparations.
KEY WORDS: budesonide, in vitro characterisation, microemulsion, nanoemulsion, nebulizer, suspension
INTRODUCTION
For nebulizing glucocorticoids, one of the most important groups of drugs used in asthma, there is currently only a single type of formulation available. Suspension formulations of glucocorticoids such as budesonide (Pulmicort Respules®, Astrazeneca, UK) and beclomethasone (Beclospin®, Chiesi, Italy) have been marketed and widely used by patients. However, several drawbacks have been reported with using these microsuspensions including:
considerable heterodispersity in concentration of the drug in the aerosolised droplets (1).
limited bioavailability of the micronized pharmaceutical products due to low solubility compared with nanoparticles
short drug residence time in the lungs because of ciliary movement (2).
inconsistency in deposition patterns when using in different nebulizers (4).
Such issues provide rational reason to consider for alternative preparations to nebulize poorly soluble drugs, including glucocorticoids.
Micro- and nanoemulsions, due to their unique characteristics (e.g. ease of preparation, clarity, stability, ability to be filtered, incorporation of drugs of different hydrophilicities in a single formulation and increased bioavailability) have been widely investigated in drug delivery systems and reviewed in several papers (e.g. (4) and (5)). A wide range of micro- and nanoemulsion-based formulations have been proposed for oral, topical and parenteral administration, which, by mimicking the physicochemical properties of solutions, “solubilise” poorly water soluble drugs in an aqueous formulation, indicating potential for use in drug delivery systems.
Nanoemulsions, because of their high solubilising and drug protection features have the potential to deliver proteins as well as other new (or traditional) active drug compounds to the lungs. Although authors have suggested using nanoemulsion formulations for metered dose inhalers (5–11), nebulizable nanoemulsions have not been reported. With the advantage of solution-like physicochemical properties of nanoemulsions, it is hypothesised that nanoemulsions perform as a solution when nebulized and will demonstrate improved aerosolisation performance over suspension formulations. The aim of this work is to evaluate the in vitro properties of a nebulized nanoemulsion-based formulation containing budesonide—as a model steroid with poor aqueous solubility—for delivery to the lungs by nebulizers. The details on preparation and optimisation procedures for the nanoemulsion formulation have been detailed in a previous report (12).
MATERIALS AND METHODS
Medium chain triglyceride (Crodamol GTCC) was a gift from Croda (UK). Budesonide (Pharm. Eur.) was purchased from Industriale Chimica s.r.l. (Italy). Pharmaceutical grade Tween 80 was from Fluka (Switzerland). Pulmicort Respules suspensions for nebulization were from AstraZeneca (UK). All other chemicals were of analytical grade and purchased from Sigma-Aldrich (USA).
The nanoemulsion preparation was prepared by mixing 10% (w/w) polysorbate 80, 1% (w/w) ethanol, 1% (w/w) medium chain triglyceride in normal saline incorporating 1.5 mg/ml of budesonide, applying 4,500 J ultra sound energy to 20 ml final preparation, as previously reported (12). Using normal saline, the preparation was then diluted to obtain a budesonide concentration of 250 μg/ml and 500 μg/ml (i.e. NE250 and NE500, respectively). The in vitro characteristics of the nebulized dose from diluted nanoemulsion preparations were then compared with those of Pulmicort Respules formulations containing 250 μg/ml and 500 μg/ml of budesonide (i.e. P250 and P500, respectively) when delivered from a jet (Porta-neb compressor (Profile Therapeutics plc, UK)) and a vibrating mesh nebulizer (Microair® U-22 (Omron, Japan)). The particles in the microsuspension preparations have been reported to be amorphous with the size of 2–3 μm (13). The primary particle size of the nanoemulsion preparations was found to be 10.9 nm (12).
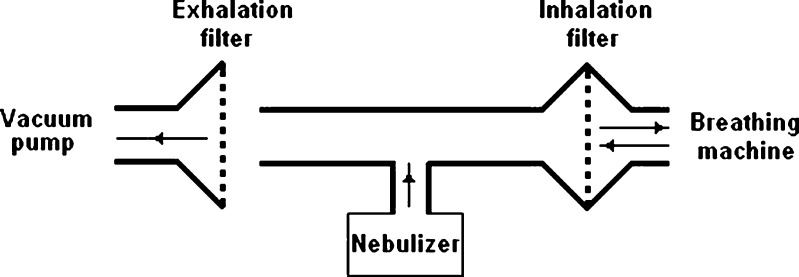
Either 4 ml of the final preparations for the jet nebulizer or 1 ml for the vibrating mesh nebulizer was put in the nebulizers and the total aerosol output from each system, as well as the aerodynamic characteristics of the nebulized droplets fraction, were determined according to the Comité Européen de Normalisation (CEN) methodology (14). Briefly, to study the aerosol outputs, the procedure describes a breathing machine creating a sinus flow of 15 breaths/min connected to a nebuliser. A vacuum pump operating at an inhalation flow of 25 L/min is used to capture the exhaled aerosol. Two electrostatic filters, positioned close to the breathing machine and the vacuum pump, take in the aerosols produced during each experiment to measure the inhaled and exhaled aerosol (see Fig. 1). To measure the aerodynamic particle size, a suction pump with a continuous flow of 13 L/min is attached to a Marple Series × 298 low-flow cascade impactor (Graseby, UK) (see Fig. 2). Stages of the cascade impactor from top to the bottom of the stack represent cut-off values of 50, 21.3, 14.8, 9.8, 6.0, 3.5, 1.55, 0.93 and 0.52 μm, respectively. The details of equipment and operating procedure have been reported previously (15). HPLC method was used to measure the amount of budesonide “trapped” on each stage of the impactor based on the procedure proposed and validated previously (16).
Fig. 1.
Setup for aerodynamic particle size measurements
Fig. 2.
Setup for aerosol output rate studies
For aerosol output studies, the nebulization continued until 1 min after the occurrence of “spluttering” in the jet nebulizer and until no aerosol was observed leaving the system in the vibrating mesh nebulizer. The nebulization time for aerodynamic particle size measurements was determined in a preliminary experiment by visual observation of the filters after running samples containing congo red dye.
To calculate the Mass Median Aerodynamic Diameter (MMAD) and Geometric Standard Deviation, the Copley inhaler testing data software version 2.00 (Copley Scientific, UK) was used. Log probability of the cumulative percentage less than the stated size was plotted against the effective cut-off diameter of each stage, and the calculations were performed. The respirable fraction was taken as cumulative amount of particles <5 μm. The fine particle fraction (FPF) was calculated using the following equation:
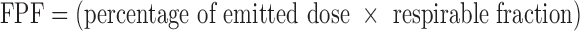 |
1 |
RESULTS AND DISCUSSION
Aerosol Output Studies
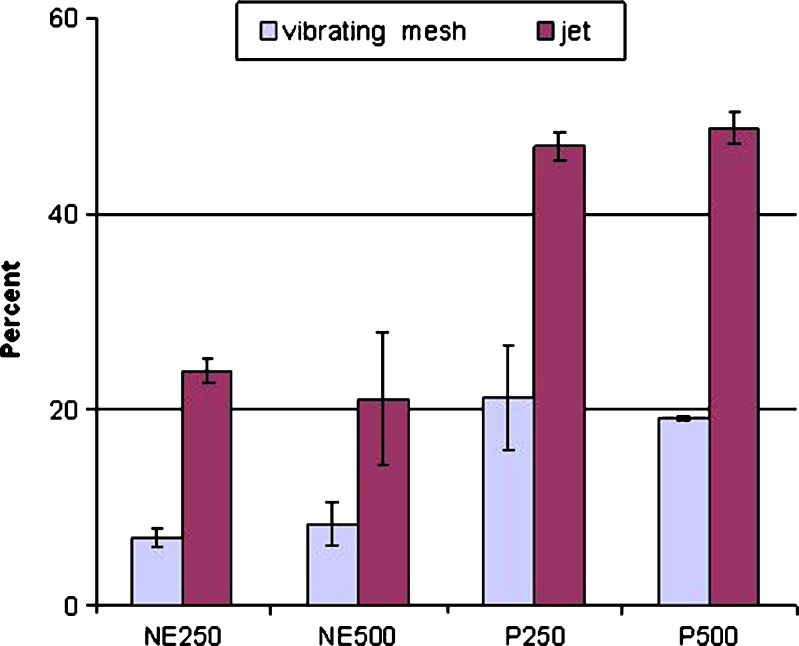
The mean (SD) in vitro aerosol output results for both the jet and the vibrating mesh nebulizers are detailed in Table I. Results were then compared using the statistical t test. Figure 3 illustrates the percentage of remaining dose in nebulizing chamber in four preparations using two nebulizer types.
Table I.
Mean (SD) Comparison of Properties of Nanoemulsion and Suspension Preparations in Jet and Vibrating Mesh Nebulizer (n = 5)
| Nebulizer | Preparation name | % inhalation filter | % exhalation filter | % left in chamber |
|---|---|---|---|---|
| Jet | NE250 | 37.5 (0.7) | 36.4 (1.2) | 23.9 (1.3) |
| NE500 | 37.5 (4.8) | 39.4 (2.1) | 21.1 (6.8) | |
| P250 | 26.6 (1.0) | 25.4 (0.6) | 46.9 (1.5) | |
| P500 | 25.8 (1.1) | 24.0 (1.6) | 48.8 (2.1) | |
| Vibrating mesh | NE250 | 43.3 (0.4) | 43.4 (1.8) | 6.9 (1.1) |
| NE500 | 42.7 (0.8) | 42.1 (0.9) | 8.3 (2.3) | |
| P250 | 34.3 (5.2) | 36.0 (3.1) | 21.2 (5.3) | |
| P500 | 33.9 (0.2) | 38.4 (1.1) | 19.1 (1.0) |
Fig. 3.
Mean (SD) of remaining dose in chamber from nebulized nanoemulsion and suspension preparations using the jet and the vibrating mesh nebulizer (n = 5)
Table I and Fig. 3 show the improved performance of NE250 and NE500 compared to the suspension formulations (i.e. P250 and P500) when nebulized by either of nebulizers. The amount of drug entrained on the inhalation and exhalation filters for NE250 and NE500 is significantly (p < 0.05) larger than corresponding values for P250 and P500, respectively. The data also show a significant decrease (p < 0.05) in the amount of drug remaining in the nebulizer chamber following nebulization for the nanoemulsion products compared to the corresponding suspension formulations.
Aerodynamic Particle Size
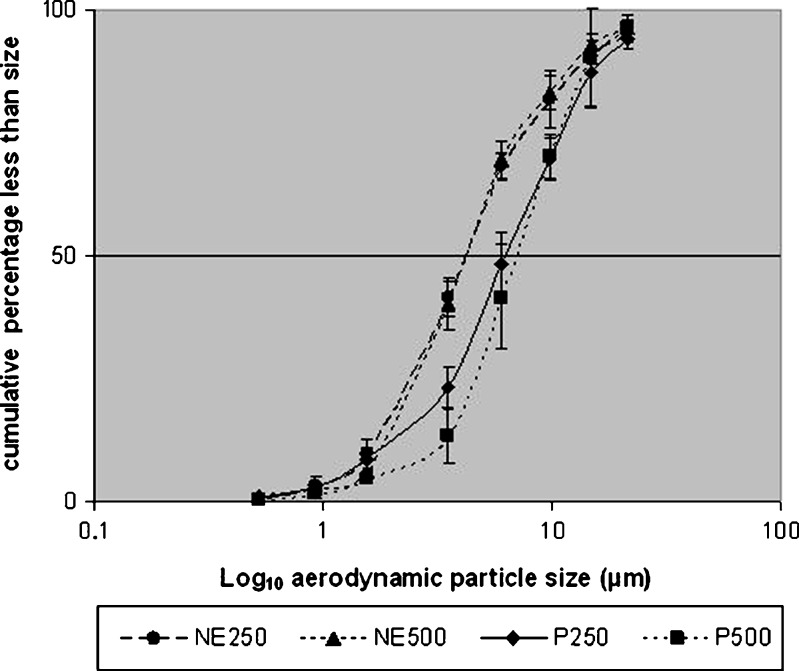
Table II provides the mean (SD) aerodynamic particle size data obtained for both nebulizer types together with the results of the statistical t test analysis. Figures 4 and 5 plot the log10 aerodynamic particles size against the cumulative percentage less than stated size. The obtained data are in good agreement with previously reported size distributions, with MMAD of 6.9 μm obtained for the suspension formulation in jet nebulizer compared with 4.8–9.9 μm—depending on the nebulizer used—(17) and 7.1 μm (18).
Table II.
Mean (SD) for Aerodynamic Particle Size Characteristics of NE250, NE500, P250 and P500 Obtained Using the Jet and the Vibrating Mesh Nebulizer
| NE250 | NE500 | P250 | P500 | ||
|---|---|---|---|---|---|
| Jet nebulizer | MMAD (μm) | 4.8 (0.9) | 5.5 (0.3) | 8.3 (0.5) | 6.9 (0.8) |
| % <5 μm | 50.6 (7.8) | 46.1 (2.6) | 22.7 (2.1) | 31.8 (7.6) | |
| Vibrating mesh nebulizer | MMAD (μm) | 4.0 (0.4) | 4.2 (0.4) | 6.2 (1.1) | 7.3 (1.9) |
| % <5 μm | 61.1 (6.9) | 60.9 (7.4) | 38.4 (9.6) | 29.9 (14.2) |
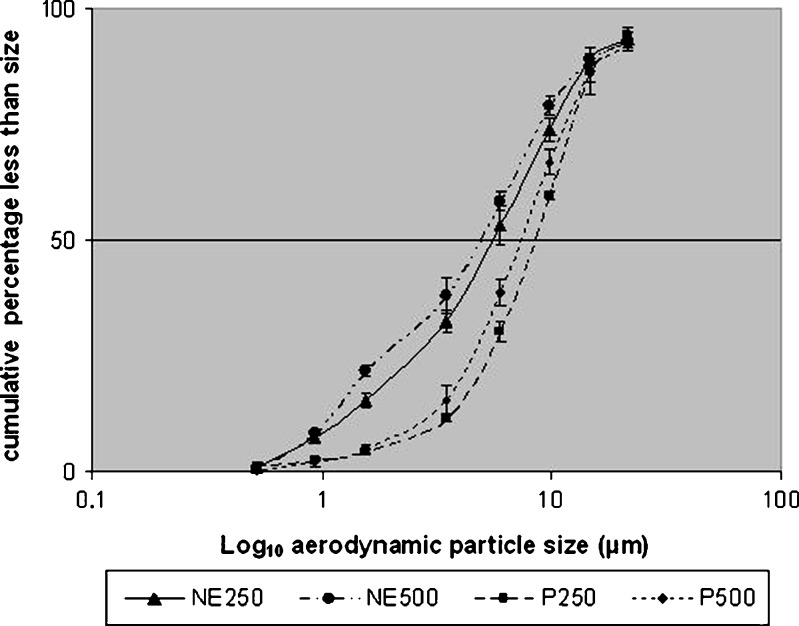
Fig. 4.
Mean (SD) of cumulative aerodynamic particle size of NE250, NE250, P250 and P500 using the jet nebulizer (n = 5)
Fig. 5.
Mean (SD) of cumulative aerodynamic particle size of NE250, NE500, P250 and P500 using the vibrating mesh nebulizer (n = 5)
Data in Table II and Figs. 4 and 5 indicate a major improvement of the in vitro aerodynamic particle size for the nebulized nanoemulsions compared to the suspension products. The MMAD of the NE250 and NE500 is significantly smaller than the P250 and P500 (p < 0.05). The respirable fraction is also significantly larger (p > 0.05) in nanoemulsion preparations compared with the suspension formulations. These findings represent a distinct improvement in in vitro characteristics of the emitted dose when nebulizing nanoemulsion preparations.
Comparing the FPF values in Table III, which represent the respirable percentage of emitted dose, a significant improvement (p < 0.05) is observed in FPF values for the nanoemulsion formulations compared with corresponding values for the suspension formulations.
Table III.
Mean (SD) FPF Values (%) for the Four Formulations Studied in Both Nebulizers
| NE250 | NE500 | P250 | P500 | |
|---|---|---|---|---|
| Jet nebulizer | 38.4 (5.8) | 34.1 (2.2) | 11.5 (1.5) | 14.0 (3.1) |
| Vibrating mesh nebulizer | 54.8 (6.5) | 53.1 (6.2) | 27.4 (9.1) | 21.6 (10.3) |
The larger MMAD values obtained for the suspension formulations using both nebulizers, as well as the lower respirable fraction and greater amount of drug left in chambers, indicate that compared with the nanoemulsion preparations, the suspension type of formulation is relatively inefficient for nebulization of this steroid. Furthermore, the respirable fraction should contain at least 50% of the emitted dose (19), since the respirable fraction values for the nanoemulsion formulations are in this range (see Table II), it is recognised that acceptable in vitro respirable fraction is obtained for NE250 and NE500. Comparing the substantially higher values for FPF for the nanoemulsions as well as smaller MMAD values—sizes which are required for steroid drug delivery to the central parts of the airways—an improved in vivo deposition performance of the nebulized nanoemulsion preparations compared with the suspension formulations can be anticipated, as reported in the literature (e.g. (20)).
The difference in the observed results between formulation types is attributed to the nanoemulsion particle size. The suspension particles in P250 and P500 are reported to be 2–3 μm using scanning electron microscopy (13), while the measured particle size of the nanoemulsion is 10.9 nm using Z-average of dynamic light scattering (12) and 10–12 nm using transmission light scattering (21). The generated aerosol droplets from nebulized microsuspensions will therefore be large aerosol droplets (i.e. larger MMAD values), each containing potentially a small number of suspension particles (2–3 μm) whereas the aerosolised nanoemulsion droplets are smaller (with lower MMAD values), containing many thousands of individual nanoemulsion particles (each 10.9 nm). Even, allowing for the coalescence of primary nanoemulsion particles because of evaporation of the aqueous phase during in vitro experiments, the aerosolised size of the nanoemulsion preparations will remain smaller than the nebulized suspension droplets. The higher concentration of the surfactant in the nanoemulsion preparation is also likely to be effective in reducing the interfacial tension and thus enable to generate smaller droplets on aerosolisation.
An additional parameter which may affect the particle size and particle size distribution of the nebulized preparations is the evaporation of the nebulized solvent (22). Using a cooled next generation impactor has been suggested to limit the effect of the evaporation during measurement (23), and thus it may be helpful to employ this approach to eliminate the effect of evaporation.
CONCLUSION
The nanoemulsion formulation containing budesonide exhibited a distinct improvement over the suspension formulation of budesonide in terms of its in vitro aerosolisation performance when evaluated, using a jet and a vibrating mesh nebulizer. Smaller MMAD and larger FPF and respirable fraction values indicate that solution-like nanoemulsion preparations are attractive alternative preparations for suspension formulations of budesonide for respiratory drug delivery via nebulisation.
Acknowledgement
The authors would like to express their gratitude to the Ministry of Health, Treatment and Medical Education of I.R. of Iran and Iranian Nanotechnology Initiative for supporting this research. We are also grateful to Mr. D.K. Nadarassan for his help in the nanoemulsion studies.
References
- 1.Knoch M, Keller M. The customised electronic nebuliser: a new category of liquid aerosol drug delivery systems. Expert Opin Drug Deliv. 2005;2:377–390. doi: 10.1517/17425247.2.2.377. [DOI] [PubMed] [Google Scholar]
- 2.Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 3.Bennet WD, Zeman KL, Kang WC, Schechter MS. Extrathoracic deposition of inhaled, coarse particles (4.5 μm) in children vs. adults. Ann Occup Hyg. 1997;41:497–502. [Google Scholar]
- 4.Nikander K, Turpeinen M, Wollmer P. The conventional ultrasonic nebulizer proved inefficient in nebulizing a suspension. J Aerosol Med. 1999;12:47–53. doi: 10.1089/jam.1999.12.47. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 6.Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst. 1999;16:461–521. [PubMed] [Google Scholar]
- 7.Courrier H, Krafft MP, Nakamura S, Shibata O, Vandamme T. Water-in-fluorocarbon reverse emulsion as a pulmonary drug delivery system: effect on the lung as modelled by a phospholipid monolayer. S.T.P. Pharma Pratiques. 2003;13:22–26. [Google Scholar]
- 8.Patel N, Marlow M, Lawrence MJ. Formation of non-ionic fluorinated surfactant microemulsions in hydrofluorocarbon 134a (HFC 134a) J Colloid Interface Sci. 2003;258:345–353. doi: 10.1016/S0021-9797(02)00072-3. [DOI] [PubMed] [Google Scholar]
- 9.Patel N, Marlow M, Lawrence MJ. Fluorinated ionic surfactant microemulsions in hydrofluorocarbon 134a (HFC 134a) J Colloid Interface Sci. 2003;258:354–362. doi: 10.1016/S0021-9797(02)00071-1. [DOI] [PubMed] [Google Scholar]
- 10.Sommerville ML, Hickey AJ. Aerosol generation by metered-dose inhalers containing dimethyl ether/propane inverse microemulsions. AAPS PharmSciTech. 2003;4:455–461. doi: 10.1208/pt040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommerville ML, Johnson CS, Cain JB, Rypacek F, Hickey AJ. Lecithin microemulsions in dimethyl ether and propane for the generation of pharmaceutical aerosols containing polar solutes. Pharm Dev Technol. 2002;7:273–288. doi: 10.1081/PDT-120005724. [DOI] [PubMed] [Google Scholar]
- 12.Amani A, York P, Chrystyn H, Clark BJ, Do DQ. Determination of factors controlling the particle size in nanoemulsions using artificial neural networks. Eur J Pharm Sci. 2008;35:42–51. doi: 10.1016/j.ejps.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Vaghi A, Berg E, Liljedahl S, Scensson JO. In vitro comparison of nebulised budesonide (Pulmicort Respules®) and beclomethasone dipropionate (Clenil® per Aerosol) Pulm Pharmacol. 2005;18:151–153. doi: 10.1016/j.pupt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.European committee for standardization (CEN) Respiratory therapy equipment—Part 1: nebulizing systems and their components. Brussles: CEN-CENELEC Management Centre; 2001. [Google Scholar]
- 15.Silkstone VL, Dennis JH, Pieron CA, Chrystyn H. An investigation of in vitro/in vivo correlation for salbutamol nebulized by eight systems. J Aerosol Med. 2002;15:251–259. doi: 10.1089/089426802760292591. [DOI] [PubMed] [Google Scholar]
- 16.Almeziny M, Clark BJ. High performance liquid chromatography assay method for simultaneous quantitation of formoterol and the two epimers of budesonide. J Pharm Pharmacol. 2007;59(Suppl. 1):76. [Google Scholar]
- 17.Berg EB, Picard RJ. In vitro delivery of budesonide from 30 jet nebulizer/compressor combinations using infant and child breathing patterns. Respir Care. 2009;54:1671–1678. [PubMed] [Google Scholar]
- 18.Amani A, Chrystyn H, Clark BJ, Abdelrahim ME, York P. Evaluation of supercritical fluid engineered budesonide powder for respiratory delivery using nebulisers. J Pharm Pharmacol. 2009;61:1625–1630. doi: 10.1211/jpp.61.12.0006. [DOI] [PubMed] [Google Scholar]
- 19.Kendrick AH, Smith EC, Wilson RS. Selecting and using nebuliser equipment. Thorax. 1997;52(2):S92–S101. doi: 10.1136/thx.52.2008.S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of ß2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–1504. doi: 10.1164/rccm.200410-1414OC. [DOI] [PubMed] [Google Scholar]
- 21.Amani A. Design, characterisation and in vitro aerosolisation performance of nanoemulsion for nebulisation of hydrophobic drugs. Ph.D. thesis, University of Bradford, 2008
- 22.Riedler J, Robertson CF. Effect of tidal volume on the output and particle size distribution of hypertonic saline from an ultrasonic nebulizer. Eur Respir J. 1994;7:998–1002. [PubMed] [Google Scholar]
- 23.Abdelrahim M, Chrsytyn H. Aerodynamic characteristics of nebulised terbutaline sulphate using the next generation impactor (NGI) and CEN method. J Aerosol Med Pulm Drug Deliv. 2008;22:19–28. doi: 10.1089/jamp.2008.0650. [DOI] [PubMed] [Google Scholar]