Abstract
Aflapin® is a novel synergistic composition derived from Boswellia serrata gum resin (Indian Patent Application No. 2229/CHE/2008). Aflapin is significantly better as an anti-inflammatory agent compared to the Boswellia extracts presently available in the market. A 90-day, double-blind, randomized, placebo-controlled study was conducted to evaluate the comparative efficacy and tolerability of 5-Loxin® and Aflapin® in the treatment of osteoarthritis (OA) of the knee (Clinical trial registration number: ISRCTN80793440). Sixty OA subjects were included in the study. The subjects received either 100 mg (n=20) of 5-Loxin® or 100 mg (n=20) of Aflapin® or a placebo (n=20) daily for 90 days. Each patient was evaluated for pain and physical functions by using the standard tools (visual analog scale, Lequesne's Functional Index, and Western Ontario and McMaster Universities Osteoarthritis Index) at the baseline (day 0), and at days 7, 30, 60 and 90. A battery of biochemical parameters in serum, urine and hematological parameters in citrated whole blood were performed to assess the safety of 5-Loxin® and Aflapin® in OA subjects. Fifty seven subjects completed the study. At the end of the study, both 5-Loxin® and Aflapin conferred clinically and statistically significant improvements in pain scores and physical function scores in OA subjects. Interestingly, significant improvements in pain score and functional ability were recorded as early as 7 days after initiation of the study in the treatment group supplemented with 100 mg Aflapin. Corroborating the improvements in pain scores in treatment groups, our in vitro studies provide evidences that Aflapin® is capable of inhibiting cartilage degrading enzyme MMP-3 and has the potential to regulate the inflammatory response by inhibiting ICAM-1. Aflapin® and 5-Loxin® reduce pain and improve physical functions significantly in OA subjects. Aflapin exhibited better efficacy compared to 5-Loxin®. In comparison with placebo, the safety parameters were almost unchanged in the treatment groups. Hence both 5-Loxin® and Aflapin® are safe for human consumption.
Keywords: Aflapin®, 5-Loxin®, Boswellia serrata, anti-inflammation, osteoarthritis and clinical study.
Introduction
Osteoarthritis (OA) is the commonest form of arthritic disease, characterized by articular cartilage degradation with an accompanying peri-articular bone response 1,2. OA affects nearly 21 million people in the USA, accounting for 25% of visits to primary care physicians. It is estimated that 80% of the population will have radiographic evidence of OA by age 65 years, although only 60% of those will be symptomatic 3. Clinical manifestations of OA of the knee include pain in and around the joint, stiffness of the joint, crepitation on motion and limited joint motion, among others 4. Current recommendations for managing OA focus on relieving pain and stiffness and improving physical function as important goals of therapy 5,6. Currently available medication regimens for most cases include nonopioid analgesics such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) including cyclo-oxygenase II inhibitors. These pharmaceutical agents can reduce both pain and inflammation quite effectively, but long term use of NSAIDs has been found to associate with enhanced risk for gastrointestinal bleeding, hypertension, congestive heart failure and renal insufficiency, among other adverse effects 7-9. Because of the high incidence of adverse events associated with both nonselective and cyclo-oxygenase II selective NSAID therapy, effective and safer alternative treatments for OA are urgently needed. In recent years, the gum resin extracted from the ancient herb, Boswellia serrata has gained lot of attention as a potent anti-inflammatory, anti-arthritic and analgesic agent 10,11. 3-O-acetyl-11-keto-beta-boswellic acid (AKBA) is the most active component of Boswellia extract and has been demonstrated to be a potent inhibitor of 5-lipoxygenase (5-LOX), a key enzyme in the biosynthesis of leukotrienes from arachidonic acid in the cellular inflammatory cascade 12,13. 5-Loxin® is a novel B. serrata extract enriched to 30% AKBA (US Patent publication no.: 2004/0073060A1). Affimatrix gene chip analysis demonstrated that 5-Loxin® can potently inhibit tumor necrosis factor α (TNFα) induced gene expression of matrix metalloproteinases (MMPs), adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1); and mediators of apoptosis in human microvascular endothelial cells 14, 15. Cell based in vitro studies suggest that 5-Loxin® can inhibit pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-1β 16. In the carrageenan-induced inflammation model, 5-Loxin® treatment yielded significant improvement in paw inflammation in albino Wistar rats. 5-Loxin® also exhibited significant Anti-arhtritic efficacy in FCA induced model of Sprague-Dawley rats 14, 15. Extensive acute and dose dependent subchronic safety studies on rats demonstrated that 5-Loxin® is safe even at dose levels 2,000 to 3,000 times higher than the human equivalence dose 17. In addition, 5-Loxin® was found to be non genotoxic as per the standard AMES bacterial reverse mutation assay, chromosomal aberration test in Chinese hamster cells and mouse peripheral blood micronucleus assay 18-21 The efficacy and tolerability of 5-Loxin® was assessed in a previous double blind placebo controlled clinical study. The supplementation of 5-Loxin® was well tolerated and its efficacy against osteoarthritis was found to be statistically significant. The dose dependent efficacy of 5-Loxin® was assessed against pain, joint stiffness, mobility and a cartilage degrading enzyme MMP-3 in OA subjects 22. Aflapin® is a novel synergistic composition derived from Boswellia serrata gum resin (Indian Patent Application No. 2229/CHE/2008). Interestingly it was found that the oral bioavailability of AKBA from Aflapin® was better compared to that of 5-Loxin®. Aflapin exhibited better 5-lipoxygenase inhibitory activity and MMP-3 inhibition. Various in vitro and In vivo studies were performed to compare efficacy of Aflapin and 5-Loxin®. These studies proved Aflapin to be more efficacious compared to 5-Loxin® (to be presented in a separate communication). The broad spectrum safety of Aflapin was tested using a battery of safety studies conducted according to OECD guidelines and it was found to be safe 23. Although a significant number of clinical study reports support the anti-inflammatory and anti-arthritic properties of Boswellia extract 24-27, no human clinical studies were done to prove the efficacy and tolerability of Aflapin in osteoarthritis. Hence in the present clinical study we sought to evaluate the comparative efficacy and tolerability of 5-Loxin® and Aflapin® in the treatment of OA of the knee.
Materials and Methods
Study materials
BE-30 (5-Loxin®) is a novel Boswellia serrata extract standardized to contain at least 30 percent 3-O-Acetyl-11-keto-β-boswellic acid (AKBA) using a selective enrichment process (Indian patent # 205269). The process involves selective enrichment of AKBA while simultaneously suppressing the concentration of triterpene compounds that are less active and those that antagonize the activity of AKBA. Aflapin is a novel synergistic composition containing B. serrata extract selectively enriched with AKBA and B. serrata non-volatile oil. The non-volatile oil was prepared using a special process (PCT application # PCT/IN2009/000505) involving selective removal of Boswellic acids followed by removing volatiles under high vacuum. The composition was standardized to contain at least 20% AKBA.
Study design
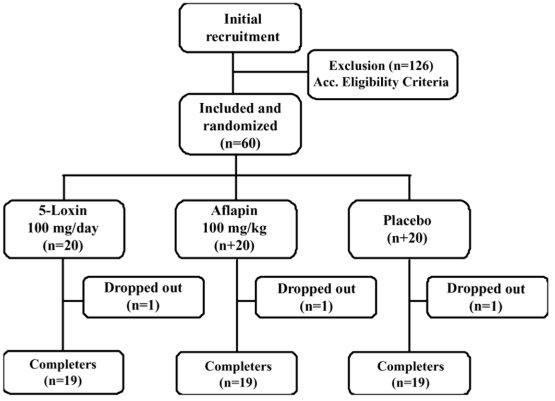
This trial was performed at Alluri Sitarama Raju Academy of Medical Sciences (ASRAM), Eluru, Andhra Pradesh, India from July 2008 to December 2008 (clinical trial registration number: ISRCTN80793440). The study protocol was evaluated and approved by the ASRAM Institutional Review Board (IRB). An overview of the clinical study is provided in Figure 1. Briefly, 186 subjects out of 283 attending the orthopaedic outpatient department of the ASRAM hospital were selected in the first phase of the screening procedure, based on the signs, symptoms and radiological changes consistent with OA. A total of 60 subjects suffering for more than 3 months with medial tibio-femoral OA were selected using inclusion/exclusion criteria summarized in Table 1. All subjects signed the IRB approved consent form. Subjects, who were otherwise healthy, were aged 40 years or older and had a diagnosis of OA, fulfilling the American College of Rheumatology classification criteria 4. After recruitment, the subjects were randomly distributed into three groups. The demographic data and baseline characteristics are summarized in Table 2.
Figure 1.
Flow chart of the subjects who participated in the clinical trial. Evaluations of physical activity and pain scores, serum biochemistry, hematology, and urine biochemistry were done at baseline (day 0) and on days 7, 30, 60 and 90 during follow up.
Table 1.
Inclusion/exclusion criteria
| Criteria | Details |
|---|---|
| Inclusion | Subjects must understand risks and benefits of the protocol and be able to give informed consent |
| Male and female subjects aged 40 to 80 years | |
| Females of child-bearing potential must agree to use an approved form of birth control and to have a negative pregnancy test result | |
| Unilateral or bilateral osteoarthritis of the knee for more than 3 months | |
| Visual analogue scale score during the most painful knee movement between 40 and 70 mm after 7 days of withdrawal of usual medication | |
| Lequesne's Functional Index score greater than 7 points after 7 days of withdrawal of usual medication | |
| Ability to walk | |
| Availability for the duration of the entire study period | |
| Exclusion | History of underlying inflammatory arthropathy or severe rheumatoid arthritis |
| Hyperuricaemia (>440 μmol/l) and/or past history of gout | |
| Recent injury in the area affected by osteoarthritis of the knee (past 4 months) and expectation of surgery in the next 4 months | |
| Intra-articular corticosteroid injections within the preceding 3 months | |
| Hypersensitivity to nonsteroidal anti-inflammatory drugs, abnormal liver or kidney function tests, history of peptic ulceration and upper gastrointestinal hemorrhage, congestive heart failure, hypertension, cancer, hyperkalaemia | |
| Major abnormal findings on complete blood count, history of coagulopathies, hematological or neurological disorders | |
| High alcohol intake (>2 standard drinks per day) | |
| Pregnant, breastfeeding, or planning to become pregnant during the study | |
| Use of concomitant prohibited medication other than ibuprofen | |
| Obesity (body mass index > 30 kg/m2) |
Table 2.
Demographic data and baseline characteristics of the subjects
| Characteristics | Placebo (n = 19) | 100 mg/day 5-Loxin® (n = 19) | 100 mg/day Aflapin® (n = 19) |
|---|---|---|---|
| Sex (male/female; n) | 9/10 | 3/16 | 7/12 |
| Age (years) | 52.4 ± 7.5 | 51.6 ± 9.9 | 53.2 ± 7.9 |
| Body weight (kg) | 62.4 ± 14.9 | 57.7 ± 10.5 | 59.1 ± 7.4 |
| Body mass index (kg/m2) | 25.3 ± 4.4 | 25.1 ± 3.8 | 25.2 ± 3.0 |
| Visual analog score | 47.7 ± 6.5 | 48.2 ± 6.1 | 47.7 ± 7.3 |
| Lequesne's Functional Index | 12.3 ± 2.8 | 12.4 ± 2.6 | 12.0 ± 2.4 |
| WOMAC scores | |||
| Pain subscale | 44.7 ± 11.5 | 46.1 ± 7.6 | 45.0 ± 13.3 |
| Stiffness subscale | 39.5 ± 11.2 | 39.5 ± 11.2 | 39.5 ± 13.3 |
| Function subscale | 42.0 ± 10.3 | 43.1 ± 7.8 | 42.0 ± 8.4 |
Before study enrollment, subjects were required to be taking an NSAID at prescription strength for at least 30 days or acetaminophen 1,200 to 4,000 mg/day on a regular basis (at least 25 of the preceding 30 days) with a history of therapeutic benefit. Eligibility requires subjects to meet specific flare criteria upon medication washout. At screening, subjects had to demonstrate a visual analog scale (VAS) score between 40 and 70 mm during the most painful knee movement, and Lequesne's Functional Index (LFI) score greater than 7 points after 7-day withdrawal of usual medication.
A total of 60 selected subjects with symptoms of moderate to mild OA were recruited into the study. Each subject was randomly assigned to a treatment group using a randomization table generated using validated computer software CODE; IDV, Gauting, Germany. The clinical trial pharmacist and statistician ensured that treatment codes remained confidential. The subjects were distributed into three groups: placebo (n=20); 5-Loxin® group, in which subjects received 50 mg encapsulated 5-Loxin® twice daily (n=20); and Aflapin group, in which subjects received 50 mg encapsulated Aflapin® twice daily (n=20). Subjects in the placebo group received two capsules of similar color, taste and appearance but filled with suitable exicipient. Each subject completed a questionnaire, providing details regarding demographics, medical history and nutritional status, at the baseline evaluation and during the follow-up evaluations on days 7, 30, 60 and 90. At the baseline evaluation, and at each visit during the 90-day follow up period, all subjects were assessed for pain and physical function using validated pain scores. Various parameters of serum biochemistry, hematology and urine analysis were carried out on each evaluation day. Safety was monitored by clinical and laboratory assessments conducted during the study visits and subject-reported adverse experiences.
Functional disability and pain score evaluation
Functional disability was assessed by the investigators at baseline and on each follow-up visit (days 7, 30, 60 and 90). Questionnaire-based assessment of pain, stiffness and physical function were done using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) index 28, LFI 29 and VAS 30. The WOMAC index produces scores for three subscales: pain, stiffness and physical function. The pain, stiffness and function subscales of the WOMAC were normalized to a scale of 0 to 100 units (NU) 31. The pain subscale was the average of the first five questions of WOMAC and measured using the NU scale from 0 ('no pain') to 100 ('extreme pain') for each question. The stiffness subscale was the average of questions 6 and 7, measured using the NU scale from 0 ('no stiffness') to 100 ('extreme stiffness') for each question. The physical function subscale was the average of questions 8 through 24 of the WOMAC and measured by NU scale from 0 ('no difficulty') to 100 ('extreme difficulty') for each question. Analyses of these end-points were based upon the time-weighted average change from baseline over 90 days.
Hematological and biochemical evaluations
For assessment of safety of 5-Loxin® and Aflapin®, several parameters were evaluated in serum, urine and whole blood of all subjects at each visit of the study duration. Serum biochemical parameters and hematological parameters were measured using an automated analyzer (HumaStar 300) and a hematological counter (Humacount, Human, Wiesbaden, Germany). The urine analysis was carried out using UroColor™10 Dip Sticks and Urometer 600 (Standard Diagnostics, Kyonggi-do, Korea) and by sediment analysis using microscopy.
In vitro studies to identify mechanisms of actions of Aflapin: Effect on expression of ICAM-1 and MMP3
Adhesion molecule (ICAM-1) expression on endothelial cells: 20,000 Endothelial cell (HDMEC, Lonza Inc., USA) per well in quadruplicate wells were treated with medium, vehicle, TNFα (20ng/ml), TNFα (20ng/ml) with 5-Loxin® or Aflapin® (4μg/ml each) for 24 hour then ICAM-1 ELISA was performed on fixed cells of these wells as per our established protocol 32.
Effect on secretion of MMP3 in TNFα induced human chondrocyte: Human primary Chondrocytes (HCH) was procured from Promo Cell GmbH (Heidelberg, Germany). HCH cells were cultivated in the growth medium (Ready-to-use; Promo Cell, Catalog number C-27101) supplemented with Supplement Mix (Promo Cell, Catalog number C-39635). Equal number of HCH cells was plated in each well of 96-well cell culture plate. Cells were treated with 5 ng/ml of TNFα in presence or absence of different concentrations of 5-Loxin® or Aflapin for 24h. Vehicle control cultures received 0.01% DMSO (v/v). MMP-3 was quantitatively measured in the cell culture supernatant by human MMP-3 EIA kit (R&D Systems, USA) following manufacturer's instructions.
Rescue medication
Subjects were prescribed ibuprofen 400 mg tablets (maximum 400 mg thrice daily; total 1,200 mg) as rescue analgesia during the study based on pain intensity reported to the study physician by the patient. However, the subjects were instructed not to take medicine at least 3 days before each evaluation. No other pain relieving interventions were allowed during the study period.
Statistical analysis
Detailed statistical analyses were performed using SAS software to evaluate the efficacy of 5-Loxin® and Aflapin® in comparison with the placebo group in terms of improvement in pain and physical function scores at baseline and on days 7, 30, 60 and 90 of treatment and serum MMP-3 levels at baseline and on day 90 of treatment. Pair-wise changes were examined by carrying out a least significant difference test for all possible pairs. The significance of the effects of the treatment groups was compared by using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. Results with P<0.05 are considered statistically significant. This is a three-arm (5-Loxin®, Aflapin® and placebo), randomized, double-blind, placebo-controlled, single-centre trial conducted over 90 days. The trial's primary objective was to determine the effects of 5-Loxin® and Aflapin® on pain, physical function and joint stiffness. For power calculations, the estimates for variability and assumed mean changes for each treatment group were based on results from previous placebo-controlled studies of celecoxib, etoricoxib and rofecoxib conducted in subjects with OA 33-36. We believe that an intervention that gives an average improvement of mean change ± 1 standard deviation, rather than mean change alone will provide results of greater significance 37. Our trial is designed to have more than 80% power to detect a situation in which either active drug dosage yields an improvement to at least mean change ± 0.9 standard deviation, under a conservative assumption, and we tested differences between groups in mean improvement using ANOVA (α=0.05, two-sided). With 20 subjects per group, we would have a 93% chance of observing at least one example of any side effect occurring in 10% or more of the patient population at a specific dosage.
Results
Baseline characteristics
Descriptive statistics comparing demographic variables, baseline disease characteristics and baseline outcome measures (LFI, VAS, WOMAC pain, function and stiffness sub-scores) are provided in Table 2. Overall, the treatment groups receiving 5-Loxin® 100 mg/day, n=19, Aflapin® 100 mg/day, n=19 and placebo n=19, were similar with respect to age, Body Mass Index and pain severity (Table 2). The subjects were randomly distributed into three groups.
Clinical efficacy
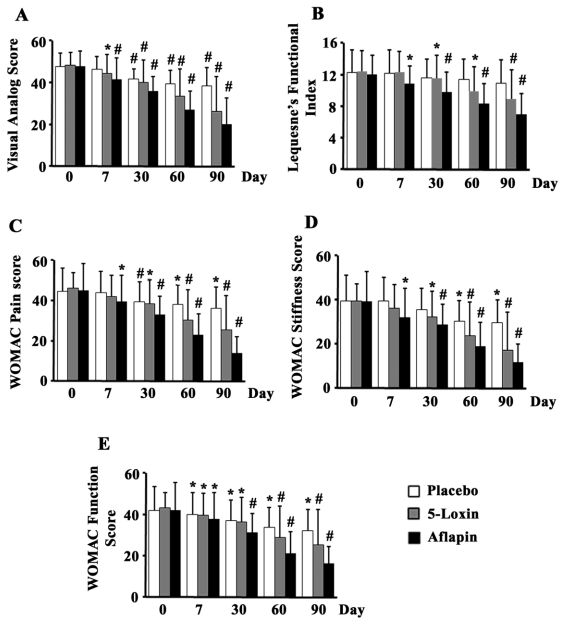
We compared the scores between the treatment groups obtained at day 90. Both the treatments with 5-Loxin® and Aflapin® conferred clinically and statistically significant improvements in pain scores and physical ability scores in OA subjects between baseline and day 90 (Table 3). Tukey's multiple comparison test revealed statistically significant improvements by 31.6% (P=0.006), 30.3% (P=0.009) and 42.2% (p=0.006) in VAS, WOMAC pain, and WOMAC stiffness scores, respectively, in the 100 mg 5-Loxin® treated group in comparison with the placebo group (Table 3). Improvements by 18.35% (P=0.060) and 21.25% (P=0.078) in LFI and WOMAC functional ability scores, respectively were also achieved in the 5-Loxin® group (Table 3). In comparison with the placebo group, the Aflapin® 100 mg treated group also exhibited statistically significant improvements in all parameters tested (Table 3). The Aflapin group showed improvements by 47.3% (P<0.0001), 35.8% (P=0.0004), 61.7% (P<0.0001), 60.1% (P=0.0001) and 49.4% (P=0.0001) in VAS, LFI, WOMAC pain, WOMAC stiffness and WOMAC functional ability scores, respectively. It is worth noting that both 5-Loxin® and Aflapin® treatment groups exhibited improvement in pain scores and physical ability scores as early as 7 days after the start of treatment, and these indices continued to improve throughout the 90 days of treatment (Figure 2). After 7 days, the 5-Loxin® treatment group exhibited 8.09% (P=0.002), 8.68% (P=0.031) and 8.35% (p=0.015) reductions in VAS, WOMAC pain and WOMAC function respectively, compared with the corresponding baseline scores. After 7 days, the Aflapin treatment group exhibited 12.8% (P=0.0004), 9.17% (P=0.003), 11.78% (P=0.012), 18.48% (P=0.012) and 10.24% (p=0.005) reductions in VAS, LFI WOMAC pain, WOMAC stiffness and WOMAC function scores respectively, compared to the corresponding baseline scores (Figure 2).
Table 3.
Student's t-test (paired) analyses for comparison of the scores obtained from the Aflapin and 5-Loxin groups at day 90
| n | Baseline | Day 90 | 95% CI (versus placebo) | p | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Visual analogue scale score | |||||||
| Placebo | 19 | 47.7 | 6.5 | 38.3 | 9.0 | 34.0, 42.7 | 0.0013 |
| 5-Loxin 100 mg/day | 19 | 48.2 | 6.1 | 26.2 | 16.5 | 18.2, 34.1 | <0.0001 |
| Aflapin 100 mg/day | 19 | 47.7 | 7.3 | 20.2 | 12.3 | 14.2, 26.1 | <0.0001 |
| Lequesne's Functional Index | |||||||
| Placebo | 19 | 12.3 | 2.8 | 10.9 | 3.0 | 9.4, 12.3 | 0.0496 |
| 5-Loxin 100 mg/day | 19 | 12.4 | 2.6 | 8.9 | 3.7 | 7.1, 10.7 | <0.0001 |
| Aflapin 100 mg/day | 19 | 12.0 | 2.4 | 7.0 | 2.6 | 7.1, 9.6 | <0.0001 |
| WOMAC pain subscale | |||||||
| Placebo | 19 | 44.7 | 11.5 | 36.3 | 10.5 | 31.2, 41.4 | 0.0021 |
| 5-Loxin 100 mg/day | 19 | 46.1 | 7.6 | 25.3 | 17.2 | 17.0, 33.6 | <0.0001 |
| Aflapin 100 mg/day | 19 | 45.0 | 13.3 | 13.9 | 8.3 | 10.0, 17.9 | <0.0001 |
| WOMAC stiffness subscale | |||||||
| Placebo | 19 | 39.5 | 11.2 | 29.6 | 9.5 | 25.0, 34.2 p | 0.0059 |
| 5-Loxin 100 mg/day | 19 | 39.5 | 11.2 | 17.1 | 16.8 | 9.0, 25.2 | 0.0001 |
| Aflapin 100 mg/day | 19 | 39.5 | 13.3 | 11.8 | 12.8 | 5.7, 18.0 | <0.0001 |
| WOMAC function subscale | |||||||
| Placebo | 19 | 42.0 | 10.3 | 32.0 | 10.8 | 26.8, 37.2 | 0.0025 |
| 5-Loxin 100 mg/day | 19 | 43.1 | 7.8 | 25.2 | 15.0 | 17.9, 32.4 | <0.0001 |
| Aflapin 100 mg/day | 19 | 42.0 | 8.4 | 16.2 | 8.1 | 12.3, 20,1 | <0.0001 |
CI, confidence interval; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 2.
Bar diagrams represent the mean scores of (a) visual analog scale (VAS) (a); Lequesne's Functional Index (LFI) (b); Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)-pain (c); WOMAC-stiffness (d); and WOMAC-function (e) in placebo, 100 mg/ day 5-Loxin® and 100 mg/day Aflapin® groups, respectively. 1 to 5, represent days of evaluations such as day 0, day 7, day 30, day 60 and day 90, respectively. Each bar represents mean ± standard deviation. In comparison with corresponding baseline data, the change in scores in the treatment groups was tested for significance using Tukey's multiple comparison test; * p<0.05; ** p<0.005.
Aflapin inhibits secretion of MMP-3 in TNFα-induced human primary chondrocytes
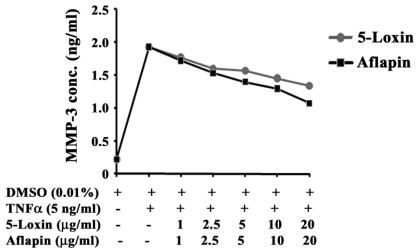
In OA, the loss of collagen from articular cartilage is proportional to the disease severity (38). Under the influence of pro-inflammatory cytokines, increased production and secretion of collagenases such as MMP-3, MMP-13 is the crucial event for enhanced collagen degradation in OA 39. Therefore, we sought to evaluate whether 5-Loxin and Aflapin can modulate MMP-3 secretion in TNFα, a potent pro- inflammatory cytokine induced human primary chondrocytes. Figure 3 shows a steep increase in MMP-3 secretion in TNFα-induced chondrocytes and dose-dependent inhibition of MMP-3 secretion in 5-Loxin and Aflapin treated cultures. Interestingly, we observed, Aflapin (IC50 at 18.5 μg/ml) provided (41.36%) better efficacy than 5-Loxin (IC50 at 31.71 μg/ml) in inhibiting MMP-3 secretion from TNFα-induced human chondrocytes.
Figure 3.
Aflapin and 5-Loxin inhibit matrix metalloproteinase-3 secretion from TNFα-induced human primary chondrocytes. Line diagram represents MMP-3 concentrations in the culture supernatants of chondrocytes treated with 5 ng/ml of human recombinant TNFα in presence or absence of different doses of either 5-Loxin or Aflapin as indicated. Vehicle control cultures received 0.01% DMSO. Each data point represents the mean of quadruplicate wells.
Aflapin inhibits ICAM-1 expression in activated endothelial cells
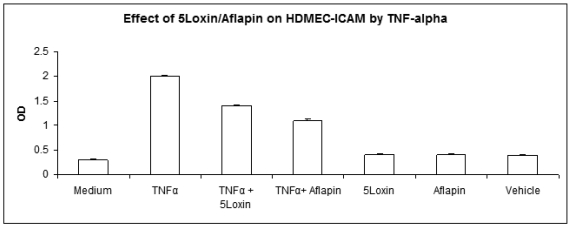
OA is a degenerative joint disorder. However, there are migrations of inflammatory cells in the synovial fluid. Adhesion molecule expression on endothelial cells helps in the diapedesis of these cells. Therefore, in order to determine whether 5-Loxin® and Aflapin® treatments can ameliorate the ICAM-1 expression, we evaluated the ICAM levels on HDMEC. Figure 4 depicts that 5-Loxin® and Aflapin® significantly reduce TNFα induced ICAM-1 expression (p<0.01, student t-test). Interestingly, Aflapin® shows more capability to reduce ICAM-1 secretion than that of 5-Loxin®.
Figure 4.
Aflapin and 5-Loxin inhibit TNFα-induced ICAM-1 expression on human dermal microvascular endothelial cells (HDMEC). Bar diagrams represent the ICAM-1 expression on HDMEC treated with 20ng/ml of human recombinant TNF-α in presence or absence of either 5-Loxin (4µg/ml) or Aflapin (4µg/ml) as indicated. Vehicle control cultures received 0.01% DMSO. Each experiment is done in quadruplicate wells. The results are expressed as the mean±SD of five experiments in quadruplicate wells. 5-Loxin and Aflapin significantly inhibits ICAM-1 expression induced by TNF-α (p<.01, student t-test).
Biochemical evaluations
As a part of the safety evaluation, laboratory tests were performed to evaluate different biochemical parameters (serum and urine) and hematological parameters. The significance of the differences between baseline and 90 days was tested by using repeated measures ANOVA. The f ratio is considered significant if P<0.05. Although minor changes were observed in some of the parameters, they remained within the normal laboratory range. Statistical analyses of these parameters did not indicate any significant changes. Similarly, no significant changes in hematological and urinary parameters were observed in the active treatment groups when compared to the placebo (data not shown). These findings further demonstrate the safety of 5-Loxin® and Aflapin® in humans.
Adverse Events and Dropouts
During the course of the 90-day study, no major adverse events were reported. However, acidity was reported as a minor adverse event by two subjects during the study; one each from placebo and Aflapin supplemented groups, respectively.
Three subjects one from each placebo, 5-Loxin® and Aflapin® supplemented groups were dropped out from the study due to their un-availability during the entire study period.
Discussion
This is the first clinical study to evaluate the efficacy of Aflapin® in OA subjects. Aflapin is a novel synergistic composition comprising AKBA enriched B. serrata extract and non acidic gum extract of B. serrata. In a battery of preclinical studies designed in in vitro cellular models and in vivo animal models, Aflapin exhibited significantly better anti-inflammatory activities in comparison with 5-Loxin® (Data to be presented in a separate communication). 5-Loxin® is a Boswellia serrata extract standardized to 30% AKBA. Its multidirectional activities related to anti-inflammatory efficacies obtained in appropriate cellular, animal models and in human subjects have established that 5-Loxin® is a potent dietary supplement for the management of inflammatory diseases such as osteoarthritis 14-22. In a series of experiments designed in in vitro cellular and in vivo animal models, Aflapin showed significantly better efficacy in comparison with 5-Loxin®. In addition, Aflapin exhibited better AKBA bioavailability than 5-Loxin® in Wistar rat model. Broad spectrum safety of Aflapin was also established in a battery of acute and sub-acute toxicity studies in rat and rabbits. These findings altogether motivated us to evaluate efficacy of Aflapin in comparison with 5-Loxin® against osteoarthritis in human subjects. In the present 90-day clinical study, we assessed the efficacy and tolerability of Aflapin in comparison with 5-Loxin® in OA subjects. Pain, stiffness of joints, reduced joint movement and physical disability are the major clinical manifestations of OA 1,40,41. Our study demonstrates that Aflapin potentially improves pain, joint stiffness and physical function in OA subjects (Figure 2). In order to check improvements in the treatment groups, we compared the data for all parameters between the baseline and day 90. Paired t-test revealed that both treatment groups showed statistically significant improvements in all parameters.
Compared to the placebo, 5-Loxin® supplementation for 90 days, significantly reduced VAS, WOMAC-pain, WOMAC-stiffness (Table 3), which are consistent with our previous observations 22. Whereas, Aflapin supplementation for 90 days, resulted in significant reduction in all pain scores tested in comparison with placebo. These findings suggest that Aflapin has better therapeutic efficacy against OA compared to 5-Loxin®. We observed that, in comparison with baseline, there were downward trends in VAS score and WOMAC scores in the placebo group. We believe that this might be partly attributable to the placebo effect 42,43 manifested while administering the questionnaires to placebo subjects and partly due to the consumption of ibuprofen as rescue medication by more subjects in the placebo group during the study. It is noteworthy that 5-Loxin® possesses significant efficacy in lowering VAS score by 8.09% (P=0.022), WOMAC pain score by 8.68% (0.031) and WOMAC function score by 8.35% (P<0.015) in OA subjects as early as 7 days after the initiation of treatment. In comparison, Aflapin showed significant reduction in all the pain scores assessed including VAS score by 12.8% (P=0.0004), LFI score by 9.17% (P=0.003), WOMAC pain score by 11.78% (P=0.012), WOMAC stiffness score by 18.48% (P=0.012) and WOMAC function score by 10.24% (P=0.005) (Figure 2). These findings therefore indicate that 5-Loxin® and Aflapin® confers prompt and significant pain relief, improvement in physical ability and quality of life in OA subjects. However, Aflapin showed better reduction in all the tested pain scores and hence can be considered superior to 5-Loxin®.
Pathogenesis of osteoarthritis is a complex process. These include mechano-transduction, the interplay between metalloproteases (MMP3, MMP13), protease inhibitors and cytokines on cartilage degradation and mechanisms of cartilage repair 40,44,45. MMP-3 is over-expressed in OA and cause degeneration of cartilage tissue 44,45. Cytokines act via autocrine and endocrine functions to alter cartilage homeostasis. Interleukin-1 (IL-1) and TNF-α are perhaps the best characterized cytokines for cartilage degradation (46,47). They are synthesized by chondrocytes and FLS. These cytokines act in various ways in the pathogenesis of OA such as inhibition of synthesis of type 2 (articular) cartilage and activation of catabolic metalloproteases including MMP-3 which plays a critical role in cartilage degradation 44,45. A role of synovitis in OA can't be disregarded either. It is a well established clinical observation that pain and swelling in OA improves for months following intra-articular corticosteroid injection. In addition, histologic studies suggest that localized inflammatory changes characterized by foci of inflammatory cells occur in up to 50% of OA patients 48. In this study to find out possible mechanism of actions of Aflapin we carried out in vitro studies to evaluate whether Aflapin can inhibit metalloprotease secretion or influence the inflammatory component of osteoarthritis. We observed: (1) Aflapin inhibits TNFα induced MMP-3 secretion in chondrocytes; (2) Aflapin inhibits TNFα induced expression of ICAM-1 in endothelial cells.
Overall, the foregoing data together demonstrates the better ability of Aflapin compared with 5-Loxin® in terms of reducing the pain, improving physical function, quality of life and joint health. Presumably these improvements might occur through down regulation of cartilage degrading enzymes such as MMP-3 in OA subjects. The present study also demonstrates no major changes in the hematological parameters, serum biochemical parameters and in urine analysis in the treatment groups compared to placebo. In addition, no major adverse effect was reported by the subjects in the treatment groups. Taken together, these observations further demonstrate that 5-Loxin® and Aflapin® are potentially safe in the treatment of OA in humans and more specifically Aflapin® is more efficacious in the management of osteoarthritis than 5-Loxin®.
Conclusion
In summary, the present study provides the evidence in support of the potential efficacy and tolerability of 5-Loxin® and Aflapin® in subjects with OA; 5-Loxin® and Aflapin significantly improved joint function. Aflapin exhibited better therapeutic efficacy over 5-Loxin® at 100 mg/day; it reduces pain rapidly, as early as after 1 week of treatment. Furthermore, in vitro studies also provide evidences that compared to 5-Loxin, Aflapin is capable of inhibiting cartilage degrading enzyme MMP-3 and has the potential to regulate the inflammatory component in by inhibiting ICAM-1. Most importantly, we have observed that 5-Loxin® and Aflapin® are safe for human consumption, even for long term supplementation. 5-Loxin® and Aflapin® are promising alternative therapeutic options, that may be used as nutritional supplements for management of OA.
Authors' contributions
KS contributed to the design of the project and data analysis, and was primarily responsible for writing the manuscript. KVA contributed to the design of the project, patient recruitment and management, and data collection. ARS and AM worked with subjects to obtain informed consent, conducted clinical evaluations, took samples and evaluated therapeutic response of 5-Loxin® and Aflapin®. TG contributed as the study coordinator and helped to review the manuscript. KVSS and DD helped in clinical data analysis. SMR helped in designing and executing the mechanisms of action studies. SPR helped in designing the study, conducting data analysis and writing the manuscript.
Acknowledgments
We sincerely thank Sri G. Ganga Raju, Chairman; Mr. G Rama Raju, Director; and Mr. B. Kiran, CEO of Laila Group of Industries, India for generous support and encouragements. This study was supported by Laila Nutraceuticals, Vijayawada, India.
Abbreviations
- AKBA
3-O-acetyl-11-keto-beta-boswellic acid
- ANOVA
analysis of variance
- ASRAM
Alluri Sitarama Raju Academy of Medical Sciences
- BMI
Body Mass Index
- ELISA
enzyme-linked immunosorbent assay
- LFI
Lequesne's Functional Index
- MMP
matrix metalloproteinase
- NSAID
nonsteroidal anti-inflammatory drug
- NU
normalized units
- OA
osteoarthritis
- VAS
visual analog scale
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index.
References
- 1.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin. North. Am. 2004;42:1–9. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Green GA. Understanding NSAIDs: from aspirin to COX-2. Clin Cornerstone. 2001;3:50–60. doi: 10.1016/s1098-3597(01)90069-9. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38:1541–1546. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 5.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, Cluzeau F, Cooper C, Dieppe PA, Günther KP, Hauselmann HJ, Herrero-Beaumont G, Kaklamanis PM, Leeb B, Lequesne M, Lohmander S, Mazieres B, Mola EM, Pavelka K, Serni U, Swoboda B, Verbruggen AA, Weseloh G, Zimmermann-Gorska I. EULAR recommendations for the management of osteoarthritis: report of task force standing committee for International Clinical Studies including Therapeutic Trials (ESCISIT) Ann Rheum. Dis. 2000;59:936–944. doi: 10.1136/ard.59.12.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am. J. Med. 1998;105:31S–38S. doi: 10.1016/s0002-9343(98)00072-2. [DOI] [PubMed] [Google Scholar]
- 8.Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug associated gastrointestinal injury. Am. J. Med. 1998;104:23S–29S. doi: 10.1016/s0002-9343(97)00207-6. [DOI] [PubMed] [Google Scholar]
- 9.Wright JM. Double-edged sword of COX-2 selective NSAIDs. CMAJ. 2002;167:1131–1137. [PMC free article] [PubMed] [Google Scholar]
- 10.Singh GB, Atal CK. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986;18:407–412. doi: 10.1007/BF01965005. [DOI] [PubMed] [Google Scholar]
- 11.Ethan B, Heather B, Theresa DH, Ivo F, Sadaf H, Jens H, David S, Catherine U. Boswellia: An evidence-based systematic review by the natural standard research collaboration. J Herbal Pharmacother. 2004;4:63–83. [PubMed] [Google Scholar]
- 12.Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon HPT. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol. Exp. Ther. 1992;26:1143–1146. [PubMed] [Google Scholar]
- 13.Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HPT, Safayhi H. Acetyl-11-keto-β-boswellic acid (AKBA): structure requirements or binding and 5-lipoxygenase inhibitory activity. Br. J Pharmacol. 1996;117:615–618. doi: 10.1111/j.1476-5381.1996.tb15235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy S, Khanna S, Shah H, Rink C, Phillips C, Preuss H, Subbaraju GV, Trimurtulu G, Krishnaraju AV, Bagchi M, Bagchi D, Sen CK. Human genome screen to identify the genetic basis of the anti-inflammatory effects of Boswellia in microvascular endothelial cells. DNA Cell Biol. 2005;24:244–255. doi: 10.1089/dna.2005.24.244. [DOI] [PubMed] [Google Scholar]
- 15.Roy S, Khanna S, Krishnaraju AV, Subbaraju GV, Yasmin T, Bagchi D, Sen CK. Regulation of vascular response to inflammation: Inducible Matrix Metalloprotenase-3 expression in Human Microvascular Endothelial Cells is sensitive to anti-inflammatory boswellia. Antioxidant and Redox Signaling. 2006;8:653–660. doi: 10.1089/ars.2006.8.653. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta K, Golakoti T, Marasetti A K, Tummala T, Ravada S R, Krishnaraju AV, Raychaudhuri SP. Inhibition of TNFα Production and Blocking of Mitogen-Activated Protein Kinase/NFkB Activation In Lipopolysaccharide-Induced THP-1 Human Monocytes By 3-O-Acetyl-11-Keto-β-Boswellic Acid. J Food Lipids. 2009;16:325–344. [Google Scholar]
- 17.Lalithakumari K, Krishnaraju AV, Sengupta K, Subbaraju GV, Chatterjee A. Safety and toxicological evaluation of a novel, standardized 3-O-acetyl-11-keto-β-boswellic acid (AKBA)-enriched Boswellia serrata extract (5-Loxin®) Toxicol. Mech. Meth. 2006;16:199–226. doi: 10.1080/15376520600620232. [DOI] [PubMed] [Google Scholar]
- 18.Indrani BK. Bacterial reverse mutation test with 5-Loxin®; Study No.4477/05, Toxicology Department, Advinus Therapeutics Private Limited, Post Box No.5813, Plot Nos 21 & 22, Peenya Industrial Area, Phase II, Bangalore, India 560 058. Bangalore, India: Advinus Therapeutics Private Limited; 2006. [Google Scholar]
- 19.Chang JT. Micronucleus assay in mice 5-Loxin®; Study number MN00075, Center of Toxicology and Preclinical Sciences, Development Center for Biotechnology, 101, Lane 169, Kangning St, Xizhi City, Taipei County 221, Taiwan. Taiwan: Development Center for Biotechnology; 2007. [Google Scholar]
- 20.Jung-Ti Chang. Chromosomal abberation test; Study number CA00094, Center of Toxicology and Preclinical Sciences, Development Center for Biotechnology, 101, Lane 169, Kangning St, Xizhi City, Taipei County 221, Taiwan. Taiwan: Development Center for Biotechnology; 2007. [Google Scholar]
- 21.Trimurtulu G, Sen CK, Krishnaraju AV, Sengupta K. In: Bagchi D, Lau FC, Bagchi M, eds. Genomic basis of anti-inflammatory properties of Boswellia extract: Genomics, Proteomics and Metabolomics in Nutrational and Functional Foods. MA: Wiley-Blackwell; 2010. pp. 155–174. [Google Scholar]
- 22.Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, Sarma KV, Dey D, Raychaudhuri SP. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008;10(4):R85. doi: 10.1186/ar2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnaraju AV, Sundararaju D, Vamsikrishna U, Suryachandra R, Machiraju G, Sengupta K, Trimurtulu G. Safety and Toxicological Evaluation of Aflapin®: a Novel Boswellia- Derived Anti-inflammatory Ingredient. Toxicol Mechanisms Methods. 2010 doi: 10.3109/15376516.2010.497978. in press. [DOI] [PubMed] [Google Scholar]
- 24.Joos S, Rosemann T, Szecsenyi J, Hahn EG, Willich SN, Brinkhaus B. Use of complementary and alternative medicine in Germany- a survey of subjects with inflammatory bowel disease. BMC Comp. Altern. Med. 2006;6:19. doi: 10.1186/1472-6882-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Muller S, Senninger N, Russell J, Jauch J, Bergmann J, Granger DN, Krieglstein CF. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am. J Physiol. Gastrointest. Liver Physiol. 2006;290:G1131–G1137. doi: 10.1152/ajpgi.00562.2005. [DOI] [PubMed] [Google Scholar]
- 26.Gupta I, Parihar A, Malhotra P, Gupta S, Ludtke R, Safayhi H, Ammon HP. Effects of gum resin of Boswellia serrata in subjects with chronic colitis. Planta Med. 2001;67:391–395. doi: 10.1055/s-2001-15802. [DOI] [PubMed] [Google Scholar]
- 27.Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee: a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3–7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy N, Buchnan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in subjects with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 29.Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee validation-value in comparison with other assessment tests. Scand J Rheumatology. 1987;65:85–89. doi: 10.3109/03009748709102182. [DOI] [PubMed] [Google Scholar]
- 30.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 31.Bellamy N, Bell MJ, Goldsmith CH, Pericak D, Walker V, Raynauld JP, Torrance GW, Tugwell P, Polisson R. The effectiveness of hylan G-F 20 in subjects with knee osteoarthritis: an application of two sets of response criteria developed by the OARSI and one set developed by OMERACT-OARSI. Osteoarthritis Cartilage. 2005;13:104–110. doi: 10.1016/j.joca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Raychaudhuri SK, Raychaudhuri SP, Weltman H, Farber EM. Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch Dermatol Res. 2001;293(6):291–295. doi: 10.1007/s004030100224. [DOI] [PubMed] [Google Scholar]
- 33.Day R, Morrison B, Luza A, Castaneda O, Strusberg A, Nahir M, Helgetveit KB, Kress B, Daniels B, Bolognese J, Krupa D, Seidenberg B, Ehrich E. A randomized trial of the efficacy and tolerability of the COX-2 inhibitor rofecoxib vs ibuprofen in subjects with osteoarthritis. Arch. Intern. Med. 2000;160:1781–1787. doi: 10.1001/archinte.160.12.1781. [DOI] [PubMed] [Google Scholar]
- 34.Gottesdiener K, Schnitzer T, Fisher C, Bockow B, Markenson J, Ko A, DeTora L, Curtis S, Geissler L, Gertz BJ. Results of a randomized, dose-ranging trial of etoricoxib in subjects with osteoarthritis. Rheumatology (Oxford) 2002;41:1052–1061. doi: 10.1093/rheumatology/41.9.1052. [DOI] [PubMed] [Google Scholar]
- 35.Bingham CO, Sebba AI, Rubin BR, Ruoff GE, Kremer J, Bird S, Smugar SS, Fitzgerald BJ, O'Brien K, Tershakovec AM. Efficacy and safety of etoricoxib 30 mg and celecoxib 200 mg in the treatment of osteoarthritis in two identically designed, randomized, placebo-controlled, non-inferiority studies. Rheumatology (Oxford) 2007;46:496–507. doi: 10.1093/rheumatology/kel296. [DOI] [PubMed] [Google Scholar]
- 36.Reginster JY, Malmstrom K, Mehta A, Bergman G, Ko AT, Curtis SP, Reicin AS. Evaluation of the efficacy and safety of etoricoxib compared with naproxen in two, 138-week randomized studies of osteoarthritis subjects. Ann. Rheum. Dis. 2007;66:945–951. doi: 10.1136/ard.2006.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power analysis for ANOVA designs. http://www.math.yorku.cal/SCS/Online/power/
- 38.Pelletier JP, Martel-Pelletier J, Howell DS, Ghandur-Mnaymneh L, Enis JE, Woessner JF Jr. Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983;26:63–68. doi: 10.1002/art.1780260110. [DOI] [PubMed] [Google Scholar]
- 39.Poole AR. Arthritis and Allied Conditions: a Textbook of Rheumatology. Baltimore: Williams and Wilkins; 1997. [Google Scholar]
- 40.Garnero P, Rousseau J, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage and synovium in joint diseases. Arthritis Rheum. 2000;43:953–968. doi: 10.1002/1529-0131(200005)43:5<953::AID-ANR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Felson DT. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 42.Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA. 1994;271:1609–1614. [PubMed] [Google Scholar]
- 43.Walach H, Sadaghiani C, Dehm C, Bierman D. The therapeutic effect of clinical trials: understanding placebo response rates in clinical trials: a secondary analysis. BMC Med Res Methodol. 2005;5:26–37. doi: 10.1186/1471-2288-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 45.Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery CR, Caterson B. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 46.Campbell IK, Piccolo DS, Roberts MJ. et al. Effects of tumour necrosis factor-alpha and beta on resorption of human articular cartilage and production of plasminogen activator by human articular chondrocytes. Arthritis Rheum. 1990;33:542. doi: 10.1002/art.1780330412. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52(1):128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 48.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]