Abstract
Over the years, in vitro Franz diffusion experiments have evolved into one of the most important methods for researching transdermal drug administration. Unfortunately, this type of testing often yields permeation data that suffer from poor reproducibility. Moreover, this feature frequently occurs when synthetic membranes are used as barriers, in which case biological tissue-associated variability has been removed as an artefact of total variation. The objective of the current study was to evaluate the influence of a full-validation protocol on the performance of a tailor-made array of Franz diffusion cells (GlaxoSmithKline, Harlow, UK) available in our laboratory. To this end, ibuprofen was used as a model hydrophobic drug while synthetic membranes were used as barriers. The parameters investigated included Franz cell dimensions, stirring conditions, membrane type, membrane treatment, temperature regulation and sampling frequency. It was determined that validation dramatically reduced derived data variability as the coefficient of variation for steady-state ibuprofen permeation from a gel formulation was reduced from 25.7% to 5.3% (n = 6). Thus, validation and refinement of the protocol combined with improved operator training can greatly enhance reproducibility in Franz cell experimentation.
KEY WORDS: franz cell, in vitro diffusion cell, reproducibility, standardisation, validation, variability
INTRODUCTION
Transdermal drug delivery is advantageous in comparison to other administration routes as it generally facilitates avoidance of first pass metabolism, decreased toxicity, fewer side effects as well as greater patient compliance. Within this context, the use of in vitro static diffusion cells to assess skin permeability has evolved into a major research methodology, providing key insights into the relationships between skin, drug and formulation (1,2). Such testing is highly useful not only for the design and development of novel formulations but also for toxicity screening and quality-control purposes (3–6).
Crucially, Franz-type diffusion studies frequently involve the use of synthetic membranes to model real skin. Although the artificial membranes will not model the lipid perturbation effects undergone by biological samples, inferences regarding partitioning and diffusion phenomena can be made. Synthetic membranes may be preferred to skin tissue as they are more easily resourced, less expensive and structurally simpler. This means large-scale studies can be more readily undertaken while mechanisms can be deconvoluted more readily (7). Furthermore, synthetic membranes exhibit superior permeation data reproducibility as in vivo variables such as skin age, race, sex and anatomical site are eliminated (8). Nevertheless, the results of artificial membrane studies tend to still yield significant (9). One study examined the intra-laboratory variation in the rates of testosterone penetration through sections of polydimethylsiloxane (PDMS) membrane (10). The study consisted of 63 replicates. It was found that the steady-state flux of testosterone exhibited a coefficient of variation (CV) of 32%. Sequential intra-laboratory measurements of solute flux across PDMS membranes yielded CV values of 45.5%, 30.7%, 15.1% and 13.1% for adenosine, aldosterone, corticosterone and estradiol, respectively (11). PDMS was also used in a different study involving 18 different participating laboratories worldwide. Methylparaben was the test penetrant, while a minimally prescriptive protocol was employed to obtain mean flux values. Interestingly, the CV between laboratories was 35% (12). These variabilities have been explained as being caused by differential experimental setup and operator training, as the synthetic membranes are of precise manufacture (12). Current regulatory guidelines offer only partial standardisation of in vitro skin absorption studies (13). This aspect gives each laboratory a broad margin of flexibility in terms of equipment design and experimental protocol, so each laboratory has its own individual set of Franz diffusion cells with varying physical dimensions and design characteristics. Also, methodologies often vary with respect to dose levels, membrane treatment, sampling intervals and temperature regulation. However, if Franz cell systems are validated before experiments, then drug penetration profiles and values should not deviate significantly between and within laboratories.
The aim of this current paper was to assess the influence of a comprehensive validation protocol on the performance and data output of a tailor-made array of Franz diffusion cells available in our laboratory. Ibuprofen was used as a model hydrophobic drug while synthetic membranes were used as model barriers in order to eliminate biological tissue variability. The investigated parameters included stirring conditions, membrane chemistry, temperature effects and sampling frequency.
MATERIALS
Diffusion Cell Equipment
Unless otherwise stated, the tests described below were undertaken on a tailor-made Franz cell array donated by GlaxoSmithKline (Harlow, UK). This apparatus consisted of 27 tailor-made donors, receptors and four heated magnetic stirrer chambers each containing eight blocks into which the Franz cells are placed. Figure 1a presents a sketch of a typical Franz cell from this array. A speed control, displaying arbitrary numbers 1 to 15, allowed modulation of the rate of magnetic stirring. A few measurements were conducted on an entirely different set of cells, namely three arrays of V-series nine-station compact Franz cells purchased from Permegear Inc. (Bethlehem, PA). These three arrays consisted of 27 pairs of donor and receptor cells, each positioned in a magnetic stirrer block. The receptor cells were water-jacketed, and the water supply of each array was connected to a thermostatically controlled water bath (Haake DC10, Karlsruhe, Germany).
Fig. 1.
a Sketch of typical Franz cell (tailor-made) and b diagram to indicate the physical characteristics investigated. The location of the thermocouples during temperature validation is indicated on (b) by the letters A, B and C
Chemicals
Periodic acid (purity 98%), glycerol (purity >99%), acetic acid (purity >99%), 37% hydrochloric acid, phosphate-buffered saline, pH 7.4 tablets and basic Fuchsin (pararosaniline) were purchased from Sigma–Aldrich (Dorset, UK). Sodium hydroxide and sodium metabisulphite (purity >90%) were obtained from BDH Laboratory Supplies (Poole, UK), potassium permanganate from Fisher Scientific (Loughborough, UK) and methylparaben, ≥99% from Fluka (Buchs, Switzerland). Ibuprofen (99%) was obtained from Medex (Naseby, UK), Phorpain gel containing 5% ibuprofen from Goldshield Pharmaceuticals (Croydon, UK) and helium gas for deaeration from BOC Gases Ltd (Guildford, UK).
Other Materials
A teflon cylindrical magnetic stirring bar (dimension 10 × 3 mm), a PTFE cylindrical magnetic bar (12 × 4.5 mm) and a PTFE cylindrical magnetic bar with pivot ring (12 × 6 mm) were purchased from VWR International (Lutterworth, UK). The PTFE octagonal magnetic stirring bar with pivot ring (38 × 10 mm) and benzoylated dialysis cellulose tubing (MW cut-off 2,000 Da) were obtained from Sigma–Aldrich (Dorset, UK). Both Visking seamless cellulose tubing (MW cut-off 12,000–14,000 Da) and Cuprophan flat sheet membrane (MW cut-off 10,000 Da) were supplied by Medicell (London, UK), and polyacrylamide membrane (AN69) was obtained from Gambro Hospal (Lyon, France). PDMS membranes (product code 19T0.3–1000–60M1) were obtained from Samco (Nuneaton, UK) while the phosphate-buffered saline (pH 7.4) sachets which had been used in a previous inter-laboratory study described by Chilcott and co-workers , were kindly provided by that author.
METHODS
Franz Cell Dimension Evaluations
Dimensional measurements were undertaken on both the tailor-made and the Permegear Franz cells. A pictorial summary of the variables investigated are shown in Fig. 1b. A pair of callipers (Mitutoyo, Hampshire, UK) was used to measure, in triplicate, the height of each donor compartment while a protractor was used to determine the angle between each receptor cell chamber and side arm. Visual observations were used to classify the receptor cell bases as either flat or convex. To determine receptor volume, a cylindrical magnetic stirring bar (12 × 4.5 mm) was placed into the receptor compartment. Distilled water was carefully filled to the lip of the compartment and the exact volume was recorded (n = 4). For measuring the internal diameters of the donor and receptor cells, a pair of callipers was placed on the inside lip, 1 to 2 mm from the opening. External diameter measurements of the receptor cells were performed by laying the calliper tips on the outside of the lip. External diameter measurements of tailor-made donor cells were not possible because the lip at the edge of the opening is curved. The effective diffusion area (EDA) was calculated for both donor and receptor cells using the formula πr2, where r was the internal radius of the donor cell, but was the mean of both internal and external radii of the receptor cell.
One immediate outcome of these measurements was that it was apparent that for the tailor-made Franz cells, the receptor chambers had much larger EDA values than the donor chambers. Hence, if the receptors and donors were matched randomly, contact areas would vary with each experiment. In order to avoid this, receptor and donors of similar EDA were matched for use in all the validated diffusion experiments but were paired randomly in unvalidated experiments.
Stirrer Efficiency and Dye Spreading Measurements
These studies, which were conducted only on the tailor-made diffusion cells, consisted of four different types of experiments. In the first series of experiments, the influence of stirring rate on dye mixing times was assessed. The receptor cells, each containing a cylindrical PTFE stirring bar of 12 × 4.5 mm, were placed randomly into the stirrer blocks and filled with distilled water at room temperature. A 10-mg mass of finely ground potassium permanganate powder was gently deposited into each receptor cell. The times taken for the dye to spread uniformly throughout the main receptor cell chamber and sidearm at arbitrary low (1.5, 3), medium (5, 7), high (10, 11) and very high (12, 14) speeds were recorded.
In the second set of experiments, three types of bars of differing dimensions were tested for stirring efficiency. These were: Type I—Teflon cylindrical magnetic stirring bar, 10 × 3 mm; Type II—PTFE cylindrical magnetic stirring bar, 12 × 4.5 mm; and Type III—PTFE pivot ring cylindrical magnetic stirring bar, 12 × 6 mm. A 10-mg mass of finely ground potassium permanganate dye powder was gently loaded into the receptor cell filled with distilled water. Stirring was initiated at room temperature. For each type of stirring bar, the dye distribution pattern was observed with no stirring as well as at low-speed, medium-speed and high-speed stirring.
The third set of experiments involved identification of which blocks yielded optimal stirring. Receptor cells filled with distilled water were inserted into these identified blocks and heated to 37 ± 0.1°C. The potassium permanganate dye experiment was repeated using a 12 × 4.5-mm (Type II bar) in the Franz cells, and the time for uniform dye distribution throughout the Franz cells was recorded.
The aim of the final group of experiments was to determine the number of revolutions per minute (RPM) achieved by a magnetic stirrer exposed to an arbitrary speed setting of 10. To this end, a 100-ml beaker filled with 50 ml of distilled water was marked with a vertical line drawn from the beaker lip to its base. A PTFE magnetic stirring bar, octagonal with pivot ring, of dimension 38 × 10 mm was placed in the beaker. A line was marked at one end of the stirring bar. The beaker containing the stirrer bar was immersed into a well at ambient temperature. The RPM was assessed by visually counting the number of times the line on the stirring bar aligned with the line on the beaker. Stirring bar behaviour, in terms of spinning rate uniformity as well as location, was also observed.
Membrane Characterisation Studies
Glycerol, an impurity commonly found in membranes, is generally removed by washing in water. This part of the study aimed to investigate firstly whether the glycerol content correlated with the manufacturers’ stated values and secondly, whether adequate glycerol removal was attained by applying a simple washing procedure. To this end, a periodic acid Schiff assay (PAS) was adapted from the literature (14,15) in order to quantify the glycerol content of each membrane before and after washing with water. For the preparation of Schiff reagent, 20 ml of 1 M hydrochloric acid was added to 100 ml of 1% w/v basic Fuchsin. Sodium metabisulphite (2 g) was added to this solution, which was then incubated at 37°C for 120 min. The periodic acid reagent was prepared by adding 10 ml of 50% w/w periodic acid solution to 7 ml of 7% w/v aqueous acetic acid.
Sections of Visking, Cuprophan, Benzoylated cellulose and Polyacrylonitrile (AN69) membranes, weighing between 5 and 10 mg, were cut out and the precise dry mass of each sample was recorded. Each membrane sample (n = 3) was washed by immersing in a 5-ml volume of distilled water in a vial which was capped and rotated for at least 12 h at 37°C. All samples were then assayed for glycerol by adding 0.2 mL of freshly prepared periodic acid reagent and incubating at 37 C for 120 min. Samples were allowed to cool to room temperature and 0.2 mL of the prepared Schiff reagent added to the vial. After 30 min, the membrane was removed. Subsequently, 1 ml aliquots of the remaining solution were assayed for glycerol at 555 nm using a Unicam UV1 spectrophotometer (Cambridge, UK). To generate a calibration curve, six aqueous solutions of glycerol were prepared at concentrations ranging from 6.25 × 10−4 to 0.05% w/v and assayed as above.
To validate the washing procedure, the membranes were rinsed with distilled water after removal from the vial. These membranes were re-washed by transferring to clean closed vials containing 1.2 ml of distilled water which were rotated for a further 24 h, and the washings were again assayed with PAS reagent.
The effect of receptor fluid uptake was also investigated. The membranes were cut into sections of mass 2 to 6 mg. These were soaked in a glass vial containing 5 ml of 0.1 M sodium hydroxide and stored at 25°C. The mass gain over 24 h was measured. Sodium hydroxide was employed instead of water because it was the receptor fluid used for the ibuprofen permeation study.
Temperature Measurements
The tailor-made Franz cells were subjected to temperature monitoring experiments that were performed in triplicate. To this end, the donor and receptor compartments were filled with de-aerated water while Visking sections were employed as model membranes. The heated magnetic stirrer blocks were set to 37°C. Thermocouple probes (Pico Technology Ltd., Cambridgeshire, UK) were positioned at three different sites within the Franz cell as shown in Fig. 1b. These positions were; at ~1 mm above the membrane (A), at ~1 mm below the membrane (B) and at the receptor base (C). The probes were electronically connected to a PicoLog TC08 data logger and IBM-compatible computer programmed with software (Pico Technology Ltd., Cambridgeshire, UK) designed to integrate the thermal data. The temperatures at points A, B and C were recorded over a 7-h period (n = 4), during which no sampling from the side-arm was undertaken.
The equilibration time was defined as the duration required for the receptor cell temperature to stabilise at 37°C prior to the running of the diffusion experiment. Investigations into this parameter involved heating of the stirrer blocks to 37°C followed by filling the receptor cells with de-aerated water at room temperature and stirring with a magnetic bar (12 × 4.5 mm). The thermocouple positioned at site R was activated and the temperature recorded once a minute over 30 min (n = 9).
Once drug diffusion is occurring, temperature fluctuations can develop in conjunction with sampling. In order to examine this process, a receptor compartment was filled with distilled water and covered with a Visking cellulose membrane section. A 1-ml aliquot of distilled water was deposited in the donor chamber. The system was left to equilibrate for 30 min. Sampling involved removing 1 ml of receptor phase and replenishing with fresh distilled water at the same temperature. Sampling was undertaken every 10 min for 3 h and subsequently every 30 min for the following 4 h. After each solution withdrawal but before replenishment, the temperatures at both points A and B were recorded (n = 4).
Ibuprofen Transport Studies—before and after Validation
Ibuprofen trans-membrane permeation from Phorpain gel was measured before and after validation by conducting transport studies over 7 h (n = 6). Phorpain gel is a commercial hydrogel for pain relief containing 5% ibuprofen EP, hydroxyethyl cellulose, sodium hydroxide, benzyl alcohol, isopropyl alcohol and purified water. A clean, dried receptor cell was filled with de-aerated 0.1 M sodium hydroxide and was allowed to equilibrate in the heated magnetic stirrer block set at 37°C. The previously hydrated cellulose membrane was mounted between the donor and receptor compartment and 0.8–1.2 g of gel was placed on the membrane surface in the donor compartment. All the Franz cell openings, including the donor–receptor interface were occluded with Parafilm in order to prevent evaporation. The receptor compartment was stirred at a speed setting of 10 using a magnetic stirrer. Sample volumes (1–2 ml) were taken for UV analysis at 272 nm and fresh preheated medium replacements of the same volume were reintroduced into the receiver. This was to maintain a constant volume as well as sink conditions. Intervals between sampling varied from 5 to 30 min. Air bubbles developing underneath the membrane were removed via the side arm by carefully tilting the Franz cell.
The experiments before validation involved casual pairing of the donors and receptors, which were then placed at random in the heated magnetic stirring blocks. All three types of stirrer bar were used at random at speed 10. Thus, in these initial pre-validation experiments, the stirring speed and stirring efficiency were not optimised nor was the ability of the heated magnetic stirrer block when set at 37°C to maintain this temperature in the particular receptor cell used. Sampling, which is meticulous and precise, also requires operator practice, as in a study of cumulative drug release, any initial loss of drug solution will magnify errors throughout the experiment.
For validated experiments, flat-based receptors and donors of similar EDA were matched for use. These were placed in the appropriate heated magnetic stirrer block, previously identified as providing efficient stirring at speed 10 and good temperature control when set to 37°C. The cellulose membrane was hydrated for 24 h prior to the start of the experiment and an equilibration time of 15 min prior to drug loading was ensured.
Plots of cumulative % ibuprofen drug permeation through each synthetic membrane against time were derived. Ibuprofen flux was calculated from the slope of the curve at steady state. A CV for the total ibuprofen content in the receptor chamber at each time point was calculated according to: 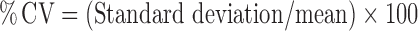 .
.
This study was then repeated but using saturated ibuprofen solutions instead of the commercial gel (n = 6). The saturated solutions were prepared by adding excess ibuprofen powder (~2 g) to 0.1 M sodium hydroxide and the suspension formed was agitated for 1 h in a water bath maintained at 60–65°C. Residues of un-dissolved ibuprofen were removed by filtration at 37°C and the solution was then allowed to cool to 33°C, which is the donor solution temperature. The flux and CV values obtained from the saturated solution experiments were compared to those obtained from commercial gel before and after validation.
Ibuprofen Transport Studies: Influence of Sampling Volume and Frequency on Sink Conditions
These studies were conducted only on the tailor-made Franz cells. The aim was to determine if sink conditions were maintained when different donor volumes and sampling time frequencies were used. Diffusion studies were undertaken employing the validated procedure (n = 3) using saturated ibuprofen solutions and Visking membrane sections that had been pre-hydrated in 0.1 M sodium hydroxide. Each receptor compartment was filled with de-gassed 0.1 M sodium hydroxide. Following a 15-min temperature equilibration period, each donor compartment was loaded with either 0.1 ml, 1 ml or filled entirely (~3 ml depending upon the individual donor cell) with saturated ibuprofen solution. After 24 h, an aliquot from each receptor compartment was withdrawn and appropriately diluted before UV analysis at 272 nm. Each measurement was performed in triplicate and receptor drug concentrations were calculated.
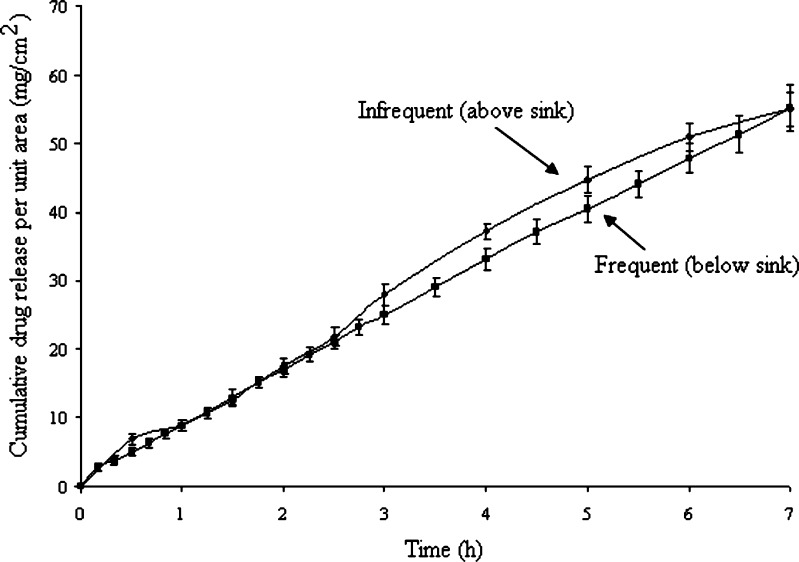
Investigations into the influence of sampling time frequency on drug penetration involved the use of polyacrylamide AN69 membranes. These were soaked for 24 h with 5 ml of receptor fluid and subsequently rinsed to remove glycerol. Six receptor cells were filled with de-aerated 0.1 M sodium hydroxide at 37°C and the pre-soaked polyacrylamide sections were inserted as model membranes. Six donor cells were then placed in position and each of these was filled with 1 ml of saturated ibuprofen solution. Aliquots of receiver solution were removed over a 7-h period and assayed for ibuprofen as described above. In three Franz cells, sampling from the receiver solution was undertaken frequently (at 0, 10, 20, 30, 40, 50, 60, 75, 90, 105, 120, 135,150, 165, 180, 210, 240, 270, 300, 330, 360, 390 and 420 min) whereas in another three Franz cells sampling was undertaken infrequently (every 30 min). Plots of cumulative amount of ibuprofen permeated per square centimetres of synthetic membrane against time were derived. Statistical analysis was conducted on both flux of frequent and infrequent sampling.
Inter-laboratory Comparison of Validated Equipment Using Methylparaben
Using the validated Franz cells, methylparaben (MP) release from saturated solution across PDMS membrane was quantified in the manner reported by Chilcott et al. The only condition that differed from that of the 2005 study was that our receptor temperature was maintained at the validated temperature of 37°C instead of 35°C. The flux and standard deviation values we obtained were compared with those reported in the earlier publication (12).
Statistical Analysis
All statistical analyses was carried out using ANOVA, and the significance level was accepted when p < 0.05.
RESULTS
Franz Cell Dimensions: GlaxoSmithKline vs PermeGear
Table I presents the derived measurement values for both types of Franz cells. It can be seen that the heights of the tailor made donor cells were greater than those of PermeGear. In terms of effective diffusion areas, the donor cell values were lower for the tailor-made cells while the receptor cell values were similar for both companies. Receiver cell volumes did not vary greatly within manufacturers. However, GlaxoSmithKline receptors exhibited greater volumes than those of Permegear but had less steep side arms. Visual observations of the tailor-made cells showed that some of the receptor cell bases were convex as a result of manufacturing variations. This feature prevented the stirrer bar from spinning centrally and achieving good mixing. As Table I shows, both types of apparatus exhibited relatively similar dimensional variability, as assessed from coefficient of variation values.
Table I.
Physical Dimension Measurements (mean ± SD) for Both Tailor-Made and Commercial Franz Cells
| Parameter | Tailor-made | Commercial | ||
|---|---|---|---|---|
| Mean ± SD | % CV | Mean ± SD | % CV | |
| Donor | ||||
| Height (cm) | 5.2 ± 0.3 | 5.8 | 1.6 ± 0.1 | 6.2 |
| EDA (mm2) | 59.6 ± 3.1 | 5.2 | 73.8 ± 3.3 | 4.5 |
| Receptor | ||||
| Volume (ml) | 11.7 ± 0.1 | 0.8 | 5.2 ± 0.1 | 1.9 |
| Angle of the arm (°) | 128 ± 2.7 | 2.1 | 135 ± 1.7 | 1.2 |
| EDA (mm2) | 78.2 ± 3.1 | 4.0 | 74.7 ± 3.5 | 4.7 |
| Shape of base | Flat to convex | – | N/A | – |
Influence of Stirring Variables
At low speed, the dye dispersed uniformly throughout the receptor chamber within approximately 8–10 min but took more than an hour to reach fully up the side arm. At high speeds (9–11 arbitrary units), the dye still took 10–12 min to disperse throughout receptor cell and no vortex was observed. From speed 12 and onwards, although the dye spread quickly throughout the receptor, a vortex could be observed at the surface of the receptor.
Type I magnetic stirring bars gave inadequate dye distribution throughout the receptor cell at high speed after 120 s, whereas Type II bars gave a similar result after 60 s. Type III bars gave the best stirring. These facilitated uniform dye distribution throughout the chamber and side arm after 60 s at high speed. However, an undesirable vortex was observed. Vortexing could even be observed at medium speed using Type III stirrer bars.
Other measurements indicated that a speed setting of 10 yielded a spinning rate of approximately 200 rpm. The speed varied from 162 to 220 rpm in the unvalidated system. It was found that only nine blocks provided effective central spinning at a rate of 197.5 ± 16.1 rpm. It was noteworthy that a certain few stirring blocks yielded consistent RPM values but caused the stirring bars to spin off-centre.
By combining all the stirring data for the tailor-made equipment, it was determined that satisfactory stirring efficiency could be obtained by using Type II stirrer bars at ~200 rpm in matched donors/receptors. Table II provides a summary of the influences of various types stirring bar.
Table II.
The Summary of Stirring Bar, Donor Volume and Sampling on Temperature Variables of Franz Cells
| Parameters | Variables | ||
|---|---|---|---|
| Stirring bar | |||
| Type I | Type II | Type III | |
| Speed | 200 rpm | 200 rpm | 200 rpm |
| Time | 120 s | 60 s | 60 s |
| Uniform dye distribution | Yes | Yes | Yes |
| Vortex | Yes | No | Yes |
| Donor volume | |||
| 0.1 mL | 1 mL | 3 mL | |
| Sink condition attained after 24 h | Yes | Yes | No |
| Temperature at various Franz cell sites | |||
| A | B | C | |
| No sampling | 31.48 ± 0.2°C | 34.12 ± 0.3°C | 36.27 ± 0.1°C |
| Temperature drop due to sampling | 1.5 ± 0.5°C* | 1.4 ± 0.2°C* | No change |
Type I Teflon cylindrical magnetic stirring bar, 10 × 3 mm; Type II PTFE cylindrical magnetic stirring bar, 12 × 4.5 mm; Type III PTFE pivot ring cylindrical magnetic stirring bar, 12 × 6 mm; A 1 mm above the membrane, B 1 mm below the membrane, C the receptor base
*p < 0.05 statistically significant different from the same site of no sampling
Influence of Membrane Type
Table III presents weight gain data following the prolonged soaking in receiver solution of each membrane type. It can be seen that appreciable membrane swelling occurred in Cuprophan, Visking and polyacrylonitrile membranes, in decreasing order of magnitude. Evidence from the PAS tests indicated that the Visking, Cuprophan and polyacrylonitrile membranes contained glycerol with recorded amounts of 56.9 ± 16.3%, 13.5 ± 2.5% and 11.6 ± 2.6%, respectively. The benzoylated cellulose membrane contained only trace amounts of glycerol (0.06 ± 0.01%). It is noteworthy that these values do reasonably correlate with the manufacturers’ stated glycerol content for those membranes (see Table III). No glycerol could be detected in any of the membranes following their rinsing and soaking for a further 24 h.
Table III.
Quantification of Glycerin Content in Various Cellulose Membranes
| Membrane | MWCO (kDa) | Surface groups | Stated glycerin content (%) | Actual glycerin content (%) | Membrane swelling after 24 h (%) |
|---|---|---|---|---|---|
| Visking | 12–14 | –OH | 9–13 | 11.6 ± 2.6 | 170 |
| Cuprophan | 10 | –OH | 13–15 | 13.5 ± 2.5 | 830 |
| Benzoylated cellulosea | 2 | –OH and benzoyl | Not stated | Trace | Not applicable |
| AN 69b | 4 | –C–N | >45 | 56.9 ± 16.3 | 130 |
aBenzoylated cellulose tubing was supplied in a hydrated state
bWhile Visking, Cuprophan and Benzoylated cellulose were all composed of cellulose derivatives, AN 69 was composed of a polyacrylonitrile-based material
Influence of Donor Solution Volume
Our results showed that ibuprofen solubility in 0.1 M sodium hydroxide was 2.18% w/v. Crucially, sink conditions were maintained with donor solution volumes of 0.1 and 1 ml, i.e. the ibuprofen concentrations were less than 0.218% w/v; but sink conditions were not maintained in diffusion cells containing fully filled (~3 mL) donor cells. This is summarised in Table II.
Overall Influence of Validation
Figure 2 shows the ibuprofen gel permeation data both before (a) and after (b) after validation of the variables. Use of the validated equipment and methodology reduced the coefficient of variation from 25.7% to 5.3% (n = 6). When the validated protocol was used to conduct permeation studies involving the same model membrane but using saturated ibuprofen solution, the coefficient of variation was only 4.1% (Fig. 3).
Fig. 2.
Ibuprofen release from model gel formulation a before (CV 25.7%) and b after validation (CV 5.3%)
Fig. 3.
Ibuprofen release from saturated solution after validation (CV 4.1%)
Influence of Sampling Rate
The rate of sampling from the receiver compartment did not greatly influence ibuprofen permeation. As Fig. 4 shows, the measured drug fluxes for frequent and infrequent sampling experiments were not statistically significant (p > 0.05). However, infrequent sampling did allow ibuprofen concentrations in the receptor chamber to reach 11.1% of saturation concentration at 7 h, which means sink conditions (<10%) were no longer maintained. In contrast, frequent sampling permitted receiver drug concentrations to attain a maximal value of 9.8%, which is just within sink conditions.
Fig. 4.
Ibuprofen release from saturated solution through AN69 membrane. Frequent sampling (varies from 5 to 30 min); Infrequent sampling (varies between 15 to 30 min)
The receptor cells took approximately 8–10 min to equilibrate to 37°C. A temperature drop was observed at point A and B (see Fig. 1b) during sampling and the mean differences recorded were 1.5 ± 0.5°C and 1.4 ± 0.2°C, respectively. Without sampling, the temperature was stable at all three points with A at 31.48 ± 0.2°C; B at 34.12 ± 0.3°C and C at 36.27 ± 0.1°C. The temperature drops caused by sampling were significantly different from those without sampling (p < 0.05, n = 10). This is summarised in Table II.
Figure 5 shows the plot of MP flux obtained from our study using validated equipment. The identified steady state exhibited a good linear fit (r2 ≥ 0.99), and the calculated steady-state flux value was 36.4 ± 0.6 μg cm−2 h−1, representing a CV of 1.7%. Table IV shows the flux and standard error values obtained from a very similar inter-laboratory comparative study published in 2005. It can be concluded that our mean flux value falls very closely to the mean flux value obtained by five labs in that earlier study, although our CV was lower.
Fig. 5.
Methylparaben release from saturated solution through PDMS membrane (CV 1.7%)
Table IV.
Average Flux for Methyl Parabens
| CV | Average flux ± SE (μg cm−2 h−1) | Nos. of labs |
|---|---|---|
| <5% | 40.7 ± 4.3 | 5 |
| <10% | 58.0 ± 4.8 | 16 |
| <20% | 60.0 ± 4.6 | 22 |
For laboratories with CV less than 5%, 10% and 20% (12)
DISCUSSION
The popularity of Franz-type diffusion cells for permeation studies has resulted in a high demand for this equipment, leading to the emergence of several Franz cell manufacturers as well as some FDA standardisation (16,17). However, this apparatus is generally expensive. Commercially manufactured sets theoretically provide the least variability at the greatest cost and are, therefore, concentrated in industrial laboratories. Hand-blown Franz cells constitute a less expensive alternative in which variability depends upon the skill of the individual technician. Consistent with this paradigm, we determined that receptor and donor EDA values were similar (~1 mm2 difference) for the commercially manufactured array but varied greatly (~20 mm2 difference) for the tailor-made array. However, the variability of other physical dimensions was surprisingly similar in magnitude for both types of apparatus. In fact, appreciable variation was observed in all physical characteristics measured.
With respect to specific elements of the Franz cell, tall donor chambers may be favourable. This is because small volumes (<0.5 mL) allow evaporation, thus producing time-dependent alterations in formulation composition that change drug solubility in the vehicle. Differences in receptor volume were also apparent with the tailor-made diffusion cells. Variations in side-arm steepness also existed. GlaxoSmithKline cells with steeper side arms showed slower dye dispersion within the side arm. Inadequate mixing in the side arm hinders receptor phase homogeneity yielding high variability when sampling. This problem was resolved by standardising the stirrer bar type and speed within the receptor. The diameter of the side arm orifice is also important because sufficient space is required to eliminate air bubble generation during sampling. Air bubbles are detrimental as their presence at the membrane underside reduces the EDA.
Stirring is another important issue to consider in validation. Stirring of the receptor fluid is critical for the maintenance of both uniform drug distribution as well as temperature equilibrium (18, 19). The time taken to achieve such uniformity determines the minimum time for the first sampling interval as well as times between subsequent samples. It is noteworthy that inefficient stirring can occur when there is fast stirring near the base of the receptor chamber but negligible stirring in the upper parts of the chamber. Poor stirring may also be associated with efficient fluid mixing throughout the bulk of the chamber but not within or in the vicinity of the side arm (18). Such effects cause local deviation from sink conditions as the drug is not distributed evenly throughout the receptor cell. Furthermore, stirring should not cause vortex formation, which is undesirable due to its potential to disrupt the static fluid layer adjacent to the membrane. Such an effect changes one of the assumptions of Fick’s law, namely that the calculation of the diffusion coefficient includes a contribution from the boundary layer (20, 21). Thus, the ideal stirrer bar-speed combination for any Franz cell system is the one that yields the fastest mixing without a vortex. In this work, a simple type II stirrer bar operating at 200 rpm was found to be optimal.
Another key consideration relates to the condition of the barrier membrane. Glycerol is generally incorporated into cellulose-based and polyacrylonitrile-based membranes to enhance suppleness and reduce brittleness during transportation and handling. Unfortunately, glycerol can block membrane pores, altering the drug diffusion coefficient and/or leach into the receptor chamber thus interfering with drug analysis (22). Hence, it is important to remove the excipient before experimentation. This can be achieved by prolonged soaking or boiling of the membrane in water or receptor fluid. However, full hydration may be crucial in itself. When a non-hydrated hydrophilic membrane is employed, the membrane absorbs fluid from both compartments and becomes saturated with a mixture of donor and receptor solvents. This will alter the membrane’s partition coefficient as well as its thickness. In order to avoid such changes, hydrophilic membranes should be allowed to fully hydrate over ~24 h with receptor fluid prior to use. This issue was particularly important in our investigated system where the Visking cellulose membrane, possessing surface hydroxyl groups, absorbed water to up to 170% of its original mass. This material also had high glycerol content. Therefore, hydration for ~24 h was necessary in order to optimise experimental reproducibility.
Temperature, which directly affects drug diffusivity as well as solubility, represents another key validation parameter. As a general rule of thumb, percutaneous flux tends to double for every 10°C rise in temperature (23). More recently, it was shown that the flux of methylparaben, butylparaben and caffeine through synthetic membranes doubled with every 7–8°C rise in receptor cell temperature (24). In our current study, validation of the heating systems revealed good temperature control in both the receptor chamber and membrane. However, it was determined that sampling induced temperature fluctuations. Sampling caused air bubble formation just below the membrane which led to a temporary (<1 min) drop in temperature. Air bubbles could be eliminated by tilting the Franz cell facilitating bubble escape via the side arm. This manoeuvre should be performed as swiftly as possible in order to minimise the extent of cooling. Within this context, de-aeration of receptor fluid prior to experimentation will prevent air bubble-associated cooling as well as the EDA decrease mentioned above.
Franz cell studies generally assume that steady state drug diffusion is conducted under perfect sink conditions, i.e. the receptor chamber has a zero drug concentration. Although this is not strictly possible with a static diffusion cell, there is a general agreement that in for in vitro experimental purposes, sink conditions occur when penetrant concentrations in the receiver are not more than 10% of their saturation solubility concentration. Sink conditions can be promoted by various means including; reducing drug concentration in the donor chamber, using a less permeable barrier membrane, employing larger receptor chamber volumes and/or increasing the rate of sampling. Yet, it is clear that altering any of these conditions will likely cause concomitant changes in temperature, levels of membrane excipient, stirring patterns and other factors described above. Hence, it can be seen that implementing an effective validation protocol involves the modulation of potentially interacting variables, indicating that the procedure may be quite complex.
In conclusion, we were able to show that validation of Franz cell experiments provided a dramatic decrease in permeation data variability. Specifically, validation resulted in the coefficient of variation for ibuprofen permeation being reduced from 25.7% to 5.3% (n = 6). This study clearly demonstrates that variability in in vitro experiments is not purely a consequence of biological tissue barrier variability. Thus, validation of the equipment and methodology as well as improvements in operator training can greatly enhance the reproducibility of these types of study.
REFERENCES
- 1.Franz TJ. Percutaneous absorption. On the relevance of in vitro data. J Invest Dermatol. 1975;64:190–5. doi: 10.1111/1523-1747.ep12533356. [DOI] [PubMed] [Google Scholar]
- 2.Franz T. The finite dose technique as a valid in vitro model for the study of percutaneous absorption. Curr Probl Dermatol. 1978;7:58–68. doi: 10.1159/000401276. [DOI] [PubMed] [Google Scholar]
- 3.Farinha A, Toscano C, Campos R, Bica A, Hadgraft J. Permeation of naproxen from saturated solutions and commercial formulations through synthetic membranes. Drug Dev Ind Pharm. 2003;29:489–94. doi: 10.1081/DDC-120018383. [DOI] [PubMed] [Google Scholar]
- 4.Shah V, Elkins J, Lam S, Skelly J. Determination of in vitro drug release from hydrocortisone creams. Int J Pharm. 1989;53:53–9. doi: 10.1016/0378-5173(89)90361-X. [DOI] [Google Scholar]
- 5.Siewert M, Dressman J, Brown C, Shah V. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSci Tech. 2003;4:7. doi: 10.1208/pt040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah V, Elkins J, Williams R. Evaluation of the test system used for in vitro release of drugs for topical dermatological drug products. Pharm Dev Technol. 1999;4:377–85. doi: 10.1081/PDT-100101373. [DOI] [PubMed] [Google Scholar]
- 7.Wester R, Maibach H. Review: percutaneous absorption of drugs. Clin Pharmacokinet. 1992;23:253–66. doi: 10.2165/00003088-199223040-00002. [DOI] [PubMed] [Google Scholar]
- 8.Michaels A, Chandrasekaran SK, Francis W, Meredith M. Drug permeation through human skin: theory and in vitro experimental measurement. A I Ch E J. 1975;21:985–96. [Google Scholar]
- 9.Henning A, Schaefer UF, Neumann D. Potential pitfalls in skin permeation experiments: influence of experimental factors and subsequent data evaluation. Eur J Pharm Biopharm. 2009;72:324–31. doi: 10.1016/j.ejpb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Khan G, Frum Y, Sarheed O, Eccleston GM, Meidan VM. Assessment of drug permeability distribution in two different model skins. Int J Pharm. 2005;303:81–7. doi: 10.1016/j.ijpharm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Frum Y, Eccleston GM, Meidan VM. Evidence that drug flux through synthetic membranes is assocated with normally distributed permeability coefficients. Eur J Pharm Biopharm. 2007;67:434–9. doi: 10.1016/j.ejpb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Chilcott R, Barai N, Beezer A, Brain S, Brown M, Bunge A, et al. Inter- and intra-laboratory variation of in vitro diffusion cell measurements: an international multi-centre study using quasi-standardised methods and materials. J Pharm Sci. 2005;94:632–8. doi: 10.1002/jps.20229. [DOI] [PubMed] [Google Scholar]
- 13.Diembeck W, Beck H, Benech-Kieffer F, Courtellemont P, Dupuis J, Lovell W, et al. Test guidelines for in vitro assessment of dermal absorption and percutaneous penetration of cosmetic ingredients. Food Chem Toxicol. 1999;37:191–205. doi: 10.1016/S0278-6915(98)00114-8. [DOI] [PubMed] [Google Scholar]
- 14.Gale E, Folkes J. The Incorporation of glycerol and lysine into the lipid fraction of staphyloccoccus aureus. Biochem J. 1965;94:390–400. doi: 10.1042/bj0940390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He P, Davis S, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–68. doi: 10.1016/S0378-5173(98)00027-1. [DOI] [Google Scholar]
- 16.Skelly J, Shah V, Maibach H, Guy R, Wester R, Flynn GL, et al. FDA and AAPS report of the workshop on principles and practices of in vitro percutaneous penetration studies—relevance to bioavailability and bioequivalence. Pharm Res. 1987;4:265–7. doi: 10.1023/A:1016428716506. [DOI] [Google Scholar]
- 17.FDA-SUPAC-SS. Guidance for Industry. SUPAC-SS non-sterile semisolid dosage forms. Scale-up and postapproval changes: chemistry, manufacturing and controls. In vitro release testing and in vivo bioequivalence documentation. 1997.
- 18.Gummer C, Hinz R, Maibach H. The skin penetration cell: a design update. Int J Pharm. 1987;40:101–4. doi: 10.1016/0378-5173(87)90053-6. [DOI] [Google Scholar]
- 19.Rolland A, Demichelis G, Jamoule J, Shroot B. Influence of formulation, receptor fluid, and occlusion on in vitro drug release from topical dosage forms, using an automated flow-through diffusion cell. Pharm Res. 1992;9:82–8. doi: 10.1023/A:1018935912097. [DOI] [PubMed] [Google Scholar]
- 20.Diez-Sales OACW, Herraez-Dominguez M, Javaloyes C, Hadgraft J. A mechanistic investigation of the in vitro human skin permeationi enhancing effect of Azone. Int J Pharm. 1996;129:33–40. doi: 10.1016/0378-5173(95)04237-7. [DOI] [Google Scholar]
- 21.Gallagher SLT, Carter T, Heard C. Effects of membrane type and liquid/liquid phase boundary on in vitro release of Ketoprofen from gel formulations. J Drug Target. 2003;11:373–9. doi: 10.1080/10611860310001636890. [DOI] [PubMed] [Google Scholar]
- 22.Haigh J, Smith E. The selection and use of natural and synthetic membranes for in vitro diffusion experiments. Eur J Pharm Sci. 1994;2:311–30. doi: 10.1016/0928-0987(94)90032-9. [DOI] [Google Scholar]
- 23.Scheuplein R. The Skin as a barrier. In: Jarrett A, editor. The physiology and pathophysiology of skin. London: Academic; 1978. pp. 1669–1692. [Google Scholar]
- 24.Akomeah F, Nazir T, Martin G, Brown M. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur J Pharm Sci. 2004;21:337–45. doi: 10.1016/j.ejps.2003.10.025. [DOI] [PubMed] [Google Scholar]