Abstract
This review summarizes recent findings on the metabolism and biological functions of saturated fatty acids (SFA). Some of these findings show that SFA may have important and specific roles in the cells. Elucidated biochemical mechanisms like protein acylation (N-myristoylation, S-palmitoylation) and regulation of gene transcription are presented. In terms of physiology, SFA are involved for instance in lipogenesis, fat deposition, polyunsaturated fatty acids bioavailability and apoptosis. The variety of their functions demonstrates that SFA should no longer be considered as a single group.
Keywords: Dietary saturated fatty acids, Myristic acid, Metabolism, N-myristoylation
Introduction
Observational studies have shown that high intake of saturated fatty acids (SFA) is positively associated with increased levels of blood cholesterol and high coronary heart disease mortality rates [1, 2]. However, more recent studies have shown inverse association [3, 4], and the meta-analysis by Siri-Tarino et al. [5] reopened the debate when writing that “there is no significant evidence for concluding that dietary saturated fat is associated with an increased risk of CHD or CVD”. Without entering this interesting epidemiological debate on the deleterious effects of some of saturated fatty acids, it seems that they can be no longer considered as a single group in terms of structure, metabolism [6, 7] and cellular function. In this context, this review will focus only on recent findings suggesting that individual SFA possess specific properties associated with important biological functions.
New Aspects on the Metabolism of Saturated Fatty Acids
Cellular Origin of Saturated Fatty Acids
SFA usually account for 30–40% of total FA in animal tissues, distributed in palmitic acid (15–25%), stearic acid (10–20%), myristic acid (0.5–1%) and lauric acid (less than 0.5%). Palmitic and stearic acids are universally found in natural fats. Lauric acid is specifically abundant in copra (39–54%) and palmist oils (44–51%). Myristic acid and short-chain fatty acids (including butyric acid) represent each about 10% of FA in milk fat. Apart from the dietary sources, it is well known that the body is capable of synthesizing SFA. Because of their multiple potential origins, it has been difficult to quantify the real importance of dietary SFA when compared with endogenous SFA, especially for palmitic acid. Adipose tissue concentrations of C15:0, C17:0 but also C14:0 have been used as quantitative markers of dairy consumption [8]. With regard to myristic acid, the amount of its endogenous biosynthesis [9] is indeed far smaller than the amounts consumed from dietary sources (4 g/day in a Swedish population) [10].
Desaturation of Saturated Fatty Acids
Recent findings on SFA concern their respective capacities to be desaturated. Every SFA from C12:0 to C18:0 is converted to its respective monounsaturated product through the action of Δ9-desaturase (Stearoyl–CoA desaturase, SCD) but with varying efficiency. In the rat [11], a clear increase in hepatic Δ9-desaturase activity is related to the carbon chain length, from C12:0 to C18:0. Evidence was also recently presented that palmitic acid, already known as a substrate of Δ9-desaturase, can also be desaturated by the mammalian Δ6-desaturase (Fatty acid desaturase 2: FADS2) [12]. Palmitic acid Δ6-desaturation produces sapienic acid (C16:1n-10) in the preputial gland of SCD1−/− mice [13] and in human sebaceous glands in which the expression of SCD is low [14]. The importance of this human physiological specificity remains to be determined.
Specific Biochemical Functions of Individual SFA
Regulation of Protein Activation by N-terminal Myristoylation
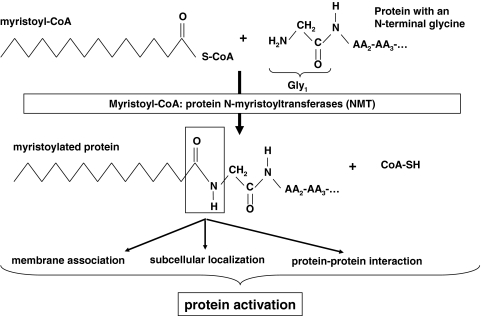
Myristic and palmitic acids are directly involved in the two classes of protein fatty acid acylation, N-terminal myristoylation and side-chain palmitoylation [15]. Protein N-myristoylation (Fig. 1) refers to the highly specific covalent attachment of myristic acid, by an amide linkage, to the NH2-terminal glycine residue of increasing number of eukaryotic and viral proteins [16]. Myristoyl–CoA: protein N-myristoyltransferase (NMT, EC 2.3.1.97), the enzyme catalyzing this stable acylation, has been identified in many organisms. Genetic advances have revealed that mammals possess two distinct NMT genes, named type 1 and 2 [17–19]. The contribution of each gene transcript to NMT expression and activity in vivo, and the specific role of each NMT isoform in cellular replication, proliferation, and other cellular processes, is still under investigation [20–22]. They both seem to have a similar high substrate selectivity for myristic acid [17, 19]. The myristoyl moiety has been shown to mediate protein subcellular localization, protein–protein interaction or protein–membrane interactions (Fig. 1) required for the biological activities of the myristoylated proteins [16]. In the myristoylation pathway, myristic acid therefore exhibits a specific and important role for protein activation.
Fig. 1.
N-terminal myristoylation of proteins with myristic acid by myristoyl–CoA: protein N-myristoyltransferases (NMT) and biological consequences for the myristoylated proteins
The proteins that are substrates of NMT include key components in intracellular signaling pathways, oncogenes, structural viral proteins, and common constitutive eukaryotic proteins. Computational prediction recently suggested that about 0.5% of all proteins in the human genome could be myristoylated [23]. New protein substrates identified recently in mammals are the tumor suppressor Fus1 [24], TRIF [25], the sphingolipid Δ4-desaturases type 1 and 2 [26], and truncated protein forms that become substrates after post-translational cleavage by caspases that reveals an internal myristoylation motif: BID [27], actin [28], gelsolin [29], and p21-activated protein kinase 2 [30].
Can Myristic Acid be the Rate-Limiting Molecule in the Myristoylation Pathway?
Since endogenous biosynthesis of C14:0 [9] is very low as compared with dietary sources [10], one can ask if the amount of free myristic acid could be insufficient to provide myristoyl–CoA: protein N-NMT with enough co-substrate. In yeast, studies analyzing the activity of NMT have suggested that the enzyme was able to use both exogenous and endogenous myristic acid as the substrate [31–33]. During the process of the maturation of one protein, the pool of endogenous free myristoyl–CoA would probably be sufficient [34]. However, when considering the pool of myristoylable proteins or the specific process for the maturation of viral proteins like Gag or Nef during the course of a viral, retroviral or lentiviral infection, the amount of myristoyl–CoA co-substrate needed is considerable [35]. The requirement for myristic acid suggests that in certain cases, it could be the rate-limiting molecule in this mechanism or that competition could occur. In addition, the mechanism by which myristic acid initially esterified in the TAG or PL is used for myristoylation is unknown, too. The available evidence indicates that the intracellular concentration of free myristic acid in endothelial cells is crucial for the activation of thrombospondin-1 and could be modulated by its uptake from the medium through CD36 [36].
Regulation of Protein–Membrane Interactions by Palmitoylation
Protein palmitoylation refers to the post-translational formation of a thioester linkage between the side-chain of cysteine and palmitic acid, mainly [37], or other saturated fatty acids, like myristic and lauric acids [38]. A long time ago, the ester linkage between saturated fatty acids and serine or threonine residues was also reported [39], but since then, this type of linkage has not been described again.
Palmitoylation is involved in regulatory mechanisms because the association of the protein with the palmitoyl moiety is reversible and facilitates protein–membrane interactions and subcellular trafficking of proteins. Several signal transductions depend therefore on palmitic acid, including proteins that have been shown to undergo successive myristoylation and palmitoylation, like the α subunit of many heterotrimeric G proteins [40]. Mammalian protein palmitoyltransferases (also named Protein S-AcylTransferases PAT) are still under investigation [37]. The recent biochemical evidence of the PAT activity of a family of proteins that share a DHHC motif in yeast [41–43] has opened a new field of investigation.
Regulation of Gene Transcription by Saturated Fatty Acids
While dietary PUFA are known to regulate gene expression through their influence on transcription factors (PPAR, SREBP), the effect of SFA on gene transcription has been less explored. Lin et al. [44] have shown that SFA (C10:0–C18:0) strongly elevate the PGC-1β mRNA level, that co-activates the action of SREBP family of transcription factors, and consequently increase the transcription of lipogenic target genes (FAS, SCD-1). Co-activation of the nuclear receptor LXR/RXR also promotes VLDL secretion. SFA (but also monounsaturated FA) are known to bind hepatocyte nuclear factor 4 (HNF4). In liver cells, palmitate and oleate both enhanced the transcription of glucose-6-phosphatase gene. Palmitate also induced the recruitment of several transcription factors like NF-κB, HNF4, CEBPα, and PPARα [45]. SFA, when linked to the lipid A moiety of lipopolysaccharides, or free, also indirectly induce NF-κB nuclear translocation, activation and expression of COX-2, and other pro-inflammatory cytokines, through the recently described toll-like receptor 4-derived signaling pathways [46, 47]. Finally, because it is an inhibitor of the histone deacetylase activity, butyric acid regulates the expression of several genes, like IL5, by altering the histone acetylating status of their promoter regions, with consequences for the structure of chromatin [48, 49].
New Demonstrated or Putative Physiological Roles of SFA
Stearic Acid is Neutral for Cholesterol but Could be Pro-Lipogenic
As for the other long-chain saturated fatty acids, the health impact of stearic acid has been studied, showing no deleterious effect on cardiovascular disease risk [50, 51]. The high rate of C18:0 conversion to C18:1n-9 by Δ9-desaturation was suggested to explain this neutral effect on cholesterol metabolism, compared to the other SFA [52]. On the other hand, the desaturation of dietary stearate to endogenous oleate has been described as a stimulating factor for VLDL–TAG secretion in hepatocytes [53], and an essential step mediating the induction of lipogenesis [54] and the promotion of obesity [55], showing the very important role of SCD. The evidence concerning the putative effect of stearic acid on thrombotic tendency appears inconsistent [56, 57].
Myristic Acid Could Regulate PUFA Bioavailability
It has recently been suggested that myristic acid may be an activator of the conversion of α-linolenic acid to docosahexaenoic acid (DHA). In cultured rat hepatocytes, myristic acid had a specific and dose-dependent effect on Δ6-desaturase activity [58]. In vivo, when myristic acid was supplied for 2 months in the diet of rats (from 0.2 to 1.2% of dietary energy), with similar level of dietary α-linolenic acid (1.6% of FA, 0.3% of energy), a dose–response accumulation of eicosapentaenoic acid (EPA) was shown in the liver and plasma [59].
In addition, in humans, compared with a diet containing 0.6% of myristic acid mainly in the sn-2 position in the TAG, a diet containing 1.2% of myristic acid during a 5-week consumption period significantly enhanced EPA and DHA levels in the plasma PL and DHA level in the plasma cholesteryl esters [60]. When the intake of myristic acid increased from 1.2 to 1.8% energy in the same population, EPA, DPA and DHA decreased significantly in plasma PL and EPA also decreased in cholesteryl esters [61]. This result suggest that, in humans, the effect of myristic acid on circulating (n-3) PUFA follows a U-shaped curve with a favorable turning point at around 1.2% of total daily energy.
So far as potential mechanism(s) of action are concerned, the increase in the activity of Δ6-desaturase by myristic acid was first postulated to be mediated by N-myristoylation (see below) because this enzyme exhibits an N-terminal glycine residue. However, we recently demonstrated that Δ6-desaturase is not myristoylated in vivo [26]. The hypothesis has been proposed that myristoylation of another protein of the whole complex of Δ6-desaturation, NADH cytochrome b5 reductase [62], could account for this increased activity.
Lauric Acid Could be a Precursor for ω3 Fatty Acid Biosynthesis
The low level of C12:0 conversion to C12:1n-3 in the liver of rats has led to the hypothesis that this monounsaturated fatty acid of the (n-3) series might be, under extreme physiological circumstances [for example animals deprived of (n-3) PUFA in the diet during a long period], an unusual precursor for the biosynthesis of α-linolenic acid, by successive Δ6-desaturation, elongation, Δ5-desaturation and two final elongations [11].
SFA May Play Multiple Roles in Early Events of Apoptosis
Specific histone deacetylase inhibitors, like butyric acid, selectively induce cellular differentiation, growth arrest and apoptosis in a variety of cancer cells [63]. Although contradictory effects have been reported [64], it seems that delivery of an adequate amount of butyrate to the appropriate site could protect against early tumorigenic events [65].
Several SFA may induce apoptosis via different ways. First, as already suggested above, butyrate and short-chain SFA may also have an effect on apoptosis [66]. Second, the pro-apoptotic effect of non-esterified palmitate and stearate was shown to require acyl–CoA formation and NF-κB activation [67]. Third, SFA may also influence apoptosis via the ceramide pathway by inducing ceramide de novo synthesis at several steps [68–70]. The first step catalyzed by the serine palmitoyltransferase involves serine condensation with palmitoyl–CoA [71]. The last step is catalyzed by dihydroceramide Δ4-desaturase (DES). We recently showed that both DES1 and DES2 isoforms are myristoylated and that this N-terminal modification significantly increased the activity of recombinant DES1 in COS-7 cells [26]. Compared with a recombinant unmyristoylable mutant form of DES1 (N-terminal glycine replaced by an alanine), the desaturase activity of the myristoylable wild-type DES1 was two times higher, in the presence of myristic acid incubated with the cells [26]. The description of this regulatory mechanism highlighted a new potential relationship between myristic acid, the saturated fatty acid capable of binding and activating the enzyme involved in the final de novo ceramide biosynthesis step, and lipoapoptosis induced through the ceramide pathway. Therefore, we subsequently hypothesized and showed that the myristoylation of recombinant DES 1 can target part of the enzyme to the mitochondria, leading to an increase in ceramide levels (specifically in the mitochondria) which in turn leads to apoptosis in the COS-7 cell model [72].
Medium-Chain Fatty Acids and Fat Deposition
A point of interest is the specific role played by medium-chain SFA. It has been reported that overfeeding with a medium-chain TAG diet in rats results in a diminished deposition of fat, when compared with rats overfed with isocaloric long-chain TAG [73]. This suggests an obligatory oxidation of medium-chain SFA in the liver, after transport via the portal vein, leaving no medium-chain TAG for accumulation in adipose tissue [73]. Another hypothesis for medium-chain TAG effect is their inhibitory effect on apoB synthesis, reducing the VLDL secretion by hepatocytes. This has been observed with octanoate in chicken hepatocytes [74].
Conclusion
We have reported here new knowledge on cellular and physiological functions of the different SFA. For this reason, SFA should no longer be considered as a single group in terms of structure, metabolism, and function.
Abbreviations
- FA
Fatty acids
- NMT
Myristoyl–CoA: protein N-myristoyltransferase
- PL
Phospholipids
- PUFA
Polyunsaturated fatty acids
- SCD
Stearoyl–CoA desaturase
- SFA
Saturated fatty acids
- TAG
Triglycerides
References
- 1.Keys A. Coronary heart disease in seven countries. Circulation. 1970;41:1–211. [PubMed] [Google Scholar]
- 2.Kromhout D, Bloemberg B, Feskens E, Menotti A, Nissinen A. Saturated fat, vitamin C and smoking predict long-term population all-cause mortality rates in the seven countries study. Int J Epidemiol. 2000;29:260–265. doi: 10.1093/ije/29.2.260. [DOI] [PubMed] [Google Scholar]
- 3.Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA. 1997;278:2145–2150. doi: 10.1001/jama.278.24.2145. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Rimm EB, Herrigton DM. Dietary fats, carbohydrate, and the progression of coronary atherosclerosis in post menopausal women. Am J Clin Nutr. 2004;80:1175–1184. doi: 10.1093/ajcn/80.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes TA, Heimberg M, Wang X, Wilcox H, Hughes SM, Tolley EA, Desiderio DM, Dalton JT. Comparative lipoprotein metabolism of myristate, palmitate, and stearate in normolipidemic men. Metabolism. 1996;45:1108–1118. doi: 10.1016/S0026-0495(96)90010-4. [DOI] [PubMed] [Google Scholar]
- 7.Rioux V, Lemarchal P, Legrand P. Myristic acid, unlike palmitic acid, is rapidly metabolized in cultured rat hepatocytes. J Nutr Biochem. 2000;11:198–207. doi: 10.1016/S0955-2863(00)00065-6. [DOI] [PubMed] [Google Scholar]
- 8.Warensjö E, Jansson JH, Berglund L, Boman K, Ahren B, Weinehall L, Lindahl B, Hallmans G, Vessby B. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction. A prospective case-control study. Br J Nutr. 2004;91:635–642. doi: 10.1079/BJN20041080. [DOI] [PubMed] [Google Scholar]
- 9.Rioux V, Catheline D, Legrand P. In rat hepatocytes, myristic acid occurs through lipogenesis, palmitic acid shortening and lauric acid elongation. Animal. 2007;1:820–826. doi: 10.1017/S1751731107000122. [DOI] [PubMed] [Google Scholar]
- 10.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–833. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 11.Legrand P, Catheline D, Rioux V, Durand G. Lauric acid is desaturated to C12:1n-3 by rat liver homogenate and hepatocytes. Lipids. 2002;37:569–572. doi: 10.1007/s11745-002-0934-y. [DOI] [PubMed] [Google Scholar]
- 12.Guillou H, Rioux V, Catheline D, Thibault JN, Bouriel M, Jan S, D’Andrea S, Legrand P. Conversion of hexadecanoic acid to hexadecenoic acid by rat Δ6-desaturase. J Lipid Res. 2003;44:450–454. doi: 10.1194/jlr.C200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki M, Gomez FE, Ntambi JM. Lack of stearoyl–CoA desaturase-1 function induces a palmitoyl-CoA delta-6 desaturase and represses the stearyl–CoA desaturase-3 gene in the preputial glands of the mouse. J Lipid Res. 2002;43:2146–2154. doi: 10.1194/jlr.M200271-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Ge L, Gordon JS, Hsuan C, Stenn K, Prouty SM. Identification of the delta-6 desaturase of human sebaceous glands: expression and enzyme activity. J Invest Dermatol. 2003;120:707–714. doi: 10.1046/j.1523-1747.2003.12123.x. [DOI] [PubMed] [Google Scholar]
- 15.Towler DA, Gordon JI, Adams SP, Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DR, Bhatnagar RS, Knoll LJ, Gordon JI. Genetic and biochemical studies of protein N-myristoylation. Annu Rev Biochem. 1994;63:869–914. doi: 10.1146/annurev.bi.63.070194.004253. [DOI] [PubMed] [Google Scholar]
- 17.Giang DK, Cravatt BF. A second mammalian N-myristoyltransferase. J Biol Chem. 1998;273:6595–6598. doi: 10.1074/jbc.273.12.6595. [DOI] [PubMed] [Google Scholar]
- 18.Rundle DR, Rajala RVS, Anderson RE. Characterization of type I and type II myristoyl–CoA: protein N-myristoyltransferase with the acyl–CoAs found on heterogeneously acylated retinal proteins. Exp Eye Res. 2002;75:87–97. doi: 10.1006/exer.2002.1189. [DOI] [PubMed] [Google Scholar]
- 19.Rioux V, Beauchamp E, Pedrono F, Daval S, Molle D, Catheline D, Legrand P. Identification and characterization of recombinant and native rat myristoyl–CoA: protein N-myristoyltransferases. Mol Cell Biochem. 2006;286:161–170. doi: 10.1007/s11010-005-9108-0. [DOI] [PubMed] [Google Scholar]
- 20.Yang SH, Shrivastav A, Kosinski C, Sharma RK, Chen MH, Berthiaume LG, Peters LL, Chuang PT, Young SG, Bergo MO. N-myristoyltransferase 1 is essential in early mouse development. J Biol Chem. 2005;280:18990–18995. doi: 10.1074/jbc.M412917200. [DOI] [PubMed] [Google Scholar]
- 21.Ducker CE, Upson JJ, French KJ, Smith CD. Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation, and apoptosis. Mol Cancer Res. 2005;3:463–476. doi: 10.1158/1541-7786.MCR-05-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvakumar P, Smith-Windsor E, Bonham K, Sharma RK. N-myristoyltransferase 2 expression in human colon cancer: cross-talk between the calpain and caspase system. FEBS Lett. 2006;580:2021–2026. doi: 10.1016/j.febslet.2006.02.076. [DOI] [PubMed] [Google Scholar]
- 23.Maurer-Stroh S, Gouda M, Novatchkova M, Schleiffer A, Schneider G, Sirota FL, Wildpaner M, Hayashi N, Eisenhaber F. MYRbase: analysis of genome-wide glycine myristoylation enlarges the functional spectrum of eukaryotic myristoylated proteins. Genome Biol. 2004;5:1–16. doi: 10.1186/gb-2004-5-3-r21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uno F, Sasaki J, Nishizaki M, Carboni G, Xu K, Atkinson EN, Kondo M, Minna JD, Roth JA, Ji L. Myristoylation of Fus1 protein is required for tumor suppression in human lung cancer cells. Cancer Res. 2004;64:2969–2976. doi: 10.1158/0008-5472.CAN-03-3702. [DOI] [PubMed] [Google Scholar]
- 25.Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O’Neill LA, Fitzgerald KA, Golenbock DT. The myristoylation of TRIF-related molecule is essential for toll-like receptor 4 signal transduction. Proc Natl Acad Sci USA. 2006;103:6399–6404. doi: 10.1073/pnas.0510041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beauchamp E, Goenaga D, Le Bloc’h J, Catheline D, Legrand P, Rioux V. Myristic acid increases the activity of dihydroceramide Δ4-desaturase 1 through its N-terminal myristoylation. Biochimie. 2007;89:1553–1561. doi: 10.1016/j.biochi.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 28.Utsumi T, Sakurai N, Nakano K, Ishisaka R. C-terminal 15 kDa fragment of cytoskeletal actin is posttranslationally N-myristoylated upon caspase-mediated cleavage and targeted to mitochondria. FEBS Lett. 2003;539:37–44. doi: 10.1016/S0014-5793(03)00180-7. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai N, Utsumi T. Posttranslational N-myristoylation is required for the anti-apoptotic activity of human tGelsolin, the C-terminal caspase cleavage product of human gelsolin. J Biol Chem. 2006;281:14288–14295. doi: 10.1074/jbc.M510338200. [DOI] [PubMed] [Google Scholar]
- 30.Vilas GL, Corvi MM, Plummer GJ, Seime AM, Lambkin GR, Berthiaume LG. Posttranslational myristoylation of caspase-activated p21-activated protein kinase 2 (PAK2) potentiates late apoptotic events. Proc Natl Acad Sci USA. 2006;103:6542–6547. doi: 10.1073/pnas.0600824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duronio RJ, Rudnick DA, Johnson RL, Johnson DR, Gordon JI. Myristic acid auxotrophy caused by mutation of S. cerevisiae myristoyl–CoA: protein N-myristoyltransferase. J Cell Biol. 1991;113:1313–1330. doi: 10.1083/jcb.113.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duronio RJ, Reed SI, Gordon JI. Mutations of human myristoyl–CoA: protein N-myristoyltransferase cause temperature-sensitive myristic auxotrophy in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:4129–4133. doi: 10.1073/pnas.89.9.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson DR, Knoll LJ, Levin DE, Gordon JI. Saccharomyces cerevisiae contains four fatty acid activation (FAA) genes: an assessment of their role in regulating protein N-myristoylation and cellular lipid metabolism. J Cell Biol. 1994;127:751–762. doi: 10.1083/jcb.127.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutin JA. Myristoylation. Cell Signal. 1997;9:15–35. doi: 10.1016/S0898-6568(96)00100-3. [DOI] [PubMed] [Google Scholar]
- 35.Hill BT, Skowronski J. Human N-myristoyltransferases form stable complexes with lentiviral nef and other viral and cellular substrate proteins. J Virol. 2005;79:1133–1141. doi: 10.1128/JVI.79.2.1133-1141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J Lipid Res. 2006;47:1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Rioux V, Galat A, Jan G, Vinci F, d’Andrea S, Legrand P. Exogenous myristic acid acylates proteins in cultured rat hepatocytes. J Nutr Biochem. 2002;13:66–74. doi: 10.1016/S0955-2863(01)00196-6. [DOI] [PubMed] [Google Scholar]
- 39.Grand RJA. Acylation of viral and eukaryotic proteins. Biochem J. 1989;258:625–638. doi: 10.1042/bj2580625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–1652. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 41.Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- 42.Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smotrys JE, Schoenfish MJ, Stutz MA, Linder ME. The vacuolar DHHC-CRD protein Pfa3p is a protein acyltransferase for Vac8p. J Cell Biol. 2005;170:1091–1099. doi: 10.1083/jcb.200507048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 45.Xu C, Chakravarty K, Kong X, Tuy TT, Arinze IJ, Bone F, Massillon D. Several transcription factors are recruited to the glucose-6-phosphatase gene promoter in response to palmitate in rat hepatocytes and H4IIE cells. J Nutr. 2007;137:554–559. doi: 10.1093/jn/137.3.554. [DOI] [PubMed] [Google Scholar]
- 46.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 47.Lee JY, Ye J, Gao Z, Lee WH, Zhao L, Sizemore L, Hwang DH. Reciprocal modulation of toll-like receptor-4 signaling pathways involving My88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 48.Smith JG, Yokoyama WH, German JB. Dietary butyric acid: implications for gene expression. Crit Rev Food Sci Nutr. 1998;38:259–297. doi: 10.1080/10408699891274200. [DOI] [PubMed] [Google Scholar]
- 49.Han S, Lu J, Zhang Y, Cheng C, Li L, Han L, Huang B. HDAC inhibitors TSA and sodium butyrate enhanced the human IL-5 expression by altering histone acetylation status at its promoter region. Immunol Lett. 2007;108:143–150. doi: 10.1016/j.imlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Yu S, Derr J, Etherton TD, Kris-Etherton PM. Plasma cholesterol-predictive equations demonstrate that stearic is neutral and monounsaturated fatty acids are hypocholesterolemic. Am J Clin Nutr. 1995;61:1129–1139. doi: 10.1093/ajcn/61.4.1129. [DOI] [PubMed] [Google Scholar]
- 51.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated and unsaturated fatty acids : a systematic review. Am J Clin Nutr. 2010;91:46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 52.Bonanome A, Grundy SM. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N Engl J Med. 1988;318:1244–1248. doi: 10.1056/NEJM198805123181905. [DOI] [PubMed] [Google Scholar]
- 53.Legrand P, Catheline D, Fichot MC, Lemarchal P. Inhibiting delta9-desaturase activity impairs triacylglycerol secretion in cultured chicken hepatocytes. J Nutr. 1997;127:249–256. doi: 10.1093/jn/127.2.249. [DOI] [PubMed] [Google Scholar]
- 54.Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl–CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fatty acids. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 55.Paillard F, Catheline D, Le Duff F, Bouriel M, Pouchard M, Deugnier Y, Daubert JC, Legrand P, et al. Δ9-desaturase, new candidate implied in the expression of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2008;18:436–440. doi: 10.1016/j.numecd.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 56.Tholstrup T, Miller GJ, Bysted A, Sandstrom B. Effect of individual dietary fatty acids on postprandial activation of blood coagulation factor VII and fibrinolysis in healthy young men. Am J Clin Nutr. 2003;77:1125–1132. doi: 10.1093/ajcn/77.5.1125. [DOI] [PubMed] [Google Scholar]
- 57.Thijssen MA, Hornstra G, Mensink RP. Stearic, oleic and linoleic acids have comparable effects on markers of thrombotic tendency in healthy human subjects. J Nutr. 2005;135:2805–2811. doi: 10.1093/jn/135.12.2805. [DOI] [PubMed] [Google Scholar]
- 58.Jan S, Guillou H, D’Andréa S, Daval S, Bouriel M, Rioux V, Legrand P. Myristic acid increases Δ6-desaturase activity in cultured rat hepatocytes. Reprod Nutr Dev. 2004;44:131–140. doi: 10.1051/rnd:2004020. [DOI] [PubMed] [Google Scholar]
- 59.Rioux V, Catheline D, Bouriel M, Legrand P. Dietary myristic acid at physiologically relevant levels increases the tissue content of C20:5n-3 and C20:3n-6 in the rat. Reprod Nutr Dev. 2005;45:599–612. doi: 10.1051/rnd:2005048. [DOI] [PubMed] [Google Scholar]
- 60.Dabadie H, Peuchant E, Bernard M, Le Ruyet P, Mendy F. Moderate intake of myristic acid in sn-2 position has beneficial lipidic effects and enhances DHA of cholesteryl esters in an interventional study. J Nutr Biochem. 2005;16:375–382. doi: 10.1016/j.jnutbio.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Dabadie H, Motta C, Peuchant E, Le Ruyet P, Mendy F. Variations in daily intakes of myristic and α-linolenic acids in sn-2 position modify lipid profile and red blood cell membrane fluidity. Br J Nutr. 2006;96:283–289. doi: 10.1079/BJN20061813. [DOI] [PubMed] [Google Scholar]
- 62.Borgese N, Aggujaro D, Carrera P, Pietrini G, Bassetti M. A role for N-myristoylation in protein targeting: NADH-cytochrome b5 reductase requires myristic acid for association with outer mitochondrial but not ER membranes. J Cell Biol. 1996;135:1501–1513. doi: 10.1083/jcb.135.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J, Ghazawi FM, Bakkar W, Li Q. Valproic acid and butyrate induce apoptosis in human cancer cells through inhibition of gene expression of Akt/protein kinase B. Mol Cancer. 2006;5:71. doi: 10.1186/1476-4598-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stempelj M, Kedinger M, Augenlicht L, Klampfer L. Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. J Biol Chem. 2007;282:9797–9804. doi: 10.1074/jbc.M609426200. [DOI] [PubMed] [Google Scholar]
- 65.Sengupta S, Muir JG, Gibson PR. Does butyrate protect from colorectal cancer? J Gastroenterol Hepatol. 2006;21:209–218. doi: 10.1111/j.1440-1746.2006.04213.x. [DOI] [PubMed] [Google Scholar]
- 66.Belakavadi M, Prabhakar BT, Salimath BP. Purification and characterization of butyrate-induced protein phosphatase involved in apoptosis of Ehrlich ascites tumor cells. Biochim Biophys Acta. 2007;1770:39–47. doi: 10.1016/j.bbagen.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Staiger K, Staiger H, Weigert C, Haas C, Haring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-κB activation. Diabetes. 2006;55:3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 68.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728. doi: 10.1016/S0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 69.Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr. 2005;37:143–153. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stiban J, Fistere D, Colombini M. Dihydroceramide hinders ceramide channel formation: implications on apoptosis. Apoptosis. 2006;11:773–780. doi: 10.1007/s10495-006-5882-8. [DOI] [PubMed] [Google Scholar]
- 71.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 72.Beauchamp E, Tekpli X, Marteil G, Lagadic-Gossmann D, Legrand P, Rioux V. N-Myristoylation targets dihydroceramide Delta4-desaturase 1 to mitochondria: partial involvement in the apoptotic effect of myristic acid. Biochimie. 2009;91:1411–1419. doi: 10.1016/j.biochi.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 73.Geliebter A, Torbay N, Bracco ET, Hashim SA, Itallie TB. Overfeeding with medium chain triglyceride diet results in diminished deposition of fat. Am J Clin Nutr. 1983;37:1–4. doi: 10.1093/ajcn/37.1.1. [DOI] [PubMed] [Google Scholar]
- 74.Tachibana S, Sato K, Cho Y, Chiba T, Schneider WJ, Akiba Y. Octanoate reduces very low-density lipoprotein secretion by decreasing the synthesis of apolipoprotein B in primary cultures of chicken hepatocytes. Biochim Biophys Acta. 2005;1737:36–43. doi: 10.1016/j.bbalip.2005.09.001. [DOI] [PubMed] [Google Scholar]