Abstract
Mortality due to seasonal and pandemic influenza could be reduced by increasing the speed of influenza vaccine production and distribution. We propose that vaccination can be expedited by (1) immunizing with influenza virus-like particle (VLP) vaccines, which are simpler and faster to manufacture than conventional egg-based inactivated virus vaccines, and (2) administering vaccines using microneedle patches, which should simplify vaccine distribution due to their small package size and possible self-administration. In this study, we coated microneedle patches with influenza VLP vaccine, which was released into skin by dissolution within minutes. Optimizing the coating formulation required balancing factors affecting the coating dose and vaccine antigen stability. Vaccine stability, as measured by an in vitro hemagglutination assay, was increased by formulation with increased concentration of trehalose or other stabilizing carbohydrate compounds and decreased concentration of carboxymethylcellulose (CMC) or other viscosity-enhancing compounds. Coating dose was increased by formulation with increased VLP concentration, increased CMC concentration, and decreased trehalose concentration, as well as increased number of dip coating cycles. Finally, vaccination of mice using microneedles stabilized by trehalose generated strong antibody responses and provided full protection against high-dose lethal challenge infection. In summary, this study provides detailed analysis to guide formulation of microneedle patches coated with influenza VLP vaccine and demonstrates effective vaccination in vivo using this system.
KEY WORDS: coating formulation, influenza, intradermal immunization, microneedle, virus-like particle vaccine
INTRODUCTION
Influenza vaccination is a critical public health measure to protect against morbidity and mortality related to seasonal and pandemic influenza (1). Currently, inactivated virus and live-attenuated virus vaccines are predominantly used and administered by intramuscular injection using a hypodermic needle and nasal spray using a special syringe, respectively (2). The conventional seasonal influenza vaccines are trivalent, including two influenza A subtypes (H1N1 and H3N2) and one variant of influenza B virus (3).
There are, however, limitations to current vaccines and vaccination methods. Speed of vaccine production and distribution is very important to effective vaccination, because antigenic drift requires development of new vaccines every year to treat seasonal disease and, in some cases, to address a novel pandemic strain (3). As a result, seasonal vaccines are manufactured according to a strict 6-month timetable and then administered to almost 250 million of patients by medical personnel over the course of a number of additional months during influenza season (4). The whole process of seasonal influenza vaccine production and distribution takes close to 1 year. During the recent pandemic of novel H1N1 “swine” influenza, production was expedited, but vaccine still did not become available until after 6 months and vaccine was in short supply for a number of months after that (5). Thus, there is a need to improve the speed of vaccine production and distribution. As discussed below, we propose that development of VLP influenza vaccines can expedite vaccine production and their administration using a microneedle patch can expedite distribution.
Various novel vaccines have been developed such as recombinant vaccines, DNA vaccines, vaccines developed by reverse genetics, and subunit vaccines such as virus-like particles (VLP) (2,6,7). VLPs are attractive because they can expedite vaccine production and also offer the potential for improved immunogenicity and safety due to their cell-based production methods that avoid the delays and safety concerns of conventional egg-based influenza vaccine production (8). Although there are no marketed VLP vaccines against influenza, there are VLP vaccines against hepatitis B and human papillomavirus in widespread use (9).
VLP vaccines might especially benefit from administration using a microneedle patch to expedite distribution to large populations. Microneedles have been fabricated by adapting manufacturing tools from the microelectronics industry to produce micron-scale needles that painlessly pierce the outer barrier layer of the skin to administer vaccine in a simple manner using a patch-like format. Microneedle patches are small and simple enough to use that they could be distributed through pharmacies or the mail and might be self-administered by patients (10)
Solid microneedle patches have been coated with vaccines, including the model antigen ovalbumin (11), hepatitis B surface antigen (12), and inactivated influenza virus (13–15). In addition, hollow microneedles have previously been used to inject influenza vaccine into the skin of human subjects (16) and solid microneedles have been used to scrape the skin for delivery of DNA and other vaccines (17), although these methods do not lend themselves as easily to rapid distribution or self-administration.
In addition to simplified logistics, microneedle vaccination may offer immunologic advantages due to targeting delivery to the skin, which is replete with antigen-presenting cells (18). For example, intradermal injection has shown evidence for dose sparing (19–22) and improved immunogenicity in the elderly (23). However, intradermal vaccination by conventional methods can be unreliable and requires the expertise of a trained medical professional (24). In contrast, microneedles inherently target delivery to the skin using a simple device, which can ultimately lead to local effects on depositing vaccines within the skin and systemic effects on transferring vaccines to the draining lymph nodes through the vasculature or lymphatics after uptake by antigen presenting cells such as dendritic and/or Langerhans cells. Animal studies have shown that microneedles coated with inactivated influenza vaccine can generate stronger humoral and cellular immune responses than intramuscular vaccination (25,26). Microneedles have also been shown to be painless and well tolerated in humans subjects (27–29).
Building off this body of literature demonstrating possible advantages of VLP vaccination using microneedles, this study investigated the effect of vaccine formulation, as well as the microneedle material and coating process parameters, on the amount and integrity of influenza VLP vaccine coated onto microneedles. This is the first detailed study to examine and optimize coating of a VLP vaccine onto microneedles.
MATERIALS AND METHODS
Preparation of Influenza VLP Vaccine
VLPs were prepared as described previously (30). Briefly, to produce VLPs containing influenza virus M1 matrix protein and hemagglutinin (HA), Sf9 cells were co-infected with rBVs expressing M1 and hemagglutinin derived from the strain of A/PR/8/34 H1N1 virus. Culture supernatants were harvested at 3 days post-infection and purified by low-speed centrifugation (6,000 rpm for 20 min at 4°C) to remove cells. VLPs in the supernatant were pelleted by ultracentrifugation (28,000 rpm for 60 min). The sedimented particles were resuspended in phosphate-buffered saline (PBS) at 4°C overnight and further purified through a 15-30-60% discontinuous sucrose gradient at 28,000 rpm for 1 h at 4°C. The VLP bands were collected and analyzed using Western blot and hemagglutination assays. The size of VLP particles ranges from 80 to 120 nm (30).
For visualization of VLP vaccine delivery by microneedles, VLPs were labeled by lipid staining with a red fluorescent dye. To perform staining, 200 μL VLP at a concentration of 3 mg/ml was mixed with 10 μL of octadecyl rhodamine B chloride (R18, Invitrogen, Carlsbad, California, USA) and incubated at 25°C for 1 h. To remove unbound R18 molecules, the labeled VLPs were suspended in 10 mL PBS and precipitated by ultracentrifugation (28,000 rpm for 1 h, Optima L-80 XP, Beckman Coulter, Fullerton, California, USA) with a 20% sucrose layer (31). The precipitated VLPs were again washed in PBS by ultracentrifugation to further remove residual R18 dye.
Fabrication and Coating of Microneedles
Arrays of solid metal microneedles were fabricated by cutting needle structures from stainless steel sheets (McMaster-Carr, Atlanta, Georgia, USA) using an infrared laser (Resonetics Maestro, Nashua, New Hampshire, USA) as described previously (32). Individual rows containing five microneedles were dip coated by horizontally dipping the microneedles into a coating solution containing 3-8 mg/ml VLP vaccine. The coating solution was composed of 0.25-1.0% (w/v) carboxymethylcellulose (CMC) sodium salt (USP grade, Carbo-Mer, San Diego, California, USA), 0.5% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, New Jersey, USA) in PBS as described previously (32,33). Additional studies used other viscosity-enhancing agents, including 1% (w/v) alginic acid, polyvinylpyrrolidone, gum ghatti, karaya gum (Sigma-Aldrich, St. Louis, Missouri, USA), and xanthan gum (Fluka, Buchs, Switzerland). Trehalose at a concentration of 5-30% (w/v; Sigma-Aldrich) was used as a stabilizer in most studies. Additional studies used other stabilizers, including 15% (w/v) sucrose, glucose, inulin from dahlia tubers and chicory, and dextrans with molecular weights of 9 and 36 kDa (Sigma-Aldrich). Unless otherwise stated, the standard coating solution contained 1% (w/v) CMC sodium salt and 0.5% (w/v) Lutrol F-68 NF in PBS buffer solution either with or without 15% (w/v) trehalose.
Imaging and Histology
Microneedles were imaged by scanning electron microscopy (LEO 1530, Carl Zeiss, Oberkochen, Germany), bright field microscopy (SZX12, Olympus America, Tokyo, Japan) with a CCD camera (DC 300, Leica Microsystems, Bannockburn, Illinois, USA), and fluorescence microscopy (IX70, Olympus) with a CCD camera (RT Slider, Diagnostic Instruments, Sterling Heights, Michigan). Histological skin sections were imaged using a Zeiss LSM/NLO 510 Confocal/Multi-Photon Microscope (Zeiss, Oberkochen, Germany) with a 100× oil-immersion objective used in conjunction with oil having an index of refraction of 1.51. To image delivery of viral antigen into skin, microneedles coated with R18-labeled virus were inserted into human cadaver skin for 10 min and fixed by freezing in histology mounting compound (Tissue-Tek®, Sakura Finetek, Torrance, California, USA) for 10 min, after which microneedles were removed and skin was sectioned using a cryostat (Cryo-Star HM 560 MV, Microm, Hésingue, France) for imaging. This use of human skin was approved by the Georgia Institute of Technology Institutional Review Board.
In vitro HA Activity Testing of Influenza VLPs after Microneedle Coating
We measured HA activity of VLPs to test their stability after coating onto microneedles. To avoid the more time-consuming process of coating microneedles, we instead coated 3 × 3 mm pieces of the same stainless steel used to make microneedles. Additional studies used other materials, including titanium, nickel, copper, glass, polystyrene, and polycarbonate (Sigma-Aldrich). Coatings were produced by mixing 1 μL of coating solution with 1 μL of VLP vaccine on the metal piece, which was allowed to air dry at room temperature overnight. The metal piece was then dissolved in 100 μL of PBS for 12 h. Validation experiments showed that VLP HA activity after coating pieces of stainless steel was similar to that after coating stainless steel microneedles (data not shown).
To determine HA titers, VLP vaccine dissolved from metal pieces was serially diluted in 100 μL of PBS deficient in Mg2+ and Ca2+, mixed with an equal volume of a fresh 0.5% suspension of chicken red blood cells (Lampire Biological Laboratories, Pipersville, Pennsylvania, USA), and incubated for 1 h at 25°C. HA titers were determined as the endpoint dilutions inhibiting the precipitation of red blood cells (34).
In vivo Immunogenicity Testing of Influenza VLPs After Vaccination Using Microneedles
Balb/c mice (n = 4 animals per group, 8-10 weeks old, female, Charles River Laboratories, Wilmington, Massachusetts, USA) were anesthetized intramuscularly with 110 mg/kg ketamine HCl and 11 mg/kg xylazine. Hair at the site of treatment was removed by depilatory cream (Nair, Princeton, New Jersey, USA) applied with a moisturized cotton stick. After washing with a cotton ball soaked with 70% ethanol and drying with hair dryer, microneedles coated with 4 μg of influenza VLP vaccine were inserted into the skin on the dorsal surface. In our previous study, depilatory cream and 70% ethanol was shown not to effect skin permeability to influenza virus vaccine (35). Microneedles were coated using the standard coating formulation with or without trehalose. Microneedles were left in the skin for 10 min to allow dissolution of the vaccine antigen from the microneedles.
At weeks 2 and 4 after vaccination, influenza virus-specific antibody (IgG) titer was determined using standard protocols. Briefly, 96-well microtiter plates (Nunc-Immuno Plate MaxiSorp; Nunc Life Technologies, Basel, Switzerland) were coated with 100 μl of inactivated A/PR8 virus at a concentration of 4 μg/ml in coating buffer (0.1 M sodium carbonate, pH 9.5) at 4°C overnight. After washing, 100 times diluted serum samples were added into plates and plates were incubated at 37°C for 1 h. After additional washing, the plates were then incubated with horseradish peroxidase-labeled goat anti-mouse IgG (Southern Biotechnology, Birmingham, Alabama, USA) at 37°C for 1.5 h and then the substrate ophenylenediamine (Zymed, San Francisco, California, USA) in citrate-phosphate buffer (pH 5.0) containing 0.03% H2O2 (Sigma-Aldrich) was used to develop color. The optical density at 450 nm was read using an ELISA reader (model 680; Bio-Rad, Hercules, California, USA).
For challenge infections, isoflurane-anesthetized mice were infected with 1,000 pfu live influenza viruses (A/PR8/34, 20 × LD50) intranasally in 50 μl of PBS per mouse on week 5 after vaccination. Naïve mice that received no vaccination were included as a negative control group. Mice were observed daily to monitor changes in body weight and to record death. Mice were euthanized if their body weight loss exceeded 25% to minimize suffering. All animal experiments and husbandry involved in studies presented in this manuscript were conducted under the guidelines of the Emory University Institutional Animal Care and Use Committee (IACUC). Emory IACUC operates and is conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations, per the spirit of Association for Assessment and Accreditation of Laboratory Animal Care International's expectations for animal care and use/ethics committees.
Statistical Analysis
Every assay was measured using at least three samples, from which the arithmetic mean and standard error of the mean were calculated. A two-tailed Student’s t test (α = 0.05) was performed when comparing two different conditions. When comparing three or more conditions, a one-way analysis of variance (ANOVA; α = 0.05) was performed. A value p < 0.05 was considered statistically significant.
RESULTS
Fabrication of Microneedles
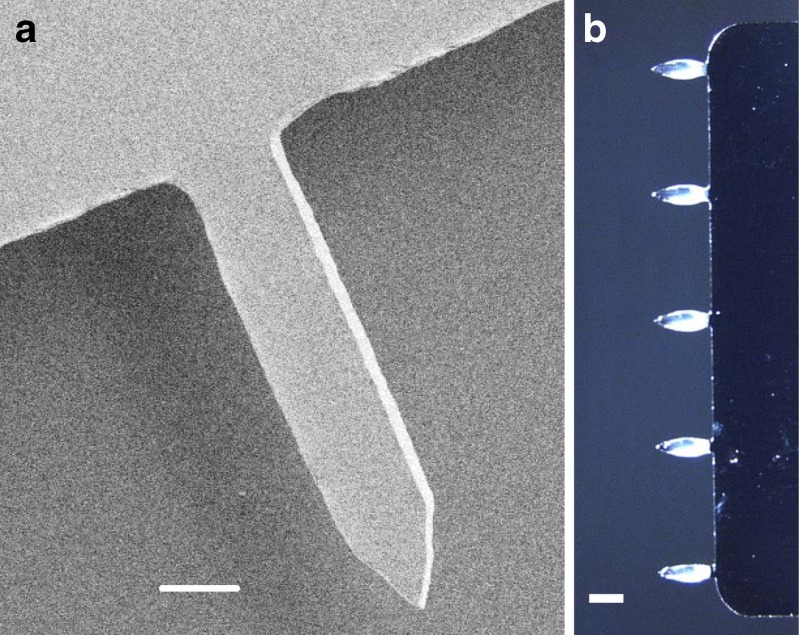
We fabricated solid stainless steel microneedles by laser cutting and electropolishing. The microneedles used in this study were prepared as single rows containing five microneedles each measuring 700 μm long, 160 μm wide at the base, and 50 μm in thickness (Fig. 1a).
Fig. 1.
Microneedles coated for vaccine delivery. a Scanning electron micrograph of a microneedle (scale bar 100 μm), b microneedle array containing five microneedles coated with influenza virus-like particle (VLP) vaccine in standard coating solution containing trehalose (scale bar 400 μm)
Coating and Delivery of Influenza VLP Vaccine
Previous studies have addressed coating of microneedles with compounds including calcein, vitamin B, bovine serum albumin, and plasmid DNA (11,32,36), but this is the first study to examine and optimize coating with a VLP vaccine. Guided by previous studies, we designed the coating formulation to contain a surfactant (i.e., Lutrol F-68 NF) to generate uniform coatings by reducing surface tension, a viscosity enhancer (i.e., CMC) to enable thicker coatings by increasing coating solution residence time on the microneedle surface during the drying process, and a stabilizer (i.e., trehalose) to prevent the loss of VLP HA activity during drying.
This standard coating formulation was able to coat influenza VLP vaccine onto microneedles (Fig. 1b). The coating was thick due to the large amount of stabilizer in the formulation (i.e., 87% of dissolved solids). Nevertheless, when coated microneedles were inserted into the skin of mice, the vaccine coating was efficiently dissolved and released into the skin almost completely.
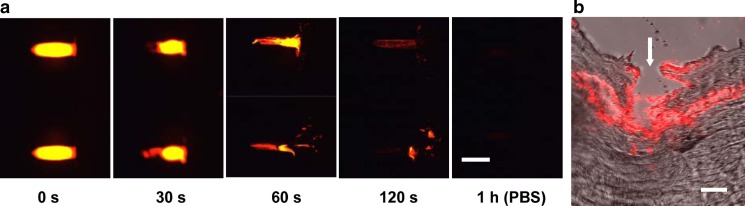
The speed of VLP vaccine release into skin is also important. To assess the release kinetics, influenza VLPs were labeled with a red fluorescent compound and visualized using fluorescence and multi-photon microscopy. As shown in Fig. 2a, coated VLP vaccine was efficiently released from microneedles after insertion into human cadaver skin within 2 min. As a comparison, a VLP-coated microneedle incubated in PBS for 1 h demonstrates complete release.
Fig. 2.
Influenza VLP vaccine delivery from coated microneedles into skin. a Representative fluorescence micrograph of microneedles coated with red-fluorescent, R18-stained VLPs (left) and after insertion into human cadaver skin for 30, 60, and 120 s. As a positive control to confirm complete release of VLPs from the microneedles, microneedles were incubated in PBS for 1 h (right; scale bar 500 μm). b Multiphoton fluorescence micrograph of cryosectioned human cadaver skin after insertion of R18-stained VLP-coated microneedle (white arrow microneedle insertion site, scale bar 300 μm)
To assess the localization of VLP vaccine after delivery into skin, histological sections were prepared after microneedle delivery of fluorescently tagged VLP into human cadaver skin and viewed by multi-photon microscopy. The representative image in Fig. 2b shows the microneedle insertion point (white arrow) and the deposition of VLP vaccine along the needle track in the skin. Some vaccine appears to have diffused horizontally away from the insertion site. Altogether, these results indicate that VLPs can be coated onto the surface of metal microneedles and efficiently released into the skin.
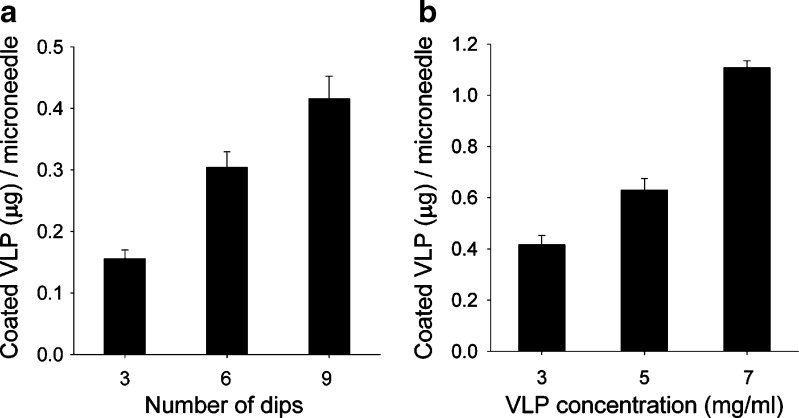
Finally, we wanted to determine the dose of VLPs that could be coated onto microneedles. We therefore varied two process parameters: VLP concentration in the coating solution and the number of times microneedles were dipped into the coating solution. As shown in Fig. 3a, increasing the number of dips increased the dose of VLP coated onto microneedles in an approximately proportional manner from 0.16 to 0.42 μg per microneedle after increasing from three to nine dips (ANOVA, p < 0.05). Figure 3b shows that increasing VLP concentration further increased the coated dose in an approximately proportional manner from 0.42 to 1.1 μg per microneedle at concentrations of 3 and 7 mg/ml, respectively (ANOVA, p < 0.05). Guided by these data, protocols can be developed to coat up to more than 1 μg of VLP vaccine per microneedle, which corresponds to hundreds of micrograms of vaccine on a patch containing hundreds of coated microneedles. Given that VLP vaccines are generally given at doses well below this (9), coated microneedles appear to be capable of administering suitable doses of VLP vaccines.
Fig. 3.
Determination of VLP dose coated onto microneedles. Amount of VLP coated per microneedle as a function of a the number of dips into coating solution containing 2 mg/mL VLPs and b the VLP concentration in the coating solution after 6 dips. Standard coating solution was used. Replicate data from n = 4 samples is shown as the mean value with error bars indicating the standard error of the mean (SEM)
HA Activity of Influenza VLP
VLPs used in this study contain influenza matrix protein and glycoproteins such as hemagglutinin as described previously (37). In contrast to chemically inactivated influenza virus vaccines, VLPs contain unmodified native hemagglutinin antigens. Thus, it is important to protect HA activity during the drying process when coating microneedles, which we assessed by measuring red blood cell HA activity of influenza VLPs after coating and dissolution in PBS.
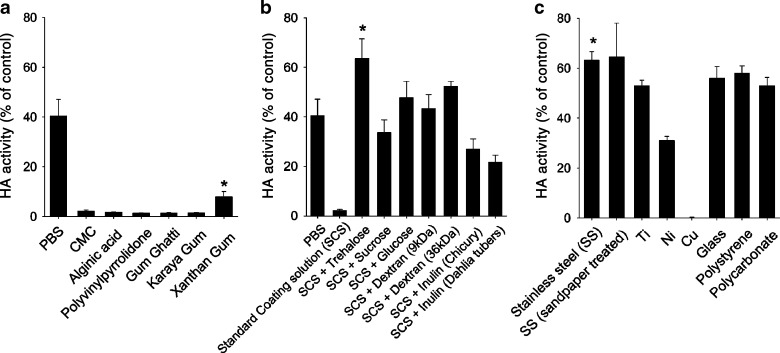
Drying influenza VLPs in PBS reduced HA activity to 40% relative to the control sample of untreated VLPs (Fig. 4a). This shows that drying VLPs can damage their activity. Drying VLPs in our standard coating formulation containing CMC and a surfactant caused even greater loss of HA activity to just 2%. Replacing CMC with other viscosity enhancing excipients, including alginic acid, polyvinylpyrrolidone, gum ghatti, karaya gum, and xanthan gum, yielded similar results, although xanthan gum provided somewhat improved results compared to CMC. However, the coating dose achieved when using xanthan gum was significantly lower than that with CMC (data not shown). We therefore continued to coat using CMC as the viscosity enhancer.
Fig. 4.
The effect of microneedle formulation and material on HA activity. HA activity of VLPs is shown after coating and dissolution in PBS. Coatings were made using a standard coating solution containing 15% trehalose and substituting CMC for various stabilizers (*p < 0.05 for comparison between xanthan gum and other viscous enhancers); b standard coating solution containing various carbohydrate stabilizers at a concentration of 15% (w/v) (*p < 0.05 for comparison between trehalose and other stabilizers); and c microneedles made from various materials as indicated in the figure legend using standard coating solution containing 15% trehalose (*p < 0.05 for comparison between stainless steel and other metals; n = 4, mean ± SEM)
Sugar compounds are known to stabilize influenza viral vaccines during storage in the dried or frozen state (38) and previously found to stabilize inactivated influenza virus during microneedle coating (13). We therefore assessed whether a set of different stabilizers, including trehalose, sucrose, glucose, dextran, inulin, could similarly stabilize influenza VLPs (Fig. 4b). Trehalose was found to retain 100% HA activity of VLPs dried in PBS without CMC (data not shown) and more than 60% HA activity in the standard CMC-containing coating solution, which was the best result among the stabilizers tested. These results indicate that trehalose is a promising candidate stabilizer for influenza VLP vaccine coated onto microneedles.
The choice of microneedle material could also affect the HA activity after coating. We therefore tested seven different materials including our standard material, stainless steel, as well as other metals (titanium, nickel, and copper), polymers (polystyrene, polycarbonate), and glass (Fig. 4c). HA activity of influenza VLPs coated onto stainless steel was found to be significantly higher than nickel, copper, titanium, and statistically indistinguishable from the polymers and glass. To test the effect of surface roughness on HA activity, stainless steel roughened with sandpaper was compared with untreated stainless steel. There was no difference in HA activity between these two conditions.
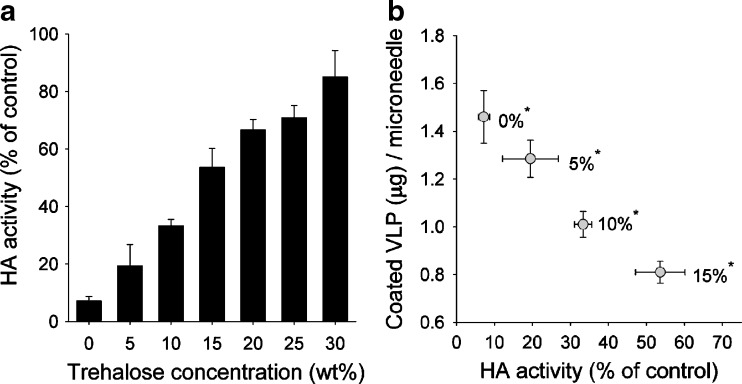
The Effect of Trehalose Concentration on VLP HA Activity and Coating Dose
Trehalose was found to play a major role in maintaining HA activity of influenza VLP vaccine during the microneedle coating process. To determine the effects of trehalose concentrations in the coating solution on retaining HA activity, trehalose concentrations from 0% to 30% were tested (Fig. 5a). HA activity increased with trehalose concentration (ANOVA, p < 0.05). However, addition of trehalose significantly decreased the dose of VLP vaccine coated onto microneedles, which makes a direct trade-off between VLP receptor-binding activity and dose (Fig. 5b). This is probably because the total amount of material that can be coated onto a microneedle is limited. Therefore, as more trehalose is added, there is less room for VLP vaccine. As a compromise, we have used 15% trehalose throughout the rest of the study to maintain HA activity while still coating sufficient vaccine doses onto microneedles.
Fig. 5.
The effect of trehalose stabilizer concentration on HA activity and coating dose of VLPs. a HA activity of VLPs is shown after coating and dissolution in PBS. Coatings were made using standard coating solution with trehalose added over a range of concentrations at 25°C. b Trade-off between HA activity and coated dose of VLPs is shown at different trehalose concentrations (*trehalose concentration, n = 4, mean ± SEM)
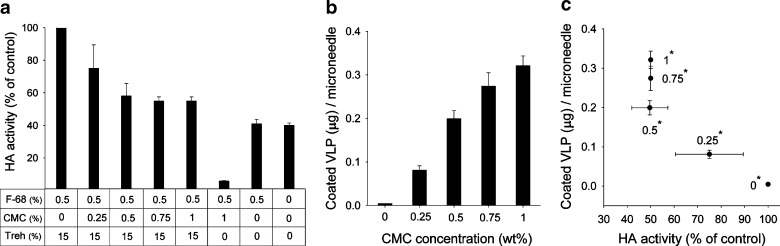
The Effect of CMC Concentration on VLP HA Activity and Coating Dose
Because the presence of CMC significantly reduced HA activity after coating, we studied the effect of CMC concentration in greater detail (Fig. 6). Using a coating solution containing 0.5% surfactant and 15% trehalose without CMC retained 100% HA activity (Fig. 6a). The addition of 0.25% CMC decreased HA activity to 85%. At 0.5-1% CMC, approximately 50% HA activity was retained. Coating with 1% CMC without trehalose destroyed almost all HA activity. Altogether, this further shows that CMC significantly damages HA activity and trehalose can significantly prevent that damage.
Fig. 6.
The effect of CMC concentration on HA activity and coating dose of VLPs. a HA activity and b coating dose of VLPs is shown after coating and dissolution in PBS. Coatings were made by use of standard coating solution containing 15% trehalose and modified to contain various concentrtations of CMC. c Trade-off between HA activity and coated dose of VLPs is shown at different CMC concentrations (F-68 Lutrol F-68 NF surfactant, Treh trehalose, *CMC concentration, n = 4, mean ± SEM)
To better understand the role of the surfactant, we found that coating 0.5% surfactant alone retained 41% HA activity (Fig. 6a). The addition of 15% trehalose to that formulation restored HA activity to 100%. This indicates that the stabilizing function of trehalose is not limited to counteracting the effects of CMC. Therefore, an optimal formulation should contain surfactant, in order to facilitate good wetting and spreading of the coating across the microneedle surface, and should further contain trehalose to retain HA activity.
Although CMC causes functional inactivation of hemagglutinin, CMC is an indispensable ingredient for efficient coating for influenza VLP vaccine. Without CMC, negligible amounts of VLP vaccine could be coated (Fig. 6b). The dose of VLP that could be coated increased with CMC concentration from 0% to 1% CMC (ANOVA, p < 0.05). HA activity decreased with increasing CMC concentration from 0% to 0.5% CMC, but showed no further decrease in HA activity from 0.5% to 1% CMC in the presence of 15% trehalose (Fig. 6a). These results indicate that 1% CMC is the optimal condition that balances HA activity loss and coating dose (Fig. 6c).
In vivo Immunogenicity of Influenza VLP Vaccination
Our in vitro studies show that influenza VLP vaccines can be coated onto microneedles using an optimized formulation to achieve microgram doses and retain most vaccine HA activity, as measured by HA activity. To validate these results in vivo, we assessed immunogenicity in mice after vaccination using microneedles coated with 4 μg of influenza VLP vaccine formulated using 1% CMC and 0.5% surfactant either with or without 15% trehalose.
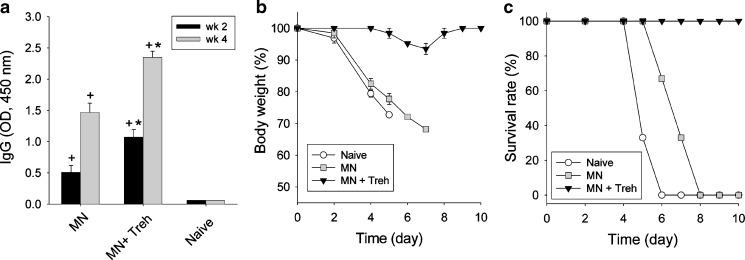
Influenza VLP immunization using microneedles formulated either with or without trehalose significantly increased virus-specific IgG compared to naïve mice (Fig. 7a). However, microneedles coated with trehalose induced significantly higher IgG responses than microneedles without trehalose, which confirmed the critical role of trehalose to stabilize the dried VLP vaccine.
Fig. 7.
Humoral antibody response and protection efficacy after microneedle immunization with influenza VLP vaccine. a Total influenza virus-specific serum antibody response (IgG) measured 2 and 4 weeks after immunization with VLP vaccine-coated microneedles. (+ p < 0.01 for comparison between microneedle and naïve, *p < 0.05 for comparison between formulation with trehalose and without trehalose). b Body weight change and c survival of mice immunized using antigen-coated microneedles after lethal challenge infection (MN microneedle, Treh trehalose)
Five weeks after vaccination, all mice were challenged with a lethal dose of A/PR/8/34 influenza virus (20 × LD50). Naïve mice rapidly lost weight and either died or had to be euthanized within 6 days of challenge (Fig. 7b, c). Mice immunized using microneedles formulated without trehalose also rapidly lost weight; all of these mice died or were euthanized within 8 days after challenge. In contrast, mice immunized using microneedles stabilized with trehalose showed minimal weight loss, quickly recovered to normal body weight, and all survived. Altogether, these immunogenicity studies show that microneedle vaccination using influenza VLPs is effective and that stabilization of the coated vaccine using trehalose is critically important to retaining protective efficacy of the vaccine.
DISCUSSION
Influenza vaccination would benefit from increased speed of vaccine production and distribution to meet both seasonal and pandemic needs (3). Influenza VLP vaccines can increase the speed of vaccine production in part because they are manufactured using cell culture instead of the cumbersome egg-based manufacturing methods in use today for inactivated virus-based vaccines (39). In this study, we propose that influenza VLP vaccination can be further expedited by administration using a microneedle patch applied to the skin. This study carried out the first detailed examination of influenza VLP vaccine coating onto microneedles with the goal of optimizing the formulation to balance the competing effects of maintaining vaccine immunogenicity and coating adequate vaccine dose onto the microneedles.
We found that influenza VLP vaccine could be coated onto microneedles and was then rapidly released into solution or into the skin. However, VLP vaccine coating onto microneedles involves a drying process and requires addition of an excipient to increase coating solution viscosity for effective coating. We found that both the drying process and the inclusion of CMC to increase coating solution viscosity decreased vaccine activity, as measured by the functional activity of hemagglutinin. The addition of trehalose to the coating formulation significantly stabilized the VLP vaccine, which increased both in vitro HA activity and in vivo protective efficacy. Overall, this study demonstrated that protective microneedle vaccination with influenza VLPs can be achieved using an optimized formulation containing an appropriate stabilizer and viscosity enhancer.
Although inclusion of a viscosity-enhancing excipient is critical for efficient microneedle coating, its presence destabilized influenza VLPs during the drying process. For example, CMC caused a loss of HA activity of coated influenza VLP vaccine in a concentration dependent manner up to 0.5% concentration in the coating solution. The VLP HA activity of undried coating solution in the liquid state was not affected by inclusion of CMC or other viscosity enhancers in the formulation (data not shown), which suggests that CMC does not interfere with the HA assay itself. A possible explanation for CMC’s adverse effects is that high molecular weight viscosity enhancers might facilitate aggregation of the VLP particles or physically block the hemagglutinin molecules on the surface of influenza VLPs during drying. We found that coating microneedles with inactivated influenza virus also led to loss of HA activity, which was associated with increased virus particle size probably due to aggregation (33).
In addition to the choice of coating excipients, the choice of microneedle material also influenced loss of HA activity. While a number of materials performed similarly to stainless steel, we found that nickel and especially copper caused additional damage to coated VLPs, although we do not know mechanism by which these materials help deactivate HA activity.
The addition of sugar molecules significantly improved influenza VLP stability by preserving HA activity during the drying process. In particular, the addition of trehalose preserved 100% HA activity of influenza VLPs in the absence of CMC and approximately 50-60% in the presence of CMC after drying. Carbohydrates have previously been shown to protect the structural integrity of soluble hemagglutinin during lyophilization, perhaps due to the formation of an amorphous glassy state after freeze drying, as shown by differential scanning calorimetry analysis (40). Other studies have similarly shown that the activity of various influenza vaccine was similarly retained after freeze drying in the presence of carbohydrates, including trehalose, dextran, and inulin (38,41,42). In contrast, our study showed that trehalose protected HA activity of influenza VLPs better than dextran and glucose and much better than inulin during the air drying process used here. Amorij et al. reported that visible aggregates were detected in a hemagglutinin vaccine lyophilized in PBS without carbohydrate after reconstitution (40). Thus, we hypothesize that influenza VLPs may similarly have been protected by trehalose during drying by preventing aggregation of VLP particles.
We elected to assess in vitro HA activity of influenza VLPs through the HA assay. In contrast, other studies have assessed conformational changes of soluble hemagglutinin subunit vaccines by fluorescence and circular dichroism spectroscopy and found changes in secondary helical conformation and tertiary structure of hemagglutinin upon freezing in PBS (40). However, unlike soluble hemagglutinin protein, VLPs resemble the wild-type virus in structure and morphology. For example, influenza VLPs contain hemagglutinin glycoprotein trimers embedded within a lipid bilayer membrane (30). Hemagglutinin is an important protective target molecule in influenza vaccine (43). Thus, the stability of influenza vaccine is dependent on maintaining the native conformation and structural integrity of hemagglutinin. Any changes in hemagglutinin structure or conformation could result in loss of receptor binding function of hemagglutinin as represented by hemagglutination of red blood cells. Because influenza VLPs are a multi-component supramolecular particles maintaining the native structure of hemagglutinin in a membrane-anchored form, determination of functional activities by HA assay is more appropriate for monitoring influenza vaccine stability than, for example, spectroscopic analysis.
As evidence for this, HA activity of influenza VLPs coated onto microneedles without trehalose was extremely low. Correspondingly, virus-specific antibodies were only weakly elevated and immunization without trehalose provided poor protection against lethal infection. In contrast, influenza VLP vaccine stabilized using trehalose had much higher HA activity and correspondingly generated much higher antibody levels and provided fully protective immunity, as measured by 100% survival and minimal weight loss after challenge. Therefore, HA activity in vitro appears to be an indicator of influenza vaccine integrity that can predict vaccine efficacy in vivo.
This study was motivated in part by the expected logistical advantages that microneedles can offer to expedite influenza vaccination. Storage, stockpiling, distribution, and disposal should be simplified by microneedles, because microneedle patches are small (i.e., much smaller than a hypodermic needle and syringe). The possibility of self-administration and distribution by mail could enable vaccination of large populations (e.g., a whole country) within a matter of days. Self-vaccination may be possible because microneedles are painless (28), do not require reconstitution, and are simple to apply to the skin. Altogether, these features of microneedles could increase coverage of influenza vaccination.
CONCLUSION
This study provides the first detailed examination of coating microneedles with influenza VLP vaccine. We found that VLPs can be coated in microgram quantities suitable for vaccination. However, drying during the coating process, especially in the presence of viscosity enhancers such as CMC, significantly damaged the VLP antigen, as measured by loss of HA activity in vitro and weaker antibody responses and poor protection in vivo. Improvement of the coating formulation, especially through the addition of trehalose, protected VLP HA activity in vitro and protective immunity in vivo. Overall, this study shows that microneedles can be coated with influenza VLP vaccine to provide adequate dose and immunogenicity for effective vaccination, as determined in mice.
Acknowledgments
This work was supported in part by NIH grants R01-EB006369 (M.R.P.) and U01-AI0680003 (R.W.C.), SERCEB (R.W.C) and the Georgia Research Alliance Program grant (S.M.K). We thank Dr. Vladimir Zarnitsyn for microneedle fabrication, Dr Young-Bin Choy for SEM imaging, and Dr. Mark Allen for use of laser microfabrication facilities. M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. These possible conflicts of interest have been disclosed and are being managed by Georgia Tech and Emory University.
Contributor Information
Richard W. Compans, Email: rcompan@emory.edu
Sang-Moo Kang, Email: skang2@emory.edu.
Mark R. Prausnitz, Phone: +1-404-8945135, Email: prausnitz@gatech.edu
References
- 1.Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine. 2009;27:D65–8. doi: 10.1016/j.vaccine.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoelscher M, Gangappa S, Zhong WM, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin Drug Deliv. 2008;5:1139–57. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- 3.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194:S111–8. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 4.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–9. doi: 10.1016/S0264-410X(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Use of influenza A (H1N1) 2009 monovalent vaccine, recommendations of the Advisory Committee on Immunization Practices. Morb Mort Wkly Rep. 2009;58:1–8. [PubMed] [Google Scholar]
- 6.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palache AM. Influenza vaccines—a reappraisal of their use. Drugs. 1997;54:841–56. doi: 10.2165/00003495-199754060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Jennings GT, Bachmann MF. Coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–36. doi: 10.1515/BC.2008.064. [DOI] [PubMed] [Google Scholar]
- 9.Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11:438–44. doi: 10.1016/S0966-842X(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 10.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009:369-93. [DOI] [PMC free article] [PubMed]
- 11.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux (R) microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/A:1013607400040. [DOI] [PubMed] [Google Scholar]
- 12.Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, et al. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci USA. 2009;106:18936–41. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201:190–8. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutsonanos DG, Martin MdP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu QY, Zarnitsyn VG, Ye L, Wen ZY, Gao YL, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106:7968–73. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–9. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 17.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–9. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 18.Debenedictis C, Joubeh S, Zhang GY, Barria M, Ghohestani RF. Immune functions of the skin. Clin Dermatol. 2001;19:573–85. doi: 10.1016/S0738-081X(00)00173-5. [DOI] [PubMed] [Google Scholar]
- 19.Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25:659–63. doi: 10.1016/j.vaccine.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum antibody responses after intradermal vaccination against influenza. New Engl J Med. 2004;351:2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 21.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. New Engl J Med. 2004;351:2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 22.Hinman AR, Orenstein WA, Santoli JM, Rodewald LE, Cochi SL. Vaccine shortages: history, impact, and prospects for the future. Annu Rev Public Health. 2006;27:235–59. doi: 10.1146/annurev.publhealth.27.021405.102248. [DOI] [PubMed] [Google Scholar]
- 23.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 24.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 25.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated-microneedles. Vaccine. 2009;27:6932–8. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS ONE. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35:193–202. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–94. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haq MI, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevices. 2008;11:35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 30.Quan FS, Huang CZ, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig K, Korte T, Herrmann A. Analysis of delay times of hemagglutinin-mediated fusion between influenza-virus and cell-membranes. Eur Biophys J. 1995;24:55–64. doi: 10.1007/BF00211399. [DOI] [PubMed] [Google Scholar]
- 32.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–37. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–95. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vyas GN, Shulman NR. Hemagglutination assay for antigen and antibody associated with viral hepatitis. Science. 1970;170:332–3. doi: 10.1126/science.170.3955.332. [DOI] [PubMed] [Google Scholar]
- 35.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24:6110–9. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Chen XF, Prow TW, Crichton ML, Jenkins DWK, Roberts MS, Frazer IH, et al. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139:212–20. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang S-M. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine. 2008;26:3352–61. doi: 10.1016/j.vaccine.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amorij JP, Huckriede A, Wischut J, Frifflink HW, Hinrichs WLJ. Development of stable influenza vaccine powder formulations: challenges and possibilities. Pharm Res. 2008;25:1256–73. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amorij JP, Meulenaar J, Hinrichs WLJ, Stegmann T, Huckriede A, Coenen F, et al. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze drying. Vaccine. 2007;25:6447–57. doi: 10.1016/j.vaccine.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 41.de Jonge J, Amorij JP, Hinrichs WLJ, Wiischuta J, Huckriedea A, Frijlinkb HW. Inulin sugar glasses preserve the structural integrity and biological activity of influenza virosomes during freeze-drying and storage. Eur J Pharm Sci. 2007;32:33–44. doi: 10.1016/j.ejps.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 42.Maa YF, Ameri M, Shu C, Payne LG, Chen DX. Influenza vaccine powder formulation development: spray freeze drying and stability evaluation. J Pharm Sci. 2004;93:1912–23. doi: 10.1002/jps.20104. [DOI] [PubMed] [Google Scholar]
- 43.Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]