Abstract
Carapa guianensis, a popular medicinal plant known as “Andiroba” in Brazil, has been used in traditional medicine as an insect repellent and anti-inflammatory product. Additionally, this seed oil has been reported in the literature as a repellent against Aedes aegypti. The aim of this work is to report on the emulsification of vegetable oils such as “Andiroba” oil by using a blend of nonionic surfactants (Span 80® and Tween 20®), using the critical hydrophilic–lipophilic balance (HLB) and pseudo-ternary diagram as tools to evaluate the system’s stability. The emulsions were prepared by the inverse phase method. Several formulations were made according to a HLB spreadsheet design (from 4.3 to 16.7), and the products were stored at 25°C and 4°C. The emulsion stabilities were tested both long- and short-term, and the more stable one was used for the pseudo-ternary diagram study. The emulsions were successfully obtained by a couple of surfactants, and the HLB analysis showed that the required HLB of the oil was 16.7. To conclude, the pseudo-ternary diagram identified several characteristic regions such as emulsion, micro-emulsion, and separation of phases.
KEY WORDS: Carapa guianensis oil, emulsions, hydrophilic–lipophilic balance (HLB), pseudo-ternary diagram, stability
INTRODUCTION
Carapa guianensis Aubl (Meliaceae) is a popular medicinal plant found in several countries. In Brazil, it is known as “Andiroba” and is an evergreen or deciduous tree that may grow up to 60 m (1–3). The specie has several uses and the quality of its wood and the oil extracted from its seeds are well-known (4). The oil from C. guianensis has been widely used for pharmaceutical and cosmetic applications (5,6). Concerning the use by traditional communities in the Amazon, the oil shows insect repellency and larvicidal effect (7–10); furthermore, it can be used for treatment of arthritis and inflammation (11). Due to the large use and therapeutic interest, the reproductive safety of the oil has also been studied (12–14).
The therapeutic use as a topical product and the “natural” source of “Andiroba” oil allow for several technologic studies (15,16). However, due to the water-insoluble nature of oil, it is best formulated as emulsions (6,16–18). Emulsions are heterogeneous mixtures that consist of droplets of a liquid dispersed in a second continuous immiscible liquid phase. The liquid/liquid immiscibility creates an interfacial tension between the two liquids that assign thermodynamic instability to such systems. In order to produce stable emulsions, surfactants are added to the system. However, most stable emulsions are achieved with emulsifiers or combination of emulsifier agents having hydrophilic–lipophilic balance (HLB) values close to that required of the oil phase (17,19). Thus, the HLB is a parameter of utmost importance in the development of pharmaceutical emulsions. This system, developed by Griffin in the 1950s (20), attempts to provide a partial answer to the search for an ideal surfactant for the stabilization of a given system. On the other hand, the Griffin’s HLB concept cannot precisely predict the best concentration of each surfactant as well as the appropriate combination for each oil phase. Thus, after a preliminary study to choose the mix of surfactants, it is necessary to determine the appropriate required HLB of the system, also known as critical HLB (17,21,22). The next step in emulsion development can be achieved by phase diagrams, which make it possible to study and characterize the systems as well as to obtain the higher stability with a smaller amount of surfactant.
The aim of this work is to report on the emulsification of vegetable oils such as C. guianensis oil by using a blend of nonionic surfactants. The determination of the critical HLB of this oil and the use of the phase diagram to evaluate disperse systems stability and physical–chemical properties are also the main goal of this research.
MATERIALS AND METHODS
Materials
The surfactants (Span 80® and Tween 20®) were purchased from Sigma; C. guianensis oil was obtained from Laboratório Santa Maria, Manaus, Amazonas, Brazil.
Methods
Hydrophilic–Lipophilic Balance Spreadsheet Design
The emulsions were prepared following the spreadsheet design shown in Tables I and II. This spreadsheet includes two surfactants: one of a lipophilic nature (Span 80®, HLB = 4.3) and the other of a hydrophilic nature (Tween 20®, HLB = 16.7). The final HLB value of each system varied according to the relative proportion of each surfactant. Therefore, the variation among the HLB total values comprises 1 U (Table I). In a second step, the six more stable emulsion systems generated a second HLB spreadsheet design with HLB values adjusted to a significant figure of two decimals (Table II).
Table I.
Hydrophilic–Lipophilic Balance Spreadsheet Design in Accordance with Individual Surfactant Contribution—First Run of Experiments
| Formulation | Tween 20® | Span 80® | Final HLB value | ||
|---|---|---|---|---|---|
| % | HLB contribution | % | HLB contribution | ||
| F1 | 100.0 | 16.7 | 0.0 | 0.0 | 16.7 |
| F2 | 91.9 | 15.4 | 8.1 | 0.3 | 15.7 |
| F3 | 83.9 | 14.0 | 16.1 | 0.7 | 14.7 |
| F4 | 75.8 | 12.7 | 24.2 | 1.0 | 13.7 |
| F5 | 67.7 | 11.3 | 32.3 | 1.4 | 12.7 |
| F6 | 59.7 | 10.0 | 40.3 | 1.7 | 11.7 |
| F7 | 51.6 | 8.6 | 48.4 | 2.1 | 10.7 |
| F8 | 43.5 | 7.3 | 56.5 | 2.4 | 9.7 |
| F9 | 35.5 | 5.9 | 64.5 | 2.8 | 8.7 |
| F10 | 27.4 | 4.6 | 72.6 | 3.1 | 7.7 |
| F11 | 19.4 | 3.2 | 80.6 | 3.5 | 6.7 |
| F12 | 11.3 | 1.9 | 88.7 | 3.8 | 5.7 |
| F13 | 3.2 | 0.5 | 96.8 | 4.2 | 4.7 |
| F14 | 0.0 | 0.0 | 100.0 | 4.3 | 4.3 |
F formulation, HLB hydrophilic–lipophilic balance
Table II.
Hydrophilic–Lipophilic Balance Spreadsheet Design—Second Run of Experiments
| Formulation | Tween 20® | Span 80® | Final HLB value | ||
|---|---|---|---|---|---|
| (%) | HLB contribution | (%) | HLB contribution | ||
| F15 | 100.000 | 16.700 | 0.000 | 0.000 | 16.7 |
| F16 | 95.968 | 16.026 | 4.032 | 0.174 | 16.2 |
| F17 | 91.936 | 15.353 | 8.064 | 0.347 | 15.7 |
| F18 | 87.904 | 14.679 | 12.096 | 0.521 | 15.2 |
| F19 | 83.871 | 14.006 | 16.129 | 0.694 | 14.7 |
| F20 | 79.839 | 13.166 | 20.161 | 1.034 | 14.2 |
| F21 | 75.807 | 12.659 | 24.193 | 1.041 | 13.7 |
| F22 | 71.775 | 11.986 | 28.225 | 1.214 | 13.2 |
| F23 | 67.742 | 11.312 | 32.258 | 1.388 | 12.7 |
| F24 | 63.710 | 10.639 | 36.290 | 1.561 | 12.2 |
| F25 | 59.678 | 9.966 | 40.322 | 1.734 | 11.7 |
| F26 | 55.646 | 9.292 | 44.354 | 1.908 | 11.2 |
F formulation, HLB hydrophilic–lipophilic balance
Preparation of the Emulsions
The emulsions were obtained by the phase inversion temperature method. For each O/W emulsions were prepared 100 ml containing 5% (w/w) of oil (5 g), 2% (w/w) of surfactants (2 g), and 93% (w/w) of water (93 ml). Initially, the continuous phase was prepared by dispersing the required Tween 20® in distilled water. The dispersed phase was obtained by melting Span 80® into the “Andiroba” oil. Both phases were heated separately to 70°C and then inter-dispersed (see Table I for percent weight/weight). Final emulsions were obtained after homogenization using an Ultra-Turrax (IKA, mod. T-25, Staufen, Germany) at 13,000 rpm for 10 min. Two batches of 14 emulsions were obtained and were stored at 25 ± 2°C and 4 ± 1°C.
Characterization of the Emulsions
Macroscopic Analysis
Each emulsion was evaluated to detect visible modifications or instabilities such as color, creaming, coalescence, and/or separation of phases. This analysis was performed for both storage conditions.
Droplet Size Analysis
For the mean diameter calculation, the diameters of 300 droplets for each emulsion were counted (n = 3), following Ferret’s method (22), by using an optical microscope (Carl Zeiss, mod. Axioscop 50, Oberkochen, Germany) equipped with a calibrated eyepiece micro-meter (1 U = 1.6 μm at ×400).
Creaming Index
The creaming rate was determined experimentally by the measurement of the creaming index (CI). The CI values were obtained by the ratio between the total height of cream layer (CC) and the total height of emulsion layer (CT) according to Eq. 1 (22,23). CC and CT were measured direct in storage glass flask with the help of a graduate scale.
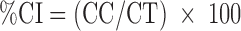 |
1 |
where CC = total height of cream layer and CT = total height of emulsion.
pH Measurements
pH measurements of the emulsions were performed using a pre-calibrated pH meter (WTW, mod. 330i, Weilheim, Germany).
Conductivity Evaluation
The electrical conductivity procedure is an easy way to evaluate the character of the emulsion. W/O emulsions present low value of conductivity. Inversely, O/W emulsions present high values that are maintained with the stability of the system. The conductivity was measured using a Conductivimeter (Tecnopon, mod. mCA150, São Paulo, Brazil).
Turbidimetric Method
This procedure is based on the works of Orafidiya and Oladimeji (21). In fact, this method is widely recognized as a tool to predict and evaluate emulsion stability because the fast dilution made during the process is unable to break the system and interferences and instabilities were completely minimized.
For turbidity, 5 ml of each sample was diluted to 25 ml with distilled water, and the percentage transmission (%T) was measured at 600 nm, previously determined for the distilled water used as the blank control (21), by using Espectrofotometer (Biochrom, mod. Libra S32, Cambridge, UK). With the blank control set at 100% transmission, the turbidity of the diluted emulsion was calculated as Eq. 2 (n = 3):
 |
2 |
Stability Studies
Long-Term Stability
The macroscopic aspect, CI, pH, and conductivity were evaluated on storage days 3, 5, 10, 15, 30, 60, 90, 120, and 180. The samples were stored at room temperature (25 ± 2°C) and at low temperature (4 ± 1°C). Regarding diluted emulsions, the flocculation rates can be determined by measuring turbidity as a function of time (24). The turbidity measurements and droplet size diameter were determined on storage day 180. These parameters were able to evaluate the emulsion system stability and therefore predict the chemical stability of the components of the emulsion. In fact, if an instability occurs for any chemical component, this will be reflected on the physicochemical stability of the system.
Short-Term Stability
The short-term stability was evaluated by the micro-emultocrit technique (MET) and centrifugal stress. Regarding the micro-emultocrit analysis, heparin-free capillary tubes were filled to 75% with each formulation and placed in a micro-centrifuge (Fanem, São Paulo, Brazil) at 11,500×g for 10 min. After the centrifugation cycle, the capillary tubes were placed against the micro-hematocrit scale, and the CI was directly measured. The visual aspect was evaluated in order to investigate phase separation. For those preparations, which were not broken, CI was measured by the micro-hematocrit reading scale (22).
The centrifugal stress was used in order to investigate the resistance of emulsions to different speeds of rotation (Fanem, mod. Excelsa Baby, São Paulo, Brazil). Wintrobe tubes were filled with the emulsions and, soon afterward, submitted to different speeds of rotations (22.4, 89.6, 201.6, 560.0, 806.4, and 1,097.6 g) for 10 min, by direct reading of the creaming layer and visual aspect in the graduated scale of the Wintrobe tubes (25).
Phase Diagram
The phase behavior was performed by a pseudo-ternary diagram (PTD) according to the method previously described (26,27). Sorbitan monolaurate (Tween 20®, Sigma) constituted the surfactant, C. guianensis oil was the oil phase, and the aqueous phase was composed of freshly distilled water. The PTD was elaborated from the mixture of surfactant and oil phase, with subsequent titration with the aqueous phase, whose concentration variation allowed the achievement of 99 formulations. The homogenization was made by Ultrasound System Sonicator (Heat Systems, model XL, New York, USA): A stirring process was maintained for the period of 1 min, with subsequent ultrasound bath (Unique, model USC-1800A, São Paulo, Brazil) for 3 min (28). This procedure was repeated twice for each sample.
The emulsions were macroscopically evaluated in order to indentify signs of instability in the formulations (creaming, coalescence, and phase separation) as well as to classify the systems according to their physical–chemical aspects in micro-emulsion, emulsion, cream, or phase separation.
RESULTS AND DISCUSSION
This section is divided into two parts named “First Run of Experiments” and “Second Run of Experiments”. The former section is dedicated to the production of emulsion systems presenting an HLB value between 4.3 and 16.7. The latter section describes the results concerning the emulsion systems produced in the HLB range of 11.2 to 16.7.
First Run of Experiments
In this work, the inversion phase process was satisfactory for production O/W emulsions containing “Andiroba” oil, which remained stable without visual modifications for 24 h. Despite the large range of HLB, no significant visual differences were observed on emulsions stored at room temperature (25 ± 2°C; Table III).
Table III.
Visual Aspect of Emulsions Stored at Room Temperature
| Formulations | D0 | D1 | D3 | D10 | D15 | D30 | D60 | D90 | D120 | D180 |
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F2 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F3 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F4 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F5 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F6 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F7 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F8 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F9 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO |
| F10 | M | M | M+CR | M+CR | M+CR | M+CR | M+CR | M(Y)+CO | M(Y)+CO | M(Y)+CO |
| F11 | M | M | M+CR | M+CR | M+CR | M+CR | M(Y)+CO | M(Y)+CO | M(Y)+CO | M(Y)+CO |
| F12 | M | M | M+CR | M+CR | M+CR | M+CR | M(Y)+CO | M(Y)+CO | M(Y)+CO | M(Y)+PS |
| F13 | M | M | M+CR | M+CR | M+CR | M+CR | M(Y)+CO | M(Y)+CO | M(Y)+CO | M(Y)+PS |
| F14 | M | M | M+CR | M+CR | M+CR | M+CR | M(Y)+CO | M(Y)+CO | M(Y)+CO | M(Y)+PS |
D day, M milky aspect, CR creaming, CO coalescence, PS phase separation, (Y) yellowish aspect, M (Y) milky and yellowish aspect
The main challenge in the formulation of an emulsion is due to the fact that the success or failure of such thermodynamically unstable systems can only be evaluated after a long-term study. Thus, the more critical stable formulations can take a very long experimental time. Additionally, the optimization of formulations becomes more complex, especially concerning the determination of the required HLB. In order to reduce the time and experimental effort, several short-term methods are suggested in the literature to evaluate emulsion destabilization phenomena such as mean droplet size (21), conductivity (29), turbidity (30,31), and/or creaming layer measurements (17). Recently, the micro-emultocrit method was proposed as a tool to predict the emulsion stability (22).
Using the centrifugal stress as the instability source, this experimental procedure provides remarkable information about the system stability since it accelerates the rate of creaming or sedimentation of the products (22). In addition, due to the low amount of sample and short time of execution, this technique can be successfully used to evaluate and/or optimize formulation parameters such as surfactant composition as well as its respective HLB value (22).
Concerning the short-term stability study, the results observed with the MET showed an important correlation between the HLB value and CI. Thus, at higher values of HLB (F1–F6), lower values of CI were observed (1%). Hence, an increase in CI was observed from F7 to F10 (1.6 to 3.33), and finally, the phase separation was detected for formulations with lower HLB (F11–F14). This behavior suggested that the method was able to detect not only the major influence of the centrifugal stress but also the individual contribution of each HLB (surfactant composition) on the system stability (Table IV).
Table IV.
Creaming Index for Products Stored at Room Temperature After Short-Term Stability Studies
| Formulations | MET | Resistance to centrifugation (g) | |||||
|---|---|---|---|---|---|---|---|
| 22.4 | 89.6 | 201.6 | 560.0 | 806.4 | 1,097.6 | ||
| F1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| F2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| F3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| F4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| F5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| F6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| F7 | 1.67 | 1.00 | 1.00 | 1.00 | 2.00 | 1.00 | 1.50 |
| F8 | 2.67 | 1.00 | 1.00 | 1.00 | 1.00 | 1.50 | 2.00 |
| F9 | 3.00 | 1.00 | 1.00 | 1.00 | 1.00 | PS | PS |
| F10 | 3.33 | 1.00 | 1.00 | 1.00 | 1.50 | PS | PS |
| F11 | PS | 1.00 | 1.00 | 1.00 | 2.50 | PS | PS |
| F12 | PS | 1.00 | 1.00 | 1.00 | 2.50 | PS | PS |
| F13 | PS | 1.00 | 1.00 | PS | PS | PS | PS |
| F14 | PS | 1.00 | 1.00 | PS | PS | PS | PS |
MET micro-emultocrit technique, PS phase separation
The analysis by centrifugal stress at several speeds, performed to confirm the MET behavior, presented a similar profile for systems with higher and lower HLB (stable and unstable emulsions, respectively). However, this traditional and recognized method showed a lower resolution response and was not appropriate to evaluate influences provided by small variations on the HLB values. Thus, besides the simplicity, the ability to detect the low variation in the surfactant composition is an important advantage of the use of MET.
Although the MET suggested the relationship between HLB values and system stability, the use of long-term stability studies is necessary to evaluate the stress provided by the storage conditions. Usually, at least one reference sample should be stored at low temperature in order to keep its original properties and minimize the environmental influences.
As mentioned above, no important visual modifications could be observed between storage conditions. Thus, the CI was observed for the first time after 3 days of storage. Already at this moment, the emulsions could be divided into three levels according to their CI: emulsion with lower (F1 to F4), intermediary (F5 to F9), and higher CI value (F10 to F14). This behavior is in accordance with the results observed with the short-term assay, and an inverse behavior was detected with the CIs and HLB values. In the course of the analysis, the instabilities improved and the CI increased. After 30 days, the CI still detected the three levels for the emulsions. The critical condition started at 60 days. At this time, the products with higher CI value and lower HLB (F11 to F14) presented coalescence and phase separation, while the others showed similar values of CI. This behavior was repeated after 90 and 120 days. Afterward, the formulation F10 was broken. Finally, the remaining emulsions presented phase separation at 180 days of storage (Table V).
Table V.
CI% for the Long-Term Stability Study at Room and Low Temperature Using the Micro-emultocrit Technique—First Run of Experiments
| Formulations | MET | Storage time (day) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D3 | D5 | D10 | D15 | D30 | D60 | D90 | D120 | D180 | |||||||||||
| R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | ||
| F1 | 1.00 | 1.47 | 0.00 | 2.98 | 0.00 | 3.03 | 2.98 | 4.48 | 2.94 | 4.61 | 3.00 | 5.17 | 3.00 | 3.33 | 3.08 | 5.36 | 3.08 | PS | 5.80 |
| F2 | 1.00 | 2.85 | 0.00 | 2.90 | 0.00 | 2.90 | 2.86 | 4.41 | 2.85 | 4.48 | 5.71 | 3.39 | 7.69 | 5.00 | 5.97 | 5.46 | 2.99 | PS | 3.03 |
| F3 | 1.00 | 1.43 | 0.00 | 1.41 | 0.00 | 2.90 | 5.80 | 2.90 | 4.28 | 2.94 | 7.14 | 4.92 | 7.81 | 3.22 | 4.48 | 5.00 | 3.03 | PS | 3.03 |
| F4 | 1.00 | 1.49 | 0.00 | 1.47 | 0.00 | 2.98 | 4.22 | 2.98 | 2.82 | 3.03 | 5.97 | 5.17 | 6.06 | 3.45 | 5.80 | 9.26 | 2.94 | PS | 4.41 |
| F5 | 1.00 | 2.85 | 0.00 | 2.86 | 0.00 | 4.35 | 5.88 | 2.90 | 2.94 | 2.94 | 4.41 | 6.56 | 6.35 | 3.17 | 6.06 | 5.08 | 4.61 | PS | 3.08 |
| F6 | 1.00 | 2.85 | 0.00 | 2.82 | 0.00 | 2.86 | 5.80 | 2.86 | 2.90 | 4.61 | 5.71 | 4.84 | 6.25 | 6.35 | 4.48 | 5.00 | 3.03 | PS | 3.03 |
| F7 | 1.67 | 2.82 | 0.00 | 2.86 | 0.00 | 4.28 | 4.41 | 4.35 | 2.94 | 6.25 | 4.41 | 5.00 | 7.94 | 5.00 | 4.54 | 5.45 | 3.08 | PS | 4.35 |
| F8 | 2.67 | 2.98 | 0.00 | 4.35 | 0.00 | 4.41 | 4.35 | 4.48 | 4.35 | 5.00 | 5.71 | 5.08 | 7.81 | 5.00 | 5.97 | 5.26 | 1.54 | PS | 4.69 |
| F9 | 3.00 | 4.28 | 0.00 | 2.90 | 0.00 | 4.35 | 4.35 | 5.88 | 4.35 | 5.97 | 4.28 | 5.00 | 6.15 | 5.00 | 5.97 | 3.45 | 3.03 | PS | PS |
| F10 | 3.33 | 5.71 | 0.00 | 5.71 | 0.00 | 5.80 | 4.28 | 7.35 | 4.28 | 7.57 | 5.71 | 6.56 | 7.69 | 8.33 | PS | PS | PS | PS | PS |
| F11 | PS | 8.82 | 2.86 | 7.35 | PS | 10.45 | PS | 9.10 | PS | 10.00 | PS | PS | PS | PS | PS | PS | PS | PS | PS |
| F12 | PS | 8.70 | 2.86 | 8.70 | PS | 10.29 | PS | 8.95 | PS | 11.30 | PS | PS | PS | PS | PS | PS | PS | PS | PS |
| F13 | PS | 8.57 | 4.48 | 10.00 | PS | 10.14 | PS | 10.29 | PS | 12.30 | PS | PS | PS | PS | PS | PS | PS | PS | PS |
| F14 | PS | 8.70 | 4.28 | 8.70 | PS | 9.10 | PS | 9.10 | PS | 11.50 | PS | PS | PS | PS | PS | PS | PS | PS | PS |
The relative standard deviations were lower than 5%
MET micro-emultocrit technique, PS phase separation, R room, L low, F formulation, D day
Regarding the formulations stored at low temperature, no creaming formation was observed for most of the emulsions for the first 5 days (F1 to F10). Only formulations F11 to F14 presented a negligible CI value. In fact, this result is in accordance with the results previously observed for the HLB influence on the stability. However, the instabilities of these last emulsions rapidly improved and showed phase separation in only a few days (Table V). After that, the remaining products showed CI between 3% and 7% until day 90, when formulation F10 was broken. Finally, phase separation was observed for formulation F9 on the last experimental day.
The pH results for emulsions stored at room temperature indicated no important influence on the surfactant composition (Fig. 1). However, the storage time promoted a decrease in the pH for all formulations. This tendency of decrease occurred for 120 days. Despite the slight decrease in the pH values, this phenomenon can be explained by fatty acids liberation after partial hydrolysis of triglycerides (24). However, a more consistent decrease in pH values was expected due to the complex and crude nature of the “Andiroba” oil. Hence, these results suggest a good ability of the systems to encage the oil component in spite of the large range of HLB.
Fig. 1.
pH results for emulsion stored at room temperature according to its HLB value
In order to study electrical conduction of nonionic O/W emulsion, a small amount of aqueous electrolyte should be added to provide the charge necessary for charge transport (32). However, the addition of salt can lead to a physicochemical change in the phase behavior, and the phase separation can be taken place (25,32). Hence, this analysis was performed without addition of electrolyte, and the assumption of the natural oil phase was the charge source. Thus, the variations in the conductivity of such systems should be attributed to constituents migration from the oil phase (alcohols and fatty alcohols, fatty acids, salts, etc.) and could be attributed to immediately system instability or to prediction of future instabilities.
The conductivity values for formulations at several HLB values are illustrated in Fig. 2. The emulsions presented similar values of conductance within the first 10 days. After that, the emulsions with HLB between 4.7 and 11.7 tended to stay at the same level. On the other hand, formulations with HLB higher than 11.7 showed a linear increase in the conductance that were proportional to the HLB value. This behavior suggested higher conductance for formulations at higher HLB values and therefore with higher concentrations of hydrophilic surfactant. In fact, the degree of hydratation of the droplet interface increased with the proportion of Tween 20® in comparison with Span 80®. This was expected since the hydrophilic head is the place in which the water is bind and its proportion increases in the formulations (29). Hence, the presence of hydrophilic head seems to be responsible for the improvement of the conductance of the nonionic droplets.
Fig. 2.
Conductivity curves versus HLB values for each experimental day. Emulsions stocked at room temperature
Second Run of Experiments
The determination of the required HLB of emulsion by short- and long-stability studies was based on the evaluation of the CI of the systems. In this present case, the formulations with higher HLB value showed the best performance and produced the more stable systems. Regarding the range of higher stability from the “First Run of Experiments” section, 12 new emulsions were prepared and evaluated in order to select the best surfactant composition (HLB from 11.2 to 16.7). The samples were stored at room (25 ± 2°C) and low temperatures (4 ± 1°C), and the CI was evaluated by micro-emultocrit and macroscopic analysis. The results of the micro-emultocrit at 1, 30, and 120 days are presented in Table VI.
Table VI.
CI% for the Long-Term Stability Study at Room and Low Temperature Using the Micro-emultocrit Technique—Second Run of Experiments
| Formulations | Micro-emultocrit | Storage at room temperature | Storage at low temperature | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 30 | Day 120 | Day 1 | Day 30 | Day 120 | Day 1 | Day 30 | Day 120 | |
| F15 | 1.00 | 1.00 | 1.00 | 1.00 | 2.98 | 4.00 | 0.00 | 1.45 | 2.98 |
| F16 | 1.00 | 1.00 | 1.00 | 1.00 | 3.08 | PS | 0.00 | 2.90 | 1.51 |
| F17 | 1.00 | 1.00 | 1.00 | 1.00 | 3.08 | PS | 0.00 | 1.47 | 3.03 |
| F18 | 1.00 | 1.00 | 1.00 | 1.00 | 3.12 | PS | 0.00 | 1.47 | 2.98 |
| F19 | 1.00 | 1.00 | 2.00 | 1.00 | 3.08 | PS | 0.00 | 2.90 | 3.03 |
| F20 | 1.00 | 1.33 | 2.00 | 1.00 | 3.08 | PS | 1.47 | 1.48 | 3.03 |
| F21 | 1.00 | 1.00 | 3.00 | 1.00 | 3.12 | PS | 1.43 | 1.45 | 4.48 |
| F22 | 1.00 | 2.00 | 1.67 | 1.00 | 3.12 | PS | 1.47 | 1.47 | 3.03 |
| F23 | 1.00 | 1.33 | 2.00 | 1.00 | 3.17 | PS | 1.43 | 1.49 | 1.54 |
| F24 | 1.67 | 2.00 | 2.00 | 1.67 | 3.03 | PS | 1.43 | 1.45 | 2.98 |
| F25 | 1.00 | 2.00 | 1.67 | 1.00 | 3.03 | PS | 1.43 | 1.44 | 2.94 |
| F26 | 1.67 | 2.33 | 3.00 | 1.67 | 3.03 | PS | 1.43 | 1.45 | 1.47 |
The relative standard deviations were lower than 5%
PS phase separation, F formulation
As expected, the data reveal very stable systems. No important difference was denoted 24 h after preparation. Regardless of the HLB value, the mean droplet size performed by optical microscopy was 2.23 ± 0.40 μm. On the 30th day, a tendency of increasing CI rate was observed for formulations from F20 to F26. In the same way, the mean particle size was improved to 3.39 ± 0.34 μm, and no correlations between HLB and particle sizes could be observed. Finally, the CI% values obtained after 120 days maintained the tendency of increasing, and formulations F19 to F26 showed the CI almost two times higher than that of the other formulations. The results of the macroscopic analysis performed at room and a low temperature was in accordance with those of the micro-emultocrit. The data reveal more stability for formulations with higher HLB values (F15–F18). Regarding the pH values, no variation could be observed in the formulations, showing a mean average of pH of 4.36 ± 0.124. For this reason, this parameter was unable to explain differences among formulations. This stability on the pH value among all formulations reflects that the surfactant used on the formulation is still stable, and no fatty acids from the lipophilic portion of the molecule were released on the medium.
The analysis of conductivity and turbidity were performed after 120 days of storage at room temperature (Fig. 3). Due to the expected stability of this range of formulations, the stress introduced by storage conditions could denote enough variability to allow for the ranking of the products. However, the formulations showed similar values of conductivity. On the other hand, the turbidity presented a slight decrease at lower HLB.
Fig. 3.
Conductivity and turbidity profile versus HLB value after 120 days of emulsion systems stored at room temperature
Although important for assessing the quality of systems, the size distribution of the droplets could not be considered due to the instabilities observed for some emulsions. Since the correlation between turbidity and droplet size was previously related (21) and that at optimum HLB the droplet size should be smallest giving higher turbidity, these assumptions were adopted to supports the supposition that the higher turbidity means better stability. Thus, the turbidity data indicate that formulations with higher HLB are more appropriate for preparing a system containing “Andiroba” oil.
To conclude, all these results suggest that the most stable emulsion system was the one produced by formulation F15, which presents an HLB value of 16.7 and is composed of water (93%, w/w), “Andiroba” oil (5%, w/w), and Tween 20® (2%, w/w).
Phase Diagram
Emulsion consists of liquid–liquid dispersions of immiscible phases exhibiting a certain resistance to their separation due to the presence of a third phase present at the interface, usually a surfactant. Thus, these three-phase systems produce emulsions easily under agitation due to the low interfacial tension. However, the stability of such systems depends on three independent variables: hydrophilic–lipophilic deviation (which corresponds to the required HLB of the oil phase at determinate temperature), the surfactant concentration, and the water/oil ratio. Thus, a three-dimensional representation can be built (33).
In this study, the required HLB at room temperature was previously measured and fixed. Using the same surfactant, the phase behavior follows a two-dimensional representation. Hence, the behavior can be represented by a triangular ternary diagram in which the independent variables are the surfactant concentration and the water/oil ratio (33–35). The isothermal ternary phase diagram of formulation F15 (water/Tween 20®/“Andiroba” oil system) was visually dominated by the O/W emulsions region (Fig. 4). This O/W single phase region was found to extend from the water/oil axis to about 0.75 of the oil phase (EM). However, increases in the oil phase at concentrations of surfactant lower than 0.5 led to the formation of a cream consistency system. This improvement in the system consistency started at an equal weight ratio of water and oil. After that, the proportion of oil led to an inversion of phases and tended to form O/W emulsions. However, the hydrophilic nature of the surfactant was inefficient in stabilizing such system, and the phase separation was considered mainly as behavior at a higher amount of oil. Finally, two other regions with similar appearance were observed. These systems were clear yellowish, which seems to indicate an isotropic dispersion of spherical droplets, leading to the assumption of microemulsions (ME).
Fig. 4.
Pseudo-ternary diagram system for the systems composed by Tween 20® (surfactant), water and Andiroba oil. ME micro-emulsion, EM emulsion, CR cream, PS phase separation
Regarding the phase diagram of micro-emulsions, it is well-known that discrete water droplets are formed in a continuous oil phase. However, the macroscopic properties of micro-emulsions can be quite similar to liquid crystals, and the difference between both products should be confirmed by cross-polarized light microscopy (36).
CONCLUSION
It has been demonstrated that the stability behavior of emulsions containing “Andiroba” oil (C. guianensis) as oil phase was in direct proportion to the HLB value. The emulsions stabilized by a couple of surfactants that presented lower values of HLB showed high creaming, coalescence, and/or phase separation. In the same way, high values of HLB implied more stability, and the final required HLB for “Andiroba” oil (C. guianensis) was found to be 16.7 by using only Tween 20®. For this study, the combination Tween 20®/Span 80® was unable to stabilize the system. On the other hand, the spreadsheet design, the characterization evaluation, and the stability study performed on this work reveal to be valuable tools to identify the best emulsion systems during the development of lipidic carriers. The phase diagram performed for the oil allowed the visual identification of several dispersed systems such as micro-emulsions, emulsions, and creams, as well as the maximum and minimum limits of component proportions for achieving the different phase behavior. Moreover, two next important steps will be necessary to improve the applicability of Andiroba oil emulsions as a new product: (a) the evaluation of chemical composition of the emulsion by HPLC analysis in order to assess chemical stability of components and (b) a scale-up study on the production of “Andiroba” oil emulsions to produce a semi-industrial batch of this product. The stable emulsions and creams from “Andiroba” oil play an important role in the development of products for topical use either by incorporation of actives or as insect repellents.
ACKNOWLEDGEMENTS
The authors are grateful for the financial support received from CNPq (477131/2007-7), FAPERN/MCT/CT-INFRA/CNPq (PPP 2007), and UFRN (Propesq/Pibic). The authors also thank Glenn Hawes, from the University of Georgia—American Language Program, for his editing this manuscript.
REFERENCES
- 1.Corrêa MP. Dicionário das plantas úteis do Brasil e das exóticas cultivadas por Manuel Pio Corrêa. Rio de Janeiro: Imprensa Nacional; 1984. [Google Scholar]
- 2.Vieira LS. Fitoterapia da Amazônia: manual de plantas medicinais. São Paulo: Ceres; 1992. [Google Scholar]
- 3.Sampaio PTB. Andiroba (Carapa guianensis) In: Clay JW, Sampaio PTB, Clement CR, editors. Biodiversidade Amazônica: exemplos e estratégias de utilização. Manaus: Amazonas; 2000. pp. 243–251. [Google Scholar]
- 4.Hammer ML, Johns EA. Tapping an Amazônian plethora: four medicinal plants of Marajó Island, Pará (Brazil) J Ethnopharmacol. 1993;40(1):53–75. doi: 10.1016/0378-8741(93)90089-N. [DOI] [PubMed] [Google Scholar]
- 5.Ferraz IDK, Camargo JLC, Sampaio PTB. Sementes e plântulas de Andiroba (Carapa guianensis Aublet e Carapa procera D. C.): aspectos botânicos, ecológicos e tecnológicos. Acta Amazônica. 2002;32(4):647–661. [Google Scholar]
- 6.Ferrari M, Oliveira MSC, Nakano AK, Rocha-Filho PA. In vitro and in vivo determinations of sun protection factor (SPF) of emulsions with andiroba oil (Carapa guianensis) Rev Bras Farmacogn. 2007;17(4):626–630. doi: 10.1590/S0102-695X2007000400023. [DOI] [Google Scholar]
- 7.Miot HA, Batistella RF, Batista KA, Volpato DEC, Augusto LST, Madeira NG, et al. Comparative study of the topical effectiveness of the Andiroba oil (Carapa guianensis) and DEET 50% as repellent for Aedes sp. Rev Inst Med Trop São Paulo. 2004;46(5):253–256. doi: 10.1590/s0036-46652004000500004. [DOI] [PubMed] [Google Scholar]
- 8.Silva OS, Romão PR, Blazius RD, Prohiro JS. The use of andiroba Carapa guianensis as larvicide against Aedes albopictus. J Am Mosq Control Assoc. 2004;20(4):456–457. [PubMed] [Google Scholar]
- 9.Mendonça FAC, Silva KFS, Santos KK, Ribeiro Junior KAL, Sant’Ana AEG. Activities of some Brazilian plants against larvae of the mosquito Aedes aegypti. Fitoterapia. 2005;76(7–8):629–636. doi: 10.1016/j.fitote.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Silva OS, Prophiro JS, Nogared JC, Kanis L, Emerick S, Blazius RD, et al. Larvicidal effect of andiroba oil, Carapa guianensis (Meliaceae), against Aedes aegypti. J Am Mosq Control Assoc. 2006;22(4):699–701. doi: 10.2987/8756-971X(2006)22[699:LEOAOC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Penido C, Conte FP, Chagas MSS, Rodrigues CAB, Pereira JFG, Henriques MGMO. Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice. Inflamm Res. 2006;55(11):457–464. doi: 10.1007/s00011-006-5161-8. [DOI] [PubMed] [Google Scholar]
- 12.Costa-Silva JH, Lyra MMA, Lima CR, Arruda VM, Araújo AV, Ribeiro e Ribeiro A, et al. Estudo toxicológico reprodutivo da Carapa guianensis Aublet (Andiroba) em ratas Wistar. Acta Farm Bonaerense. 2006;25(3):425–428. [Google Scholar]
- 13.Costa-Silva JH, Lyra MMA, Lima CR, Arruda VM, Araújo AV, Ribeiroeribeiro A, et al. A toxicological evaluation of the effect of Carapa guianensis Aublet on pregnancy in Wistar rats. J Ethnopharmacol. 2007;112(1):122–126. doi: 10.1016/j.jep.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Costa-Silva JH, Lima CR, Silva EJ, Araújo AV, Fraga MC, Costa-Silva JH, et al. Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J Ethnopharmacol. 2008;116(3):495–500. doi: 10.1016/j.jep.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Masson DS, Morais GG, Morais JM, Andrade FF, Santos ODH, Oliveira WP, et al. Polyhydroxy alcohols and peach oil addition influence on liquid crystal formation and rheological behavior of o/w emulsions. J Dispers Sci Technol. 2005;26(4):463–468. doi: 10.1081/DIS-200054579. [DOI] [Google Scholar]
- 16.Andrade FF, Santos OD, Oliveira WP, Rocha-Filho PA. Influence of PEG-12 Dimethicone addition on stability and formation of emulsions containing liquid crystal. Int J Cosmet Sci. 2007;29(3):211–218. doi: 10.1111/j.1467-2494.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 17.Rahate AR, Nagarkar JM. Emulsification of vegetable oils using a blend of nonionic surfactants for cosmetic applications. J Dispers Sci Technol. 2007;28(7):1077–1080. doi: 10.1080/01932690701524802. [DOI] [Google Scholar]
- 18.Lima CG, Vilela AFG, Silva AAS, Piannovski AP, Silva KK, Carvalho VFM, et al. Desenvolvimento e avaliação da estabilidade física de emulsões O/A contendo óleo de babaçu (Orbignya oleifera) Rev Bras Farm. 2008;89(3):239–245. [Google Scholar]
- 19.Westesen K. Emulsions. In: Herzfeldt CD, Kreuter J, editors. Grundlagen der Arzneiformenlehre 2: Galenik. 1 Auf. Berlin: Springer; 1999. [Google Scholar]
- 20.Griffin WC. Classification of surface-active agents by “HLB”. J SCC. 1949;1:311–326. [Google Scholar]
- 21.Orafidiya LO, Oladimeji FA. Determination of the required HLB values of some essential oils. Int J Pharm. 2002;237(1/2):241–249. doi: 10.1016/S0378-5173(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 22.Macedo JPF, Fernandes LL, Formiga FR, Reis MF, Nagashima T, Jr, Soares LAL, et al. Micro-emultocrit technique: a valuable tool for determination of critical HLB value of emulsions. AAPS PharmasciTech. 2006;7(1):E146–E152. doi: 10.1208/pt070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onunkwo GC, Adikwu MU. Stability of veegum/mucuna gum emulsions. STP Pharma Sciences. 1997;74:320–325. [Google Scholar]
- 24.Tadros T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv Colloid Interface Sci. 2004;108/109:227–258. doi: 10.1016/j.cis.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Formiga FR, Fonseca IAA, Souza KB, Silva AKA, Macedo JPF, Araújo IB, et al. Influence of a lipophilic drug on the stability of emulsions: an important approach on the development of lipidic carriers. Int J Pharm. 2007;344(1/2):158–160. doi: 10.1016/j.ijpharm.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliver Rev. 2000;45(1):89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 27.Aboofazeli R, Patel N, Thomas M, Lawrence MJ. Investigations into the formation and characterization of phospholipid microemulsions. IV. Pseudo-ternary phase-diagrams of systems containing water–lecithin–alcohol and oil—the influence of oil. Int J Pharm. 1995;125(1):107–116. doi: 10.1016/0378-5173(95)00125-3. [DOI] [Google Scholar]
- 28.Loffredo L. Desenvolvimento de Microemulsões contendo fotoprotetores inorgânicos nanoparticulados [Mestrado] Natal: Universidade Federal do Rio Grande do Norte; 2008. [Google Scholar]
- 29.Bisal S, Bhattacharya PK, Moulik SP. Conductivity study of microemulsions. Evaluation of hydration of oil/water microemulsions applying Bruggeman equation. J Phys Chem. 1990;94(10):4212–4216. doi: 10.1021/j100373a062. [DOI] [Google Scholar]
- 30.Song M-G, Cho S-H, Kim J-Y, Kim J-D. Rapid evaluation of water-in-oil (W/O) emulsion stability by turbidity ratio measurements. Korean J Coll and Inter Sci. 2000;230:213–215. doi: 10.1006/jcis.2000.7090. [DOI] [PubMed] [Google Scholar]
- 31.Song M-G, Cho S-H, Kim J-Y, Kim J-D. Novel evaluation method for the water-in-oil (W/O) emulsion stability by turbidity ratio measurements. Korean J Chem Eng. 2002;19(3):425–430. doi: 10.1007/BF02697151. [DOI] [Google Scholar]
- 32.Salager JL, Forgiarini A, Márquez L, Peña A, Pizzino A, Rodriguez MP, et al. Using emulsion inversion in industrial processes. Adv Colloid Interface Sci. 2004;108/109:259–272. doi: 10.1016/j.cis.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Kantarcı G, Özgüney I, Karasulu HY, Arzık S, Güneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech. 2007;8(4):75–81. doi: 10.1208/pt0804091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunieda H, Hasegawa Y, John AC, Naito M, Muto M. Phase behaviour of polyoxyethylene hydrogenated castor oil in oil/water system. Colloids Surf A. 1996;109:209–216. doi: 10.1016/0927-7757(95)03456-0. [DOI] [Google Scholar]
- 35.Testard F, Zemb T. Interpretation of phase diagrams: topological and thermodynamical constraints. Colloids Surf A. 2002;205(1/2):3–13. doi: 10.1016/S0927-7757(01)01141-4. [DOI] [Google Scholar]
- 36.Boonme P, Krauel K, Graf A, Rades T, Junyaprasert VB. Characterization of microemulsion structures in the pseudoternary phase diagram of isopropyl palmitate/water/Brij 97:1-butanol. AAPS PharmSciTech. 2006;7(2):E99–E104. doi: 10.1208/pt070245. [DOI] [PMC free article] [PubMed] [Google Scholar]






