Abstract
Insulin is a polypeptide hormone and usually administered for treatment of diabetic patients subcutaneously. The aim of this study was to investigate the efficiency of enteric nanoparticles for oral delivery of insulin. Nanoparticles were formed by complex coacervation method using chitosan of various molecular weights. Nanoparticles were characterized by drug loading efficiency determination, particle size analysis, Scanning Electron Microscopy (SEM), Zeta potential and CD spectroscopy (Circular Dichrosim). The in vitro release studies were performed at pH 1.2 and 7.4. The drug loaded nanoparticles showed 3.38% of entrapment, loading efficiency of 30.56% and mean particle size of 199 nm. SEM studies showed that the nanoparticles are non-spherical. Zeta potential increased with increasing molecular weight of chitosan. The CD spectroscopy profiles indicated that the nano-encapsulation process did not significantly disrupt the internal structure of insulin; additionally, pH-sensitivity of nanoparticles was preserved and the insulin release was pH-dependent. These results suggest that the complex coacervation process using chitosan and Eudragit L100-55 polymers may provide a useful approach for entrapment of hydrophilic polypeptides without affecting their conformation.
Key words: chitosan, complex coacervation, Eudragit L100-55, insulin, nanoparticle
INTRODUCTION
The oral delivery is the most convenient route of administration for patients (1,2). However, administering proteinous drugs orally is a formidable challenge due to their very short life in the gastric and intestinal fluids (3). The preparation of particles from polymers is based on emulsion-diffusion (4). Basically, an organic solution of the polymer is emulsified in an aqueous solution with or without a surfactant. In a second step, the organic solvent is removed by different methods such as evaporation, diffusion under stirring to allow particle formation. With these techniques, the drug has to be at least partially soluble in an organic solvent to be encapsulated. This is a major limitation to the encapsulation of hydrophilic compounds such as peptides, proteins or nucleic acids. A more common way is to use a double emulsion technique in which an aqueous solution of the hydrophilic compound is first emulsified in an organic solution of the polymer. The primary emulsion is then poured into a large volume of liquid paraffin. The double emulsion technique has fairly good encapsulation efficiency for hydrophilic compounds; however, particle size is usually larger than that of single emulsion technique (5). Once the particles are formed, a purification step is needed. This may be achieved by centrifugation or ultracentrifugation depending on the size of the particles, filtration (centrifugal filtration or crossflow filtration), gel permeation or dialysis. Finally concentration and drying are usually achieved by lyophilization. In the complex coacervation approach, two water-soluble and oppositely charged polymers are employed to form nanoparticles. This may potentially damage the conformation of the polypeptides or proteins, thus leading to a diminished activity after administration. Accordingly, a milder formulation method assists to circumvent this problem.
The aim of the present study was to develop a pH-sensitive insulin nanoparticle system employing Eudragit L100-55 and chitosan of different molecular weights. The suitability of prepared nanoparticles for oral delivery of insulin is discussed.
MATERIAL & METHODS
Materials
Re-combinant insulin was obtained from Lily (28.3 U/mg, USA), Eudragit L100-55 was obtained from Röhm Pharma GMBh (Weiterstadt, Germany). Low-molecular-weight (LMW, 40,000 Da), medium-molecular-weight (MMW, 480,000 Da) and high-molecular-weight (HMW, 850,000 Da) chitosan were purchased from Sigma-Aldrich (Germany). Glacial acetic acid, ethyl alcohol, hydrochloric acid and potassium chloride were all from Merck (Germany). All solvents and reagents were of analytical grade.
Methods
Preparation of Insulin Nanoparticles and Physical Mixtures
Insulin nanoparticles were prepared at room temperature by injecting the positively charged chitosan solution into the negatively charged Eudragit L 100-55. Nanoparticles prepared with three types of chitosan, namely, low, medium, high and mixture of low and high molecular weight chitosans (2:1 ratio). Insulin (10 mg) was dissolved in 0.4 ml of 0.1 M hydrochloric acid, and mixed with 4 ml of 0.2% (w/v) chitosan solution (pH 5.8). The above solution was then injected into 24 ml of 0.2% (w/v) Eudragit L100-55 solution in ethanol. During injection, the mixture was stirred at 500 RPM. The resulting opalescent dispersion was filtered through a 20-μm filter (Whatman, kent, UK) (6–10).
The prepared raw insulin nanoparticle dispersion was purified by discontinuous sucrose gradient centrifugation, as reported previously (6). The gradient, 80%–50%–35% (w/v) of sucrose layer was ascertained by the previous screening. Briefly, for constructing a discontinuous sucrose density gradient, 5 ml of 80% (w/v) sucrose, 5 ml of 50% (w/v), and 5 ml of 35% (w/v) sucrose were gently put into the same centrifuge tube, respectively. Finally, 20 ml of raw insulin nanoparticle dispersion was layered on the top of the sucrose solutions in tube. The tube was then centrifuged at 87,000 g for 30 min at 4°C. After centrifugation, insulin nanoparticles were concentrated in the middle layer (50% sucrose). The middle layer was then collected and dialyzed using dialysis membrane for 24 h in the dark against physiological saline (0.9% NaCl). The saline was changed every 8 h for three times. After dialysis, insulin nanoparticle dispersion in the dialysis membrane was collected, and stored at 4°C.
Physical mixture of the drug with chitosan and Eudragit L100 was prepared via tumbling method using corresponding amount of components.
Determination of Drug Loading and Entrapment Efficiency
Insulin loading percentage in nanoparticles was measured as follows: 10 mg of lyophilized insulin nanoparticles were weighed accurately, dissolved in 0.1 mol/L NaOH solutions, and adjusted to pH 7.4 immediately using 0.1 mol/L HCl. The final volume of the resultant solution was 10 ml. The resulting solution was then centrifuged at 16,000 g for 30 min. The supernatant was collected for measurement of insulin amount in the nanoparticles.
For determination of insulin entrapment efficiency, 10 ml of insulin nanoparticle suspension was ultracentrifuged at 87,000 g for 30 min. Insulin concentration in the supernatant was measured, by UV spectrophotometry at 274 nm. Insulin entrapment efficiency was expressed as the ratio of the insulin amount measured in the supernatant to the total insulin amount added (4,6,11–13). Each measurement was repeated for three times.
Scanning Electron Microscopy (SEM)
The morphology of nanoparticles was examined with a scanning electron microscope (LEO 440i, England) operating at 15 kV. The samples were mounted on a metal stub with double adhesive tape and coated with platinum/palladium alloy under vacuum.
Particle Size Analysis
A laser light scattering particle size analyzer (SALD-2101, Shimadzu, Japan) was used to determine the particle size of the nanoparticle formulations. Samples were suspended in distilled water and stirred continuously during the particle size analysis. Each measurement was done in triplicates.
Circular Dichroism Spectrophotometry
Effect of encapsulation on the conformation of insulin was evaluated with circular dichroism spectrophotometry. The samples for circular dichroism analysis were prepared as follows:
Insulin alone was dissolved in HCL solution (pH 2.5), as a standard control;
Insulin was dissolved in HCL solution (pH 2.5), and mixed with (10% w/v) Eudragit L100-55 solution as the sample 1;
Lyophilized insulin nanoparticles were dissolved in 0.1 mol/L NaOH solution. The resultant solution was then centrifuged at 16,000 g for 30 min and the supernatant was collected.
In the above solutions, the final concentration of insulin was 0.1 mg/ml for each and confirmed using a UV spectrometer at 276 nm (Jasco J-810, Tokyo, Japan). The control solutions or sample solutions were measured using a circular dichroism spectrophotometer. The measurement conditions were as follows: Temperature, 20°C; a 0.2-cm cuvette; wavelength range, 200–250 nm; resolution, 2 nm; and scanning speed, 200 nm/min with a 1 s response time. Noise reduction, blank buffer subtraction, and data analysis were performed using a standard analysis and temperature/wavelength analysis program.
Zeta Potential Analysis
Zeta potential is electric potential in the interfacial double layer (DL) at the location of the slipping plane versus a point in the bulk fluid away from the interface (14). Zeta potential of insulin nanoparticles was measured with Zetasizer (Malvern instruments, England). Insulin nanoparticles were diluted with deionized water before measurement. Each measurement was carried out in triplicate.
In vitro Release Study
Dissolution studies were carried out using a shaking incubator method at 37°C and 100 strokes per minutes. Three milliliters of 0.2 N HCl (containing 0.1 mg methyl cellulose to prevent adsorption to the vessel glass) were equilibrated at 37 ± 0.5°C. after 2 h 0.1 ml of concentrated phosphate buffered saline stoke solution, pre-equilibrated at 37°C, was added to the dissolution vial to render the pH to 7.4 (15,16). Physical mixture or purified nanoparticles (equivalent to 1.5 mg of insulin) were placed in the dissolution medium and 3 ml aliquots of medium were withdrawn at pre-set times of 0.5, 1, 2, 4, 6, and 8 h. The samples were ultracentrifuged at 22,000 g for 30 min. The insulin amount in the supernatant was measured spectrophotometrically at 276.4 nm for both of acidic and enteric buffers. Each experiment was repeated three times (16,17).
RESULTS AND DISCUSSION
The Effect of Polymer Molecular Weight on the Physical Properties of Nanoparticles
Four different formulations containing insulin with chitosan of various molecular weights were prepared. The properties of these formulations are listed in Table I. The nanoparticles were prepared by interfacial cross-linkage of chitosan and Eudragit L100-55 dissolved is the inner and outer phase of the dispersion, respectively. The drug loading and encapsulation efficiency for nanoparticle formulation with high MW chitosan were 3.38% and 30.56% respectively.
Table I.
Effect of Different Molecular Weights of Chitosan on the Drug Content, Loading Efficiency, and Particle Size of Insulin Nanoparticles and Comparison of Various Release Characteristics of Insulin from Different Nanoparticle Formulations
| Formulation | NP1 | NP2 | NP3 | NP4 |
|---|---|---|---|---|
| Chitosan type (Mw) | Low | Medium | High | Mix (low & high) 2:1 |
| Theoretical drug content (%) | 15.15 | 15.15 | 15.15 | 15.15 |
| Drug Loading (% ±SD) | 2.55 ± 0.25 | 3.07 ± 0.33 | 3.38 ± 0.45 | 1.91 ± 0.20 |
| Loading Efficiency (% ±SD) | 17.98 ± 3.21 | 23.65 ± 3.09 | 30.56 ± 2.76 | 28.0 ± 94.35 |
| Mean particle size (nm±SD) | 166.10 ± 1.70 | 189.36 ± 1.69 | 199.64 ± 1.72 | 135.76 ± 1.69 |
| aQ2 | 6.72 ± 0.33 | 15.27 ± 0.33 | 15.86 ± 0.57 | 10.38 ± 0.71 |
| bQ8 | 27.35 ± 2.87 | 48.35 ± 1.08 | 49.43 ± 2.42 | 36.62 ± 4.38 |
| ct 50%(h) | – | 8 | 8 | – |
| dDE | 11.48 | 26.37 | 27.40 | 18.34 |
| Similarity factor | 40.91 | 92.03 | –* | 50.78 |
NP1 Nanoparticles containing low MW chitosan; NP2 Nanoparticles containing medium MW chitosan; NP3 Nanoparticles containing high MW chitosan; NP4 Nanoparticles containing mixture (2:1) of low and high Mw chitosan
aQ2) amount of drug release after 2 h; bQ8) amount of drug release after 8 h; ct 50%) time for 50% dissolution. *NP3 was used as reference formulation for similarity factor determination.
The molecular weight of chitosan significantly influenced the entrapment efficiency and loading percentage, that is, higher molecular weights resulted in greater insulin entrapment efficiency and loading percent (p < 0.05).
Scanning electron microscopic photographs of nanoparticles (chitosan with high Mw) are shown in Fig. 1, which shows that the prepared nanoparticles are non-spherical. Table I and Fig. 2 indicate that nanoparticles with the size range of 135–199 nm were obtained. Table I also shows that the molecular weight of chitosan affects the particle size of nanoparticles, i.e. the higher molecular weight, the larger particle size. Volume-based size distribution of nanoparticles indicated a log–probability distribution.
Fig. 1.
SEM of insulin nanoparticles containing high molecular weight of chitosan at 1000×
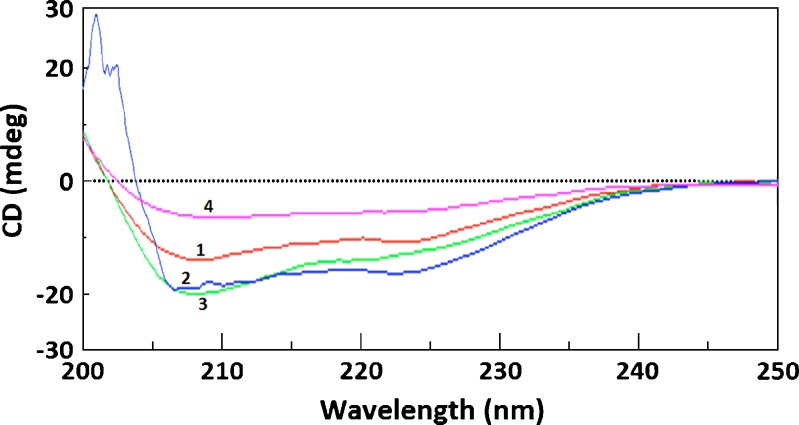
Fig. 2.
Circular dichroism spectra of (1) pure intact insulin, (2) insulin in 0.2% (w/v) chitosan solution with high Mw, (3) insulin in 0.2% (w/v) Eudragit L100-55 solution, and (4) insulin in the chitosan-Eudragit L100-55 nanoparticles. The insulin concentration was 0.2 mg/ml
Effect of the matrix materials and formulation procedure on the secondary structure of insulin is shown in Fig. 2. The circular dichroism spectrum of intact insulin showed ellipticity troughs at 208 and 222 nm. The circular dichroism spectrum of insulin in the physical mixtures and prepared nanoparticles was close to that of intact pure insulin, demonstrating that the matrix materials and formulation procedure did not significantly influence insulin conformation. Parameters of the secondary structure of insulin i.e. α-helix, β-sheet, β-turn, and random coil, are demonstrated in Table II. Compared to the conformation parameters of pure intact insulin, a slight difference can be seen in the secondary structure parameters of the physical mixtures and nanoparticles. In the nanoparticles prepared with chitosan of high MW, α-helix and β-sheet were increased by 13.7 and 1.1 percent respectively. On the other hand β-turn and random coil were decreased by 3.9 and 10.9 percent, respectively (6,9,10).
Table II.
Observed Parameters of Secondary Structure of Standard Insulin, Insulin Treated under Various Conditions, or Insulin Encapsulated in Chitosan-Eudragit L100-55 Nanoparticles
| Samples | α-helix (%) | β-sheet | β-Turn (%) | Random coil (%) |
|---|---|---|---|---|
| Insulin in pH 1.2 HCl, as a standard control | 28.5 | 24.5 | 20.5 | 26.5 |
| Insulin in 0.2% (w/v) chitosan | 23.4 (−4.1)a | 23.4(−1.1)a | 25.1 (+4.6)a | 28 (+1.5)a |
| Insulin in 0.2% (w/v) Eudragit L100-55 | 45.9 (+17.4)a | 0 (−24.5)a | 38.7 (+18.2)a | 15.4 (−11.1)a |
| Insulin in chitosan-Eudragit L100-55 nanoparticles (with high chitosan) | 42.2 (+13.7)a | 25.6 (+1.1)a | 16.6 (−3.9)a | 15.6 (−10.9)a |
aNumber in the blanket is the changed percentage value, compared with the value of the standard control; (+) reperesents the increased percentage value, and (-) the decreased, respectively. The concentration of insulin was 0.2 mg/ml
The zeta potential of nanoparticle formulations was in the range from −20.7 mV to −27.9 mV. With higher molecular weight of chitosan the zeta potential was significantly more negative (p < 0.05). In fact, zeta potential is the potential difference of the dispersion medium and stationary layer of fluid attached to the dispersed particle. A value of 25 mV (positive or negative) can be taken as the arbitrary value that separates low-charged surfaces from highly-charged surfaces. The significance of zeta potential is that its value can be related to the stability of colloidal dispersion. The zeta potential indicates the degree of repulsion between adjacent, similarly charged particles in dispersion. For molecules and particles that are small enough, a high zeta potential will confer stability, i.e. the solution or dispersion will resist aggregation (14). That is, colloids with high zeta potential such as nanoparticles with chitosan of high MW (−27.9 mV), are electrically stabilized while colloids with low zeta potentials such as nanoparticles with chitosan of low MW (−20.7 mV) tend to coagulate or flocculate.
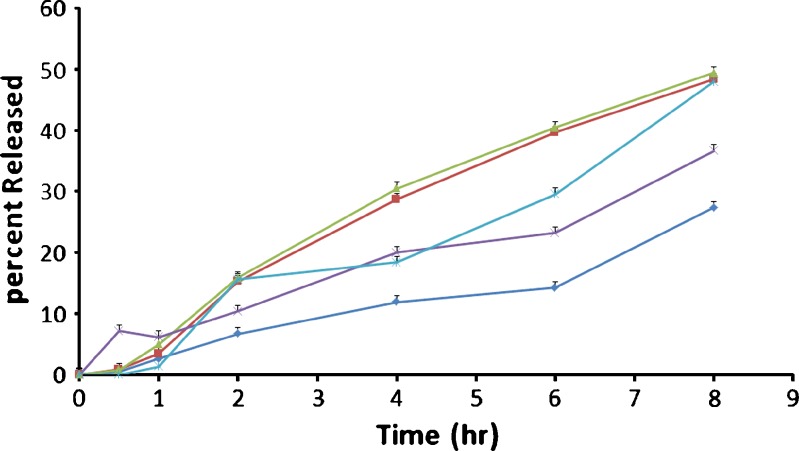
The release profiles of insulin from nanoparticles with high MW of chitosan are showed in Fig. 3. There was no burst release from nanoparticle formulations (Fig. 3), indicating a very good encapsulation of insulin inside nanoparticles resulting from the stable nature of the coacervate droplets during the solvent removal step, which may cause a very small part of the drug to relocate at the nanoparticle surface (18,19). The corresponding release rates are increased by increasing molecular weight of chitosan when comparing with other formulations (Fig. 3). This phenomenon could be attributed to the increased porosity of the polymer matrix surrounding the drug. Similar behavior has been reported for verapamil microparticles prepared with Eudragit RS100 (15). As can be seen in Fig. 3, a small portion of insulin was release in the acid stage (less than 15%). This observation could be ascribed to pH dependency of Eudragit L 100 dissolution (20–22).
Fig. 3.
Cumulative percent release of insulin nanoparticles prepared with different molecular weight of chitosan. NP 1 Nanoparticles containing low MW chitosan (-♦-); NP 2 Nanoparticles containing medium MW chitosan (-■-); NP 3 Nanoparticles containing high MW chitosan (-▲-); NP 4 Nanoparticles containing mixture (2:1) of low and high Mw chitosan (-×-); Physical mixture containing drug and polymers (Eudragit L100-55 and chitosan)(-ж-). Each data point represents mean±SD (n = 3)
Statistical analysis of release data was performed by comparing the DE (dissolution efficiency), t50% (time for 50% release); similarity factor (f2) (Table I) (23). Similarity factor is defined by the following equation:
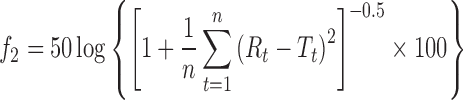 |
Where n is the number of dissolution sample times Rt and Tt are the percentage dissolved at each time point for the reference and test formulation, respectively (23). An f2 value between 50 and 100 suggests that two dissolution profiles are similar and indicates a point-to-point difference of 10% or less (23). From Table I it could be concluded that the release profile of nanoparticles prepared with high MW chitosan is similar to that of nanoparticles prepared with medium MW chitosan, but different with that of nanoparticles prepared with low MW chitosan.
Dissolution efficiency (DE) was calculated from the area under the dissolution curve at time and expressed as percentage of the area of the rectangle described by 100% dissolution in the same time (24–27). Insulin nanoparticles prepared with chitosan of high MW showed higher dissolution efficiency (Table I). Mathematical models have been used extensively for the parametric representation of dissolution data (15,28–30). The data obtained were also put in Korsemeyer-Peppas model in order to find out n value, which describes the drug release mechanism. The n value of nanoparticles of chitosan with different MW was between 0.623–1.437, indicating that the mechanism of the drug release was mass transfer following a non-Fickian model and erosion (31).
CONCLUSION
The effect of molecular weight of chitosan can be significantly observed from different properties and release profiles of insulin. Insulin release from prepared nanoparticles was pH-dependent . As the molecular weight of chitosan increases, the amount of released increased with respect to time. The encapsulating mechanism may be associated with the interactions among oppositely charged chitosan and Eudragit L 100-55 polymer.
Since the nanoparticles could be prepared by using two appositely charged polymers, the need of cross linker and homogenizer should not be considered an absolute requirement for preparation of nanoparticles. This would provide a mild procedure that would be beneficial to prevent protein denaturation. Therefore this complex coacervation approach using chitosan and Eudragit L100-55 polymer may provide a useful approach for entrapment of hydrophilic polypeptides without affecting their conformation.
Acknowledgments
The financial support from the drug applied research center of Tabriz University of Medical Sciences is greatly acknowledged.
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Zakeri-Milani P, Barzegar-Jalali M, Azimi M, Valizadeh H. Biopharmaceutical classification of drugs using intrinsic dissolution rate (IDR) and rat intestinal permeability. Eur J Pharm Biopharm. 2009;73(1):102–106. doi: 10.1016/j.ejpb.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Zakeri-Milani P, Valizadeh H, Tajerzadeh H, Azarmi Y, Islambolchilar Z, Barzegar S, et al. Predicting human intestinal permeability using single-pass intestinal perfusion in rat. J Pharm Pharm Sci. 2007;10(3):368–379. [PubMed] [Google Scholar]
- 3.Lee VH. Enzymatic barriers to peptide and protein absorption. Crit Rev Ther Drug Carrier Syst. 1988;5(2):69–97. [PubMed] [Google Scholar]
- 4.Jelvehgari M, Valizadeh H, Rezapour M, Nokhodchi A. Control of encapsulation efficiency in polymeric microparticle system of tolmetin. Pharm Dev Technol. 2010;15(1):71–79. doi: 10.3109/10837450903002173. [DOI] [PubMed] [Google Scholar]
- 5.Delie F, Berton M, Allemann E, Gurny R. Comparison of two methods of encapsulation of an oligonucleotide into poly(D, L-lactic acid) particles. Int J Pharm. 2001;214(1–2):25–30. doi: 10.1016/S0378-5173(00)00627-X. [DOI] [PubMed] [Google Scholar]
- 6.Li MG, Lu WL, Wang JC, Zhang X, Zhang H, Wang XQ, et al. Preparation and characterization of insulin nanoparticles employing chitosan and poly(methylmethacrylate/methylmethacrylic acid) copolymer. J Nanosci Nanotechnol. 2006;6(9–10):2874–2886. doi: 10.1166/jnn.2006.411. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Pei Y, Zhang X, Gu Z, Zhou Z, Yuan W, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Control Release. 2001;71(2):203–211. doi: 10.1016/S0168-3659(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 8.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70(1–2):1–20. doi: 10.1016/S0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 9.De Campos AM, Sanchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001;224(1–2):159–168. doi: 10.1016/S0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 10.Dyer AM, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, et al. Nasal delivery of insulin using novel chitosan based formulations: a comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm Res. 2002;19(7):998–1008. doi: 10.1023/A:1016418523014. [DOI] [PubMed] [Google Scholar]
- 11.Allemann E, Gurny R, Doelker E. Drug-loaded nanoparticles—Preparation methods and drug targeting issues. Eur J Pharm Biopharm. 1993;39(5):173–191. [Google Scholar]
- 12.De Jaeghere F, Doelker E, Gurny R. Nanoparticles. In: Mathiowitz E, editor. The encyclopedia of controlled drug delivery. New York: Wiley and Sons Inc.; 1999. pp. 641–664. [Google Scholar]
- 13.Allemann E, Leroux J, Gurny R. Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Deliv Rev. 1998;34(2–3):171–189. doi: 10.1016/S0169-409X(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 14.Zeta Potential of Colloids in Water and Waste Water, ASTM Standard D 4187-82, American Society for Testing and Materials, 1985.
- 15.Kilicarslan M, Baykara T. The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int J Pharm. 2003;252(1–2):99–109. doi: 10.1016/S0378-5173(02)00630-0. [DOI] [PubMed] [Google Scholar]
- 16.Fix JA. Oral controlled release technology for peptides: status and future prospects. Pharm Res. 1996;13(12):1760–1764. doi: 10.1023/A:1016008419367. [DOI] [PubMed] [Google Scholar]
- 17.Aboubakar M, Couvreur P, Pinto-Alphandary H, Gouritin B, Lacour B, Farinotti R, et al. Insulin-loaded nanocapsules for oral administration: in vitro and in vivo investigation. Drug Dev Res. 2000;49(2):109–117. doi: 10.1002/(SICI)1098-2299(200002)49:2<109::AID-DDR4>3.0.CO;2-#. [DOI] [Google Scholar]
- 18.Jameela SR, Suma N, Jayakrishnan A. Protein release from poly(epsilon-caprolactone) microspheres prepared by melt encapsulation and solvent evaporation techniques: a comparative study. J Biomater Sci Polym Ed. 1997;8(6):457–466. doi: 10.1163/156856297X00380. [DOI] [PubMed] [Google Scholar]
- 19.Kim BK, Hwang SJ, Park JB, Park HJ. Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J Microencapsul. 2002;19(6):811–822. doi: 10.1080/0265204021000022770. [DOI] [PubMed] [Google Scholar]
- 20.Pignatello R, Consoli P, Puglisi G. In vitro release kinetics of Tolmetin from tabletted Eudragit microparticles. J Microencapsul. 2000;17(3):373–383. doi: 10.1080/026520400288337. [DOI] [PubMed] [Google Scholar]
- 21.Wade A, Weller PJ. Handbook of pharmaceutical excipients. 2. London: Pharmaceutical Society of Great Britain; 1994. [Google Scholar]
- 22.Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. 5. London: Pharmaceutical Press; 2006. [Google Scholar]
- 23.Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20(6):64–74. [Google Scholar]
- 24.Dastmalchi S, Garjani A, Maleki N, Sheikhee G, Baghchevan V, Jafari-Azad P, et al. Enhancing dissolution, serum concentrations and hypoglycemic effect of glibenclamide using solvent deposition technique. J Pharm Pharm Sci. 2005;8(2):175–181. [PubMed] [Google Scholar]
- 25.Barzegar-Jalali M, Nayebi AM, Valizadeh H, Hanaee J, Barzegar-Jalali A, Adibkia K, et al. Evaluation of in vitro-in vivo correlation and anticonvulsive effect of carbamazepine after cogrinding with microcrystalline cellulose. J Pharm Pharm Sci. 2006;9(3):307–316. [PubMed] [Google Scholar]
- 26.Azarmi S, Farid J, Nokhodchi A, Bahari-Saravi SM, Valizadeh H. Thermal treating as a tool for sustained release of indomethacin from Eudragit RS and RL matrices. Int J Pharm. 2002;246(1–2):171–177. doi: 10.1016/S0378-5173(02)00378-2. [DOI] [PubMed] [Google Scholar]
- 27.Nokhodchi A, Okwudarue ON, Valizadeh H, Momin MN. Cogrinding as a tool to produce sustained release behavior for theophylline particles containing magnesium stearate. AAPS Pharmscitech. 2009 Oct 28. [DOI] [PMC free article] [PubMed]
- 28.Yuksel N, Kanik AE, Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and -independent methods. Int J Pharm. 2000;209(1–2):57–67. doi: 10.1016/S0378-5173(00)00554-8. [DOI] [PubMed] [Google Scholar]
- 29.Barzegar-Jalali M, Adibkia K, Valizadeh H, Shadbad MR, Nokhodchi A, Omidi Y, et al. Kinetic analysis of drug release from nanoparticles. J Pharm Pharm Sci. 2008;11(1):167–177. doi: 10.18433/j3d59t. [DOI] [PubMed] [Google Scholar]
- 30.Nokhodchi A, Nazemiyeh H, Khodaparast A, Sorkh-Shahan T, Valizadeh H, Ford JL. An in vitro evaluation of fenugreek mucilage as a potential excipient for oral controlled-release matrix tablet. Drug Dev Ind Pharm. 2008;34(3):323–329. doi: 10.1080/03639040701662594. [DOI] [PubMed] [Google Scholar]
- 31.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–133. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]