Abstract
Invasion of placental trophoblasts into uterine tissue and vessels is an essential process of human pregnancy and fetal development. Due to their remarkable plasticity invasive trophoblasts fulfil numerous functions, i.e. anchorage of the placenta, secretion of hormones, modulation of decidual angiogenesis/lymphangiogenesis and remodelling of maternal spiral arteries. The latter is required to increase blood flow to the placenta, thereby ensuring appropriate transfer of nutrients and oxygen to the developing fetus. Since failures in vascular changes of the placental bed are associated with pregnancy diseases such as preeclampsia or intrauterine growth restriction, basic research in this particular field focuses on molecular mechanisms controlling trophoblast invasion under physiological and pathological conditions. Throughout the years, an increasing number of growth factors, cytokines and angiogenic molecules controlling trophoblast motility have been identified. These factors are secreted from numerous cells such as trophoblast, maternal epithelial and stromal cells, as well as uterine NK cells and macrophages, suggesting that a complex network of cell types, mediators and signalling pathways regulates trophoblast invasiveness. Whereas essential features of the invasive trophoblast such as expression of critical proteases and adhesion molecules have been well characterised, the interplay between different cell types and growth factors and the cross-talk between distinct signalling cascades remain largely elusive. Similarly, key-regulatory transcription factors committing and differentiating invasive trophoblasts are mostly unknown. This review will summarise our current understanding of growth factors and signal transduction pathways regulating human trophoblast invasion/migration, as well as give insights into novel mechanisms involved in the particular differentiation process.
Keywords: human placenta, trophoblast invasion, growth factor, signal transduction
Introduction
General aspects of trophoblast differentiation
Placenta morphogenesis and formation of specialised trophoblast cell types are initiated after implantation and have to be completed during the first weeks of gestation to guarantee successful progression of pregnancy. Trophoblast progenitor cells residing at the basement membrane of placental villi give rise to distinct epithelial cell types. Fusion of cytotrophoblasts (CTBs) generates the multinucleated syncytium which covers the floating villi and is mainly responsible for protein transport and hormone production. In addition, different invasive CTB cell types develop which migrate from villous structures and hence are commonly termed extravillous trophoblasts (EVT).
Invasive differentiation program of the anchoring villus
In anchoring villi attaching to the uterine epithelium, mononuclear CTBs form cell columns through proliferation. Interaction of L-selectin with carbohydrate ligands might play a role in maintaining integrity of these columns throughout pregnancy (Prakobphol et al., 2006). However, at their distal anchoring sites CTBs detach from the columns and invade the maternal decidual stroma (Fig. 1). Cell cycle exit and differentiation into these interstitial CTBs (iCTBs) is thought to be influenced by extracellular matrix contact and endometrial components. In addition, oxygen concentrations are thought to be critically involved since hypoxia promotes trophoblast proliferation whereas normoxia inhibits proliferation and induces migration (Genbacev et al., 1997). However, spontaneous local invasion of trophoblasts in vitro as well as in ectopic pregnancies supports the hypothesis of a strongly activating, intrinsic differentiation program (Fisher et al., 1989; Wells, 2007). Production of pregnancy hormones, such as human chorionic gonadotrophin (hCG) and placental lactogen (hPL), acquisition of polyploidy, and differentiation into placental bed giant cells are some of the remarkable functions of this particular invasive trophoblast cell type (Genbacev et al., 1993; Handschuh et al., 2007a; Pijnenborg et al., 1980; Zybina et al., 2002).
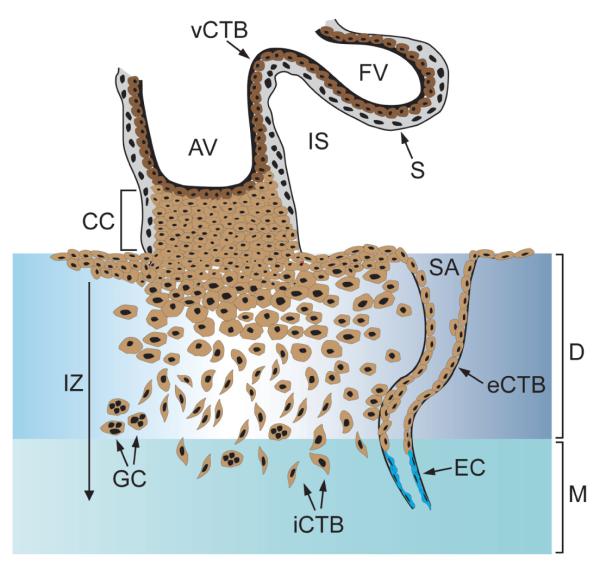
Fig. 1. Invasive differentiation of human trophoblast.
After anchorage of a mesenchymal villus (formation of anchoring villi, AV) at the uterine basement membrane villous cytotrophoblast (vCTB) precursor cells give rise to proliferative cell columns (CC). At distal sites non-proliferating, extravillous trophoblasts are formed which detach from the cell columns and migrate into stromal areas of the maternal decidua (D), i.e. formation of interstitial cytotrophoblasts (iCTB). iCTB differentiate into giant cells (GC) in deeper areas of the placental bed. Endovascular trophoblasts (eCTB) migrate into spiral arteries (SA) within the decidua and inner third of the myometrium (M), replace maternal endothelial cells (EC) and acquire endothelial characteristics. In floating villi (FV) surrounded by maternal blood of the intervillous space (IS), CTB progenitors fuse to build the multinucleated syncytium (S).
Besides iCTB another population of migratory trophoblasts develop which are thought to play an important role in vascular remodelling of maternal spiral arteries. Apoptosis of vascular smooth muscles around these vessel and displacement of maternal endothelial cells by endovascular cytotrophoblasts (eCTB) are key features of the particular invasive differentiation process (Harris and Aplin, 2007; Pijnenborg et al., 1983). The eCTBs acquire endothelial-like functions expressing typical vascular adhesion molecules (Zhou et al., 1997). These modification steps may finally result in enlargement of the vessel diameter which is thought to initiate and sustain blood flow into the intervillous space.
Failures in transformation of spiral arteries and shallow interstitial invasion were detected in the placental bed of women suffering from preeclampsia or severe intrauterine growth restriction (IUGR) indicating that the EVT differentiation program could be disturbed in these pregnancies (Khong et al., 1986; McFadyen et al., 1986; Pijnenborg et al., 1991). Poor perfusion of placental tissue is thought to result in the release of cytokines and products of oxidative stress into the maternal circulation which may cause endothelial dysfunction (Hung et al., 2002; Many et al., 2000).
Mechanisms controlling trophoblast invasion
Since in uncomplicated pregnancies interstitial/endovascular invasion do not occur beyond the decidua and the first third of the underlying myometrium, the extent of trophoblast invasiveness is thought to be precisely controlled by trophoblast-derived as well as maternal factors in a time- and distance-dependent manner (Bischof et al., 2000; Lala and Hamilton, 1996). In general, iCTBs are equipped with different protease systems allowing them to degrade extracellular matrix (ECM) proteins to promote cell migration, whereas decidua was shown to express a variety of inhibitory proteins thereby restricting invasiveness. Migratory trophoblasts express different family members of matrix metalloproteinase (MMPs), cathepsins and urokinase plasminogen activator (Bischof et al., 2000; Lala and Chakraborty, 2003; Varanou et al., 2006). However, decidual cells produce tissue inhibitors of metalloproteinases (TIMPs) and plasminogen activator inhibitor (PAI) (Lala and Graham, 1990; Schatz and Lockwood, 1993). The importance of the MMP/TIMP and the uPA/PAI system in trophoblast invasion is further emphasized by the fact TIMPs/PAI and MMPs are expressed by iCTB and decidua, respectively (Feinberg et al., 1989; Bischof, 2000; Lala and Hamilton, 1996).
Besides degradation of ECM components trophoblast are known to switch expression of adhesion molecules along the invasive differentiation pathway in vivo as well as in vitro (Damsky et al., 1994; Vicovac et al., 1995). CTB residing at the villous basement membrane express α6β4 integrin, one of the predominant laminin-5 receptors. In contrast, trophoblasts of the cell columns induce surface expression of α5β1 integrin which is though to play a role in the stabilisation of cell columns by binding to fibronectin. Distally located iCTBs, however, suppress α6β4 integrin and upregulate α1β1 integrin which is thought to promote invasiveness upon interaction with collagens and distinct laminins, such as laminin-2, which is abundantly expressed in the decidua (Church et al., 1996).
The adherens junction protein E-cadherin, another marker of the polarised epithelium, is transiently downregulated during trophoblast invasion (Zhou et al., 1997). Hence, the invasive differentiation of trophoblasts shares features with a process termed epithelial-mesenchymal transition (EMT) which is critically involved in development and tumorigenesis. Typically, iCTBs display nuclear expression of critical transcription factors involved in EMT such as Snail (S. Sonderegger and M. Knöfler, unpublished observation) and T-cell factor family (TCF) members, the latter being regulated by Wnt signalling (Pollheimer et al., 2006). However, differently to cancer cells migratory trophoblasts do not induce critical (mesenchymal) markers of EMT such as vimentin and retain epithelial characteristics such as cytokeratin 7 expression. Despite their high invasive potential it is currently unclear why iCTB do not fully undergo EMT. With respect to that it is interesting to mention that during tumorigenesis epithelial cells switch to a metastable cell phenotype, characterised by co-expression of mesenchymal and epithelial genes, before they fully transform into a mesenchymal cell. It could well be that iCTB are kept in a similar metastable phenotype (Lee et al., 2006). Maintenance of critical (epithelial) markers may ensure that cell cycle exit and differentiation occur in order to limit decidual invasion. Interestingly, invasive trophoblasts of complete hydatidiform mole (CHM) placentae, which eventually deeply infiltrate into maternal tissue, strongly express TCF molecules as well as nuclear β-catenin providing the activation domain of the Wnt-dependent transcription factor family (Pollheimer et al., 2006). Appearance of nuclear β-catenin is a typical feature of EMT and cancer cells suggesting that invasive trophoblasts of CHM placentae may have further progressed towards a tumor cell-like, mesenchymal phenotype.
Model systems of trophoblast invasion
Trophoblast cell lines
Several investigators have established trophoblast cell lines derived from first trimester placentae which are commonly used to study trophoblast invasion. Similar to choriocarcinoma cells HTR-8/SVneo cells, which had been generated by transformation of HTR-8 cells with large T antigen, display an unlimited life-span in culture. The primary HTR-8 cultures were obtained after plating and outgrowth of cells from tissue pieces of first trimester villi and share features with invasive trophoblasts such as expression of cytokeratin 18 and some EVT-specific integrins (Graham et al., 1993). Similarly, SGHPL-4 and SGHPL-5 cells have been produced after trypsinisation, gradient centrifugation and SV40 large T antigen transfection of minced first trimester placentae (Choy and Manyonda, 1998; Choy et al., 2000). The two presumptive EVT cell lines, which express cytokeratin 7 and HLA-G, particularly when plated on Matrigel, retain a senescence mechanism and are commonly used until passage 25 (G. Whitley, personal communication). SGHPL-4 cells migrate into fibrin-embedded spiral arteries in vitro suggesting that they have retained cellular functions of in vivo EVT (Cartwright et al., 2002a). HIPEC 65 represents another SV40 large T-transformed cell line derived from early chorionic villi expressing several EVT markers (Pavan et al., 2003). Whereas many researchers investigated molecular mechanism of trophoblast invasion in the above mentioned cell lines, several other putative EVT cell lines such as HT-116, or SWAN 71, exist, which however were hardly used for in vitro studies (Aplin et al., 2006; Logan et al., 1996; Zdravkovic et al., 1999). However differently to primary EVT, downregulation of cytokeratin 7 and induction of vimentin was noticed in all trophoblast cell lines. This suggests that the selection procedure has artificially converted EVT from an epithelial or presumptive metastable phenotype into a more fibroblastoid phenotype. Indeed, gene expression profiling and cluster analyses revealed that primary EVT share more similarities with villous CTB than with trophoblastic cell lines (M. Bilban and M. Knöfler, unpublished).
Trophoblast primary cultures
Besides analyses of cell lines molecular mechanisms controlling trophoblast invasion should be also investigated in appropriate primary cell models. This, however, is hampered by fact that the amount of primary trophoblasts is limited and that cultures are eventually contaminated with other placental cell types such as fibroblasts. In addition, cells rapidly cease proliferation in culture and thus are notoriously difficult to transfect by standard protocols.
Nevertheless, villous CTBs isolated by the Kliman method, i.e., trypsinization of first trimester villous material, Percoll gradient centrifugation and immunopurification have been widely used to study aspects of trophoblast invasiveness (Fisher et al., 1989; Kliman et al., 1986). Similarly, the villous explant culture system was established allowing to study column formation and EVT migration/invasion in a time- and distance-dependent manner (Genbacev et al., 1993). After plating of first trimester villous tissues on ECM-coated dishes, differentiated EVT develop expressing cell-specific markers such as HLA-G and integrins α5β1 and α1β1 (Bauer et al., 2004; Vicovac et al., 1995).
Whereas utilisation of primary cells is desirable, one also has to be aware of the disadvantages of the trophoblast models described above. Explant cultures correctly mimic the EVT differentiation program, however, the different processes, i.e., adhesion, proliferation and migration/invasion, cannot be studied separately. Depending on the immunopurification protocol Kliman-prepared CTB represent a mixture of different trophoblast cell types with villous CTB being the most prominent one. Thus, it is not anticipated that for example effects of growth factors on protease expression and invasion would be the same as on distal, migratory EVT. Rather, utilisation of these cells may reflect the initial step of the invasive differentiation process, i.e. detachment of CTB from villous basement membranes. Therefore, procedures were developed to isolate and purify primary EVT from first trimester villous tissue (Tarrade et al., 2001a).
To overcome the limitations of isolated primary cultures different attempts were also made to isolate and propagate CTB stem cells which eventually can be differentiated into the EVT lineage. Indeed, human CTB stem cells/lines have been developed from embryoid bodies of embryonic stem cells expressing critical trophoblast (stem cell)-specific markers such as Cdx2, cytokeratin 7 and HLA-G (Harun et al., 2006; Gerami-Naini et al., 2004). These cells also express endothelial-specific genes upon endometrial co-cultivation reflecting the vascular adhesion phenotype of eCTB in vivo (Harun et al., 2006). Nevertheless, further functional analyses of CTB stem cells, such as mechanisms of differentiation into EVT, are still lacking. Also, there is currently no agreement within the community whether placental villi harbour a bi-potential CTB stem cells which is capable to differentiate into both syncytium and EVT or whether each differentiated trophoblast cell type has its own progenitor cell (Baczyk et al., 2006; James et al., 2007). Hence, analyses of EVT cell lines in combination with the verification of critical steps in a primary trophoblast model currently seems the most appropriate way to gain more insights into mechanisms of trophoblast invasion.
It is also worth mentioning, that recent trophoblast models systems have paid attention to the fact that, differently to standard cell culture conditions, iCTB do not grow in two dimensions in vivo and closely interact with different cell types of the decidua. Hence, spheroid cultures of SGHPL-4 cells were established suggesting that three dimensional growth could be critical for invasiveness and expression of some MMPs (LaMarca et al., 2005). Also, co-culture systems of trophoblasts with endothelial cells, explanted spiral arteries or smooth muscle cells are being developed to unravel the role of CTB in uterine vessel modification (Aldo et al., 2007; Cartwright et al., 2002a; Harris et al., 2006).
Growth factors of the fetal-maternal interface
Numerous growth factor have been identified at the fetal-maternal interface controlling proliferative as well as invasive capacity of trophoblasts (Bischof et al., 2000; Lala and Chakraborty, 2003). Different decidual cell types which are in close contact to iCTBs, i.e. decidual stromal cells, uterine NK cells and macrophages, are thought to regulate these processes in a paracrine manner. In addition, EVT express multiple ligands and hormones as well as their receptors indicating autocrine control.
During early gestation placental development occurs in the absence of maternal arterial supply. Hence, placental growth and invasion are also potentially regulated by proteins released from endometrial glands such as EGF, vEGF or LIF which are commonly regarded as critical regulators of implantation (Burton et al., 2007). Throughout gestation growth factors such as EGF, vEGF, PDGF, placental growth factor (PlGF), CSF-1, IGF-I or IGF-II are abundantly secreted from diverse cell types of the fetal-maternal interface including CTBs and were shown to promote proliferation, adhesion and/or invasion (Bischof et al., 2000; Ferretti et al., 2007; Lala and Hamilton, 1996; Pollheimer and Knöfler, 2005b). However, decidual cells / CTBs also produce a variety of inhibitory proteins such as TGFβ family members, interferon-γ, endostatin, kisspeptin-10 or TNFα to fine-tune and limit the extent of trophoblast invasion (Bauer et al., 2004; Bilban et al., 2004; Lala and Graham, 1990; Lash et al., 2006; Pollheimer et al., 2005a).
Besides classical growth factors a plethora of cytokines and chemokines are secreted from CTBs and decidual cell types, predominantly from macrophages and fibroblasts (Hannan and Salamonsen, 2007; Jokhi et al., 1997). Many of their respective receptors were identified on CTBs (Drake et al., 2004). Chemokines such as CX3CL1, CCL14, CCL4 (Hannan et al., 2006), CXCL16 (Huang et al., 2006) or CCL21 (Red-Horse et al., 2005) were shown to increase trophoblast migration or invasion. Similarly, the interleukins IL-1, IL-6, IL-8 or IL-11 secreted from CTB/decidual NK cells were shown to promote gelatinase activity and/or trophoblast invasion (Hanna et al., 2006; Librach et al., 1994; Meisser et al., 1999; Paiva et al., 2007). Interestingly, chemokines potentially regulated by the binding of ephrin ligands to their EPH receptors may not only play a role in interstitial invasion but also in the selective remodelling of uterine spiral arteries as compared to veins (Red-Horse et al., 2005). Moreover, regulatory binding proteins, for example IGFBPs (Giudice and Irwin, 1999; Lala and Chakraborty, 2003), soluble TNF receptors (Knöfler et al., 1998), sFlt-1 (Clark et al., 1998), a vEGF/PlGF antagonist, or soluble endoglin (sEng) (Venkatesha et al., 2006), a secreted TGFβ co-receptor, have been identified at the fetal-maternal interface adding further complexity to the growth factor network regulating trophoblast invasion. Physiological levels of sFlt-1 and sEng are critical for normal progression of pregnancy since elevated concentrations of these factors are thought to be involved in the pathogenesis of preeclampsia (Tjoa et al., 2007).
Whereas an increasing number of growth factors controlling trophoblast invasion are being identified, it is largely unknown whether some of these play a more predominant role than others. Similarly, importance of an individual growth factor may vary throughout gestation depending on its temporal expression pattern and the abundance of soluble inhibitors or other receptor ligands. However, its tempting to speculate that growth factors specific to pregnancy may fulfil central functions. With respect to that it is worth mentioning that hCG may play a pivotal role in trophoblast syncytialisation. Inhibition of its receptor abolishes the fusing-promoting effects of several growth factors, i.e. EGF, TGFα and LIF, suggesting that these secreted factors signal through stimulation of hCG expression/secretion (Yang et al., 2003a). It could well be that hCG which is known to promote trophoblast motility (Handschuh et al., 2007b; Prast et al., 2008) also plays a similar key role in the invasive differentiation process. Indeed, several factors promoting trophoblast invasion, such as IGF-I, IL-1 or EGF, were shown to increase hCG expression and secretion (Fig. 2).
Fig. 2. Interplay between growth factors expressed at the fetal-maternal interface.
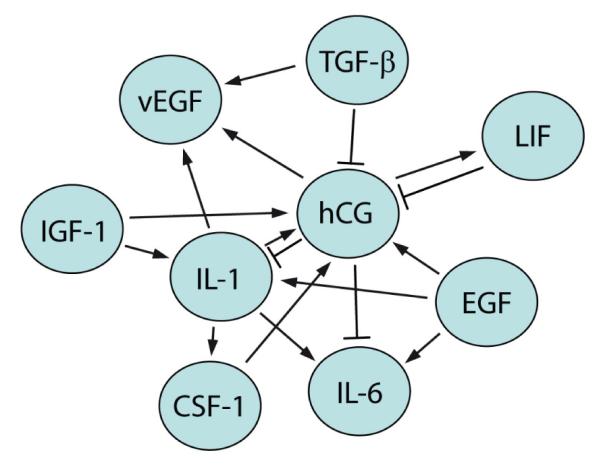
Growth factor expression/secretion is stimulated by paracrine interactions of diverse maternal and placental cell types. Predominant growth factors of the fetal-maternal interface as well as their mutual stimulations published so far are summarised. With the exception of TGFβ all factors depicted were shown to positively influence trophoblast proliferation and/or migration/invasion. Some soluble ligands such as hCG may play key roles in trophoblast motility since several growth factors trigger their secretion. On the other hand, hCG for example may also promote trophoblast migration through elevation of vEGF and LIF secretion. Hence, when studying effects of a particular growth factor on trophoblast migration direct as well as indirect effects must be considered. Stimulating (arrows) as well as inhibitory effects on expression / secretion are depicted.
Signal transduction pathways promoting trophoblast invasion
Signal transduction through sequential steps of phosphorylation represents the most common control mechanism of cellular protein function. Multiple extracellular stimuli such as growth factors, hormones, cytokines, chemokines or cell-matrix contacts initiate signalling upon interaction with receptor tyrosine kinases (RTKs), G-protein-coupled receptors (GPCRs), integrins or others. This ultimately leads to the activation of critical signalling cascades such as mitogen-activated protein kinases (MAPKs), focal adhesion kinase (FAK), the phosphoinositide 3-kinase (PI3K)-Akt pathway or Janus kinase (JAK)-Signal Transducers and Activators of Transcription (STAT) controlling a wide range of biological processes including proliferation, differentiation, migration and apoptosis. Hence, it is not surprising that the different signalling pathways also play a critical role in placental development and differentiation. Here, we will predominantly discuss signalling cascades promoting trophoblast invasion /migration. We respect to anti-invasive mechanisms we would like to refer to recent reviews discussing for example TGFβ-mediated signalling and their inhibitory targets such as PAI-1 (Lala and Graham, 1990; Lala and Hamilton, 1996; Pollheimer and Knöfler, 2005b).
Mitogen-activated protein kinases (MAPKs)
Family members and general role of MAPKs
The family of MAPKs comprises a large group of enzymes which are sequentially activated by phosphorylation at specific Ser, Thr and Tyr residues. Upon ligand binding to RTKs or GPCRs enzymes of the MAPK kinase kinase family, such as Raf, are activated through Ras. This leads to the activation of MAPK kinases, for example MEKs, which subsequently phosphorylate four different families of MAPK, i.e. extracellular regulated kinases (ERKs), ERK5, c-Jun N-terminal kinases (JNKs) and different p38 MAPKs. Whereas ERKs are mainly activated through mitogenic signals, JNK and p38 MAPK are predominantly associated with stress and inflammatory response (Kyriakis and Avruch, 2001).
MAPKs in trophoblast invasion
One particular Raf protein, Raf-B, is critically involved in vascular development of the murine placenta (Galabova-Kovacs et al., 2006). The role of Raf has not been investigated in human placenta; however, the fact that various growth factors trigger activation of ERKs in different EVT cell models suggests that Ras-Raf-MEK-ERK may control trophoblast motility. Indeed, IGF-II and IGFBP-1 activated ERK-1 and −2 and promoted migration of HTR-8/SVneo cells which could be blocked by a specific MEK inhibitor (Gleeson et al., 2001; McKinnon et al., 2001). Inhibition of MEK also diminished HGF-induced motility of SGHPL-4 cells (Cartwright et al., 2002b). Similarly, the N-terminal fragment of uPA which lacks catalytic activity, stimulated migration of HTR-8/SVneo cells which was reduced upon inhibition of MEK (Liu et al., 2003). Other factors stimulating HTR-8/SVneo cell migration and phosphorylation of ERKs are endothelin, EGF and prostaglandin E2 (Chakraborty et al., 2003; Nicola et al., 2008a; Qiu et al., 2004a). The pregnancy hormone hCG also promoted trophoblast motility through ERK phosphorylation since MEK inhibition affected migration of SGHPL-5 cells and of EVTs in first trimester villous explant cultures (Prast et al., 2008). Interestingly, these effects might be largely attributed to the hyperglycosylated form of hCG secreted from EVT since hormone released from syncytium had no effects on trophoblast motility (Handschuh et al., 2007b). The role of MAPK kinase signalling in trophoblast invasion is also emphasized by the fact that active ERKs are detectable in late-gestational EVT in situ using immunohistochemistry of placental bed biopsies (Moon et al., 2008). Presumptive roles of ERK5 and JNK in the regulation of trophoblast motility have not been investigated, whereas p38 MAPK may not be involved. Active and inactive forms of p38 MAPK were absent from EVTs in situ and chemical inhibition of the enzyme did not affect migration of SGHPL-4 cells (Cartwright et al., 2002b; Moon et al., 2008). In general, activation of ERKs through growth factors may affect numerous processes involved in cell motility such as integrin signalling, cytoskeletal dynamics or nuclear functions (Pullikuth and Catling, 2007). In trophoblasts ERKs were shown to regulate hCG- and EGF-dependent induction of MMP-2 and MMP-9, respectively, indicating that proteinases crucial for trophoblast invasion are targets of the particular signalling pathway (Prast et al., 2008; Qiu et al., 2004b).
Phosphoinositide 3-kinase (PI3K) – AKT signalling
General function of the PI3K-AKT pathway
PI3K-AKT signalling is involved in a variety of cellular processes including cell growth, proliferation, migration and survival (Manning and Cantley, 2007) Activation of RTKs or GPCRs results in membrane recruitment/activation of the p85 and p110 subunits of PI3K, respectively. Active PI3K phosphorylates phosphatidylinositol-4, 5-bis-phosphate (PIP2) at the 3’position of its inositol ring and thereby converts PIP2 to PIP3. Elevated PIP3 levels result in recruitment and activation of AKT at the membrane. Subsequently, signalling is achieved by sequential phosphorylation of downstream factors, for example, activation of the mammalian target of rapamycin (mTOR). The kinase mTOR controls cell cycle progression and cell size/mass through phosphorylation of proteins controlling protein translation, for example ribosomal S6 kinases and factors involved in translation initiation. AKT, however, also phosphorylates a wide range of other target proteins that control proliferation, cell growth and survival. In particular, the anti-apoptotic function of the signalling pathway is well known. Increased PIP3 levels occur in cancer due to hyperactivation of PI3K-AKT or loss of function mutations in PTEN (phosphatase and tensin homolog deleted on chromosome 10), the phosphatase converting PIP3 to PIP2.
Role of PI3K-AKT in trophoblast motility
Activation of PI3K-AKT signalling by anti-apoptotic factors such as EGF has also been described in human trophoblasts (Johnstone et al., 2005). However, AKT also seems to play a critical role in development and differentiation of the placenta/trophoblast. In mice, homozygous deletion of ATK1 affects placental development due to decreased numbers of proliferative trophoblasts (Yang et al., 2003b). PI3K-AKT also seems to be involved in differentiation of murine giant cells (Kamei et al., 2002).
The role of AKT in cell migration is less well understood. The three different AKT isoforms may positively or negatively influence motility depending on the cell type (Manning and Cantley, 2007). However, recent evidence from several laboratories suggests that PI3K-AKT signalling positively affects human trophoblast migration. In particular, abundant growth factors of the fetal-maternal interface, i.e. EGF and IGF-II, are potent activators of PI3K-AKT and AKT-dependent migration of trophoblastic HTR-8/SVneo cells (Qiu et al., 2005; Qiu et al., 2004a). In addition, rapamycin, which specifically blocks mTOR, was shown to decrease phosphorylation of S6 kinase and migration of these cells (Qiu et al., 2004a). In SGHPL-5 cells activation of PI3K with specific peptides resulted in increased motility, whereas inhibition of PI3K reduced basal and HGF-induced migration (Cartwright et al., 2002b). Activation of AKT through hCG was noticed in purified EVT and SGHPL-5 cells (Prast et al., 2008). Inhibition of the signalling pathway reduced migration of SGHPL-5 cells and motility of EVT in villous explant cultures (Prast et al., 2008). PI3K-AKT is also required for hCG- and EGF-dependent expression of MMP-2 and MMP-9 in trophoblasts (Prast et al., 2008; Qiu et al., 2004b), as well as of MMP-3 (J. Prast and M. Knöfler, unpublished). Since these enzymes are also targets of ERK signalling, PI3K-AKT and MAPK signalling may have synergistic effects on protease expression and CTB invasion.
Focal adhesion kinase (FAK)
General aspects of FAK signalling
FAK is a non-receptor protein tyrosine kinase (PTK) located in focal adhesions which are particular contact sites between migratory cells and the surrounding ECM. Activation of GPRCs or clustering of integrins in focal adhesions results in activation of FAK through phosphorylation at critical residues. Signalling through FAK ultimately provokes directed polymerisation/stress fiber formation of actin filaments at the leading edge of motile cells. The active kinase contains regulatory protein regions interacting with integrin-associated proteins, paxilin and talin, but also harbours contact sites binding Src-family members and other adaptor proteins with Src homology (SH) domains (Schlaepfer and Mitra, 2004). For example, activation at Tyr-397 selectively occurs at the leading edge of motile cells suggesting a critical role in cell migration. Indeed, phosphorylation of the particular amino acid promotes high-affinity binding of Src-family PTKs resulting in activation of multiple proteins kinase cascades promoting migration, i.e. MAPK, PI3K and signalling through Rho-family GTPases (Schlaepfer et al., 1999).
FAK controls trophoblast migration
Integrin-mediated signalling and FAK are also crucially involved in trophoblast migration and invasion. Integrin switching takes place during invasive trophoblast differentiation in vivo as well as in vitro (Aplin et al., 1999; Damsky et al., 1994) and active, Tyr-397 phosphorylated FAK was noticed in invasive trophoblasts expressing MMP-2 and α5 integrin (Ilic et al., 2001; MacPhee et al., 2001). Down-regulation of FAK expression in villous explant cultures or CTB reduced in vitro migration and invasion (Ilic et al., 2001; MacPhee et al., 2001). Proteins such as the amino acid transporter CD98 expressed on EVT co-localize with αvβ3 integrin and promote migration through FAK (Kabir-Salmani et al., 2008). Different growth factors were shown to activate FAK in trophoblasts. IGFBP-1-treated HTR-8/SVneo cells showed elevated FAK phosphorylation and migration (Gleeson et al., 2001). Treatment of EVTs with IGF-I induced FAK activation as well as stress fiber formation (Kabir-Salmani et al., 2002). Besides FAK, EVT express another enzyme activated through integrin-clustering in focal adhesions, i.e. integrin-linked kinase (ILK). Expression of a dominant-negative form of ILK was shown to reduce migration of HTR-8SVneo cells (Elustondo et al., 2006).
RhoGTPases and Rho-associated kinase ROCK
General role of Rho proteins
RhoA, Rac1 and Cdc42 comprise the family of Rho-GTPases regulating diverse biological processes such as proliferation, adhesion and migration (Hall, 1998). Similar to Ras, the Rho-like GTPases function as molecular switches by cycling between an active GTP-bound and an inactive GDP-bound state. Growth factor binding or focal adhesion formation result in their activation thereby promoting stress fiber formation and motility. RhoGTPases are regulated by guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs), and RhoGDP dissociation inhibitors. FAK can control Rho activity since FAK-src signalling regulates binding and phosphorylation of GAPs and Rho-GEFs (Schlaepfer and Mitra, 2004). Numerous downstream effectors of Rho were described for example the Rho-associated kinases (ROCKs) or p21-activated kinases (PAKs) controlling actin polymerisation, microtubuli and myosin motor proteins.
Rho-ROCK signalling regulates trophoblast motility
Signalling through Rho factors has also been described in trophoblasts. In mice, ROCK2 was suggested to play a role in blood flow through the labyrinth. Targeted deletion of the gene resulted in elevated PAI-1 expression, intrauterine growth restriction and fetal death (Thumkeo et al., 2003). Changes in ROCK2 expression were also noticed in preeclamptic placentae (Ark et al., 2005). With respect to trophoblast motility inhibition of Rho or ROCK were shown to decrease spreading and migration of EVT through fibronectin-coated filters (Shiokawa et al., 2002). Different growth factors may utilize the Rho-ROCK pathway to promote trophoblast motility. For example, inhibition/siRNA-mediated silencing of either Rho, Rac1, CDC42 or ROCK reduced prostaglandin E2-dependent migration of HTR-8/SVneo cells and of EVT in villous explant cultures (Nicola et al., 2008a; Nicola et al., 2008b). Interestingly, depending on the receptor type different RhoGTPases were shown to be involved in IGF-II-induced migration of HTR-8SVneo cells. Signalling of IGF-II through IGFR1 required RhoA and RhoC but not Rac1 or CDC42, whereas IGFR1-independent effects of IGF-II involved ROCK but none of the Rho factors (Shields et al., 2007).
Wnt signalling
Key components of canonical Wnt signalling
Proteins of the Wingless (Wnt) family are secreted regulators playing key roles in embryonic development and tumorigenesis (Gordon and Nusse, 2006). In the canonical pathway Wnt ligands bind to the heterodimeric Frizzled (FZD)/low-density lipoprotein receptor-related protein-5/6 (LRP-5/6) receptors thereby inhibiting the β-catenin destruction complex formed by proteins that include Axin, glycogen synthase kinase 3β (GSK-3β) and adenomatous polyposis coli (APC). In the absence of Wnt ligands active GSK-3β phosphorylates β-catenin thereby keeping cytosolic levels of β-catenin low. Wnt-dependent inactivation of the destruction complex/GSK-3β results in cytoplasmic accumulation and nuclear translocation of dephosphorylated β-catenin. Nuclear β-catenin then provides the activation domain of the lymphoid enhancer binding factor 1 (LEF-1)/T-cell factor (TCF) transcription factor family which induce growth- and invasion-associated genes such as cyclin D1, c-myc, MMP-7 and MT1-MMP. To control canonical Wnt signalling cells also produce soluble proteins such as different Dickkopf (DKK) members which inhibit the pathway upon interaction with LRP-5/6.
Role of Wnt/TCF in trophoblast differentiation and invasion
Throughout the years evidence accumulated that Wnt signalling could also be involved in placental development and trophoblast differentiation. Homozygous mutation of Wnt2, Wnt7b and LEF-1/TCF in mice resulted in different placental pathologies (Galceran et al., 1999; Monkley et al., 1996; Parr et al., 2001). Implantation in mice was shown to be associated with increased expression of Wnt4 and inhibition of canonical signalling impaired the process (Mohamed et al., 2005; Paria et al., 2001).
In human placenta, 14 out of 19 Wnt ligands and 8 out of 10 FZD receptors, respectively, were detectable indicating that Wnt signalling could be involved in trophoblast function/differentiation (Sonderegger et al., 2007). Interestingly, expression patterns of distinct Wnt ligands varied with gestational age and between different trophoblast subtypes suggesting cell-specific functions (Sonderegger et al., 2007). Indeed, trophoblast invasion was associated with nuclear accumulation of TCFs in EVTs in vitro as well as in vivo (Pollheimer et al., 2006). Activation of the pathway by a recombinant Wnt ligand induced migration and invasion of SGHPL-5 cells and CTBs which could be specifically inhibited by soluble DKK1 (Pollheimer et al., 2006).
The decidua which is generally considered as an anti-invasive environment expresses Wnt3 and DKK in a menstrual-cycle dependent manner (Tulac et al., 2003). In particular, DKK1 was shown to be highly expressed during the secretory phase and could be upregulated by progesterone in vitro (Tulac et al., 2006) suggesting that decidual expression of the inhibitor may represent another mechanism limiting trophoblast invasiveness. Indeed, inhibition with DKK1 reduced basal trophoblast migration/invasion (Pollheimer et al., 2006) indicating that autocrine Wnt ligands contribute to the inherent, invasive properties of EVT.
Besides canonical signalling recent data suggest that Wnt can also stimulate trophoblast migration through activation of the PI3K-AKT pathway (S. Sonderegger and M. Knöfler, unpublished). Since phosphorylation of AKT by a recombinant Wnt ligand could not be inhibited by DKK1, non-canonical Wnt receptor(s) but not FZD-LRP-5/6 must be involved. Evidence that aberrant Wnt signalling may play a role in human placental pathologies came from analyses of CHM placentae (Pollheimer et al., 2006). Increased nuclear expression of β-catenin in EVT of these placentae suggested that, similar to tumour cells, mutations of critical Wnt signalling components and /or overexpression of Wnt ligands may contribute to the pre-malignant status of CHM.
Transcription factors in trophoblast invasion
General aspects
In mice, critical tissue-specific transcription factors controlling development of different trophoblast subtypes have been described (Cross et al., 2003). In humans, however, it’s largely unclear which factors commit and differentiate invasive trophoblasts (Loregger et al., 2003). Although transcriptional regulators important for invasive giant cell differentiation of mice were elucidated, expression and distribution of their human counter-parts do not necessarily suggest equivalent roles (Meinhardt et al., 2005). This might be explained by differences in placental morphologies/trophoblast subtypes as well as by the fact that trophoblast differentiation of humans can only be monitored at few time-frames of gestation. For example, the basic helix-loop-helix (bHLH) protein Hand1 promotes differentiation of precursors into invasive giant cells during early murine pregnancy (Riley et al., 1998).
In humans, however, Hand1 expression can only be detected in blastocysts and upon trophectoderm differentiation in vitro but not in any of the different trophoblast cell types of first trimester placentae (Knöfler et al., 2002; Peiffer et al., 2007). Hence, it is possible that determination of human trophoblasts subtypes occurs early in development and cannot be studied in first trimester placental tissue usually obtained between 8th and 12th week of gestation. On the other hand, several homeobox genes potentially controlling commitment and differentiation were identified in human invasive trophoblasts (Quinn et al., 1997; Quinn et al., 1998).
Regulatory transcription factors in humans
Several transcription factors were shown to be involved in human trophoblast invasion based on in vitro experiments with trophoblast cell lines or isolated CTBs/EVTs. Id-2, an inhibitor of bHLH proteins, was found to be highly expressed in cell columns but decreased upon differentiation into EVT (Janatpour et al., 2000). Overexpression of Id-2 in CTBs reduced in vitro invasiveness.
Signal transducer and activator of transcription 3 (STAT3) was identified in JEG-3 cells and first trimester trophoblasts but not in term cells suggesting a role in trophoblast invasion (Corvinus et al., 2003). In general, phosphorylation, dimerisation and nuclear translocation of STATs are achieved upon growth factor/cytokine-dependent activation of receptor-associated Janus kinases (JAKs). Leptin was shown to increase protease expression and STAT3 activity and siRNA-mediated down-regulation of STAT3 reduced in vitro invasion of JEG-3 cells and CTBs (Fitzgerald et al., 2005; Poehlmann et al., 2005). Similarly, invasion-promoting effects of IL-11 on primary EVT are potentially triggered through STAT3 (Paiva et al., 2007). Another STAT factor involved in trophoblast invasion is STAT5. Human placental growth hormone (hPGH) stimulates STAT5 binding activity through activation of Janus kinases 2 (JAK2) as well as invasion of purified, primary EVT in a JAK2-dependent manner.
Invasive trophoblasts also express one isoform of the Ikaros (Ik) transcription factors, namely Ikx. Overexpression of a dominant-negative Ikx, lacking DNA binding, was shown to inhibit migration of HTR-8/SVneo cells (Yamamoto et al., 2005).
Finally, different studies indicate that peroxisome proliferator-activated receptor-gamma (PPAR-γ) plays a critical role in placental function and trophoblast invasion (Schaiff et al., 2006). Activation of PPAR-γ with synthetic ligands reduced invasion of primary CTB and trophoblastic HIPEC 65 cells whereas antagonists increased invasiveness suggesting an inhibitory role of the nuclear receptor in trophoblast motility (Pavan et al., 2003; Tarrade et al., 2001b). Interestingly, activation of PPAR-γ was recently shown to impair hCG expression and secretion from EVT suggesting that the negative effects of the transcription on trophoblast invasion could be mediated through reduced levels of the pregnancy hormone (Handschuh et al., 2007b).
Conclusion
Multiple growth factors expressed at the fetal-maternal are involved in the regulation of trophoblast migration and invasion (Fig. 3). Signalling of these factors occurs through pathways also modulating motility in other cellular systems, for example ERK, FAK or WNT signalling. Since invasiveness represents an inherent property of EVT, signal transduction pathways which are not primarily operational in controlling cell migration, such as the PI3K-AKT pathway, are also critically involved. Similar to other cell types most growth factors do not signal through a single cascades but stimulate invasiveness through a range of pathways. Whereas the number of growth factors, cytokines and chemokines affecting trophoblast motility are steadily increasing their downstream effectors such as critical transcription factors and their target genes are largely unknown. However, some of the nuclear factors, for example STATs, PPAR-γ, homeobox genes or WNT-dependent TCFs have been identified, which based on functional analyses or their expression pattern likely play key roles in trophoblast invasion. It can be speculated that these genes may control markers of the differentiated, invasive trophoblast such as integrins, EVT-specific hormones, and different protease systems. However, much remains to be learned about physiological trophoblast invasion and whether changes in activity of certain signalling cascades may contribute to the pathogenesis of pregnancy diseases with abnormal placentation or failed trophoblast differentiation.
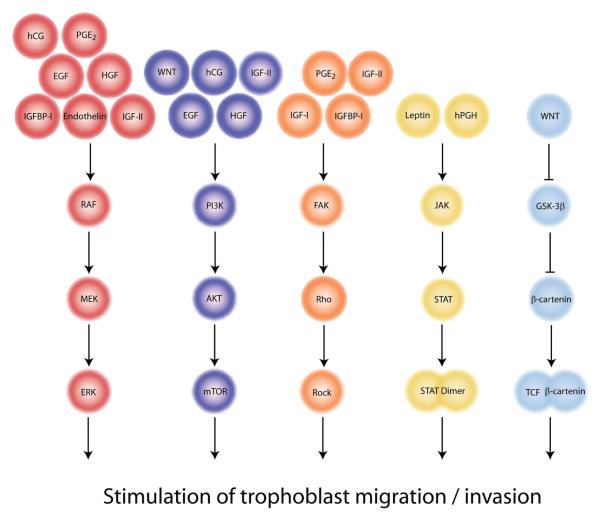
Fig. 3. Schematic presentation of signalling pathways stimulating trophoblast migration and/or invasion.
Growth factors acting through these pathways are indicated. Sequential signalling steps resulting in phosphorylation (arrows) or dephosphorylation of downstream kinases or other protein targets (STAT, β-catenin) are depicted. Several critical factors such as EGF, IGFs, hCG or WNT signal through more than one pathway.
Acknowledgements
Trophoblast research in the laboratory of M. Knöfler is supported by grant Nrs. 11772 and 12487 of the Jubiläumsfonds of the Austrian National Bank and by grant Nr. P-17894-B14 of the Austrian Science Funds.
Abbreviations used in this paper
- CHM
complete hydatidiform mole
- CTB
cytotrophoblast
- DKK
Dickkopf
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- EGF
epidermal growth factor
- ERK
extacellular regulated kinase
- EVT
extravillous trophoblast
- FAK
focal adhesion kinase.
References
- ALDO PB, KRIKUN G, VISINTIN I, LOCKWOOD C, ROMERO R, MOR G. A novel three-dimensional in vitro system to study trophoblast-endothelium cell interactions. Am J Reprod Immunol. 2007;58:98–110. doi: 10.1111/j.1600-0897.2007.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APLIN JD, HAIGH T, JONES CJ, CHURCH HJ, VICOVAC L. Development of cytotrophoblast columns from explanted first-trimester human placental villi: Role of fibronectin and integrin alpha5beta1. Biol Reprod. 1999;60:828–838. doi: 10.1095/biolreprod60.4.828. [DOI] [PubMed] [Google Scholar]
- APLIN JD, STRASZEWSKI-CHAVEZ SL, KALIONIS B, DUNK C, MORRISH D, FORBES K, BACZYK D, ROTE N, MALASSINE A, KNÖFLER M. Trophoblast differentiation: Progenitor cells, fusion and migration — a workshop report. Placenta. 2006;27(Suppl A):S141–S143. doi: 10.1016/j.placenta.2006.01.011. [DOI] [PubMed] [Google Scholar]
- ARK M, YILMAZ N, YAZICI G, KUBAT H, AKTAS S. Rho-associated protein kinase ii (rock ii) expression in normal and preeclamptic human placentas. Placenta. 2005;26:81–84. doi: 10.1016/j.placenta.2004.03.012. [DOI] [PubMed] [Google Scholar]
- BACZYK D, DUNK C, HUPPERTZ B, MAXWELL C, REISTER F, GIANNOULIAS D, KINGDOM JC. Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi. Placenta. 2006;27:367–374. doi: 10.1016/j.placenta.2005.03.006. [DOI] [PubMed] [Google Scholar]
- BAUER S, POLLHEIMER J, HARTMANN J, HUSSLEIN P, APLIN JD, KNÖFLER M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- BILBAN M, GHAFFARI-TABRIZI N, HINTERMANN E, BAUER S, MOLZER S, ZORATTI C, MALLI R, SHARABI A, HIDEN U, GRAIER W, et al. Kisspeptin-10, a kiss-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- BISCHOF P, MEISSER A, CAMPANA A. Paracrine and autocrine regulators of trophoblast invasion—a review. Placenta. 2000;21(Suppl A):S55–S60. doi: 10.1053/plac.2000.0521. [DOI] [PubMed] [Google Scholar]
- BURTON GJ, JAUNIAUX E, CHARNOCK-JONES DS. Human early placental development: Potential roles of the endometrial glands. Placenta. 2007;28(Suppl A):S64–S69. doi: 10.1016/j.placenta.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTWRIGHT JE, KENNY LC, DASH PR, CROCKER IP, APLIN JD, BAKER PN, WHITLEY GS. Trophoblast invasion of spiral arteries: A novel in vitro model. Placenta. 2002a;23:232–235. doi: 10.1053/plac.2001.0760. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT JE, TSE WK, WHITLEY GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002b;279:219–226. doi: 10.1006/excr.2002.5616. [DOI] [PubMed] [Google Scholar]
- CHAKRABORTY C, BARBIN YP, CHAKRABARTI S, CHIDIAC P, DIXON SJ, LALA PK. Endothelin-1 promotes migration and induces elevation of [ca2+]i and phosphorylation of map kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol. 2003;201:63–73. doi: 10.1016/s0303-7207(02)00431-8. [DOI] [PubMed] [Google Scholar]
- CHOY MY, MANYONDA IT. The phagocytic activity of human first trimester extravillous trophoblast. Hum Reprod. 1998;13:2941–2949. doi: 10.1093/humrep/13.10.2941. [DOI] [PubMed] [Google Scholar]
- CHOY MY, ST WHITLEY G, MANYONDA IT. Efficient, rapid and reliable establishment of human trophoblast cell lines using poly-l-ornithine. Early Pregnancy. 2000;4:124–143. [PubMed] [Google Scholar]
- CHURCH HJ, VICOVAC LM, WILLIAMS JD, HEY NA, APLIN JD. Laminins 2 and 4 are expressed by human decidual cells. Lab Invest. 1996;74:21–32. [PubMed] [Google Scholar]
- CLARK DE, SMITH SK, HE Y, DAY KA, LICENCE DR, CORPS AN, LAMMOGLIA R, CHARNOCK-JONES DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- CORVINUS FM, FITZGERALD JS, FRIEDRICH K, MARKERT UR. Evidence for a correlation between trophoblast invasiveness and stat3 activity. Am J Reprod Immunol. 2003;50:316–321. doi: 10.1034/j.1600-0897.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- CROSS JC, BACZYK D, DOBRIC N, HEMBERGER M, HUGHES M, SIMMONS DG, YAMAMOTO H, KINGDOM JC. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- DAMSKY CH, LIBRACH C, LIM KH, FITZGERALD ML, MCMASTER MT, JANATPOUR M, ZHOU Y, LOGAN SK, FISHER SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- DRAKE PM, RED-HORSE K, FISHER SJ. Reciprocal chemokine receptor and ligand expression in the human placenta: Implications for cytotrophoblast differentiation. Dev Dyn. 2004;229:877–885. doi: 10.1002/dvdy.10477. [DOI] [PubMed] [Google Scholar]
- ELUSTONDO PA, HANNIGAN GE, CANIGGIA I, MACPHEE DJ. Integrin-linked kinase (ilk) is highly expressed in first trimester human chorionic villi and regulates migration of a human cytotrophoblast-derived cell line. Biol Reprod. 2006;74:959–968. doi: 10.1095/biolreprod.105.050419. [DOI] [PubMed] [Google Scholar]
- FEINBERG RF, KAO LC, HAIMOWITZ JE, QUEENAN JT, JR., WUN TC, STRAUSS JF, 3RD, KLIMAN HJ. Plasminogen activator inhibitor types 1 and 2 in human trophoblasts. Pai-1 is an immunocytochemical marker of invading trophoblasts. Lab Invest. 1989;61:20–26. [PubMed] [Google Scholar]
- FERRETTI C, BRUNI L, DANGLES-MARIE V, PECKING AP, BELLET D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- FISHER SJ, CUI TY, ZHANG L, HARTMAN L, GRAHL K, ZHANG GY, TARPEY J, DAMSKY CH. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989;109:891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD JS, TSAREVA SA, POEHLMANN TG, BEROD L, MEISSNER A, CORVINUS FM, WIEDERANDERS B, PFITZNER E, MARKERT UR, FRIEDRICH K. Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol. 2005;37:2284–2296. doi: 10.1016/j.biocel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- GALABOVA-KOVACS G, MATZEN D, PIAZZOLLA D, MEISSL K, PLYUSHCH T, CHEN AP, SILVA A, BACCARINI M. Essential role of b-raf in erk activation during extraembryonic development. Proc Natl Acad Sci USA. 2006;103:1325–1330. doi: 10.1073/pnas.0507399103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALCERAN J, FARINAS I, DEPEW MJ, CLEVERS H, GROSSCHEDL R. Wnt3a-/—like phenotype and limb deficiency in lef1(−/−)tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENBACEV O, JENSEN KD, POWLIN SS, MILLER RK. In vitro differentiation and ultrastructure of human extravillous trophoblast (evt) cells. Placenta. 1993;14:463–475. doi: 10.1016/s0143-4004(05)80466-7. [DOI] [PubMed] [Google Scholar]
- GERAMI-NAINI B, DOVZHENK OV, DURNING M, WEGNER FH, THOMSON JA, GOLOS TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- GENBACEV O, ZHOU Y, LUDLOW JW, FISHER SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- GIUDICE LC, IRWIN JC. Roles of the insulinlike growth factor family in nonpregnant human endometrium and at the decidual: Trophoblast interface. Semin Reprod Endocrinol. 1999;17:13–21. doi: 10.1055/s-2007-1016207. [DOI] [PubMed] [Google Scholar]
- GLEESON LM, CHAKRABORTY C, MCKINNON T, LALA PK. Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein kinase pathway. J Clin Endocrinol Metab. 2001;86:2484–2493. doi: 10.1210/jcem.86.6.7532. [DOI] [PubMed] [Google Scholar]
- GORDON MD, NUSSE R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- GRAHAM CH, HAWLEY TS, HAWLEY RG, MACDOUGALL JR, KERBEL RS, KHOO N, LALA PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- HALL A. Rho gtpases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- HANDSCHUH K, GUIBOURDENCHE J, TSATSARIS V, GUESNON M, LAURENDEAU I, EVAIN-BRION D, FOURNIER T. Human chorionic gonadotropin expression in human trophoblasts from early placenta: Comparative study between villous and extravillous trophoblastic cells. Placenta. 2007a;28:175–184. doi: 10.1016/j.placenta.2006.01.019. [DOI] [PubMed] [Google Scholar]
- HANDSCHUH K, GUIBOURDENCHE J, TSATSARIS V, GUESNON M, LAURENDEAU I, EVAIN-BRION D, FOURNIER T. Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma. Endocrinology. 2007b;148:5011–5019. doi: 10.1210/en.2007-0286. [DOI] [PubMed] [Google Scholar]
- HANNA J, GOLDMAN-WOHL D, HAMANI Y, AVRAHAM I, GREENFIELD C, NATANSON-YARON S, PRUS D, COHEN-DANIEL L, ARNON TI, MANASTER I, et al. Decidual nk cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- HANNAN NJ, JONES RL, WHITE CA, SALAMONSEN LA. The chemokines, cx3cl1, ccl14, and ccl4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod. 2006;74:896–904. doi: 10.1095/biolreprod.105.045518. [DOI] [PubMed] [Google Scholar]
- HANNAN NJ, SALAMONSEN LA. Role of chemokines in the endometrium and in embryo implantation. Curr Opin Obstet Gynecol. 2007;19:266–272. doi: 10.1097/GCO.0b013e328133885f. [DOI] [PubMed] [Google Scholar]
- HARRIS LK, APLIN JD. Vascular remodeling and extracellular matrix breakdown in the uterine spiral arteries during pregnancy. Reprod Sci. 2007;14:28–34. doi: 10.1177/1933719107309588. [DOI] [PubMed] [Google Scholar]
- HARRIS LK, KEOGH RJ, WAREING M, BAKER PN, CARTWRIGHT JE, APLIN JD, WHITLEY GS. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169:1863–1874. doi: 10.2353/ajpath.2006.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARUN R, RUBAN L, MATIN M, DRAPER J, JENKINS NM, LIEW GC, ANDREWS PW, LI TC, LAIRD SM, MOORE HD. Cytotrophoblast stem cell lines derived from human embryonic stem cells and their capacity to mimic invasive implantation events. Hum Reprod. 2006;21:1349–1358. doi: 10.1093/humrep/del017. [DOI] [PubMed] [Google Scholar]
- HUANG Y, ZHU XY, DU MR, WU X, WANG MY, LI DJ. Chemokine cxcl16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum Reprod. 2006;21:1083–1091. doi: 10.1093/humrep/dei436. [DOI] [PubMed] [Google Scholar]
- HUNG TH, SKEPPER JN, CHARNOCK-JONES DS, BURTON GJ. Hypoxia-reoxygenation: A potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- ILIC D, GENBACEV O, JIN F, CACERES E, ALMEIDA EA, BELLINGARD-DUBOUCHAUD V, SCHAEFER EM, DAMSKY CH, FISHER SJ. Plasma membrane-associated py397fak is a marker of cytotrophoblast invasion in vivo and in vitro. Am J Pathol. 2001;159:93–108. doi: 10.1016/S0002-9440(10)61677-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES JL, STONE PR, CHAMLEY LW. The isolation and characterization of a population of extravillous trophoblast progenitors from first trimester human placenta. Hum Reprod. 2007;22:2111–2119. doi: 10.1093/humrep/dem144. [DOI] [PubMed] [Google Scholar]
- JANATPOUR MJ, MCMASTER MT, GENBACEV O, ZHOU Y, DONG J, CROSS JC, ISRAEL MA, FISHER SJ. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development. 2000;127:549–558. doi: 10.1242/dev.127.3.549. [DOI] [PubMed] [Google Scholar]
- JOHNSTONE ED, MACKOVA M, DAS S, PAYNE SG, LOWEN B, SIBLEY CP, CHAN G, GUILBERT LJ. Multiple anti-apoptotic pathways stimulated by egf in cytotrophoblasts. Placenta. 2005;26:548–555. doi: 10.1016/j.placenta.2004.08.012. [DOI] [PubMed] [Google Scholar]
- JOKHI PP, KING A, LOKE YW. Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface. Cytokine. 1997;9:126–137. doi: 10.1006/cyto.1996.0146. [DOI] [PubMed] [Google Scholar]
- KABIR-SALMANI M, FUKUDA MN, KANAI-AZUMA M, AHMED N, SHIOKAWA S, AKIMOTO Y, SAKAI K, NAGAMORI S, KANAI Y, SUGIHARA K, et al. The membrane-spanning domain of cd98 heavy chain promotes alpha(v)beta3 integrin signals in human extravillous trophoblasts. Mol Endocrinol. 2008;22:707–715. doi: 10.1210/me.2007-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABIR-SALMANI M, SHIOKAWA S, AKIMOTO Y, HASAN-NEJAD H, SAKAI K, NAGAMATSU S, NAKAMURA Y, HOSSEINI A, IWASHITA M. Characterization of morphological and cytoskeletal changes in trophoblast cells induced by insulin-like growth factor-i. J Clin Endocrinol Metab. 2002;87:5751–5759. doi: 10.1210/jc.2002-020550. [DOI] [PubMed] [Google Scholar]
- KAMEI T, JONES SR, CHAPMAN BM, KL MC, DAI G, SOARES MJ. The phosphatidylinositol 3-kinase/akt signaling pathway modulates the endocrine differentiation of trophoblast cells. Mol Endocrinol. 2002;16:1469–1481. doi: 10.1210/mend.16.7.0878. [DOI] [PubMed] [Google Scholar]
- KHONG TY, DE WOLF F, ROBERTSON WB, BROSENS I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- KLIMAN HJ, NESTLER JE, SERMASI E, SANGER JM, STRAUSS JF., 3RD Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- KNÖFLER M, MEINHARDT G, BAUER S, LOREGGER T, VASICEK R, BLOOR DJ, KIMBER SJ, HUSSLEIN P. Human hand1 basic helix-loop-helix (bhlh) protein: Extra-embryonic expression pattern, interaction partners and identification of its transcriptional repressor domains. Biochem J. 2002;361:641–651. doi: 10.1042/0264-6021:3610641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNÖFLER M, STENZEL M, HUSSLEIN P. Shedding of tumour necrosis factor receptors from purified villous term trophoblasts and cytotrophoblastic bewo cells. Hum Reprod. 1998;13:2308–2316. doi: 10.1093/humrep/13.8.2308. [DOI] [PubMed] [Google Scholar]
- KYRIAKIS JM, AVRUCH J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- LALA PK, CHAKRABORTY C. Factors regulating trophoblast migration and invasiveness: Possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–587. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- LALA PK, GRAHAM CH. Mechanisms of trophoblast invasiveness and their control: The role of proteases and protease inhibitors. Cancer Metastasis Rev. 1990;9:369–379. doi: 10.1007/BF00049525. [DOI] [PubMed] [Google Scholar]
- LALA PK, HAMILTON GS. Growth factors, proteases and protease inhibitors in the maternal-fetal dialogue. Placenta. 1996;17:545–555. doi: 10.1016/s0143-4004(96)80071-3. [DOI] [PubMed] [Google Scholar]
- LAMARCA HL, OTT CM, ZU BENTRUP KHONER, LEBLANC CL, PIERSON DL, NELSON AB, SCANDURRO AB, WHITLEY GS, NICKERSON CA, MORRIS CA. Three-dimensional growth of extravillous cytotrophoblasts promotes differentiation and invasion. Placenta. 2005;26:709–720. doi: 10.1016/j.placenta.2004.11.003. [DOI] [PubMed] [Google Scholar]
- LASH GE, OTUN HA, INNES BA, KIRKLEY M, DE OLIVEIRA L, SEARLE RF, ROBSON SC, BULMER JN. Interferon-gamma inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J. 2006;20:2512–2518. doi: 10.1096/fj.06-6616com. [DOI] [PubMed] [Google Scholar]
- LEE JM, DEDHAR S, KALLURI R, THOMPSON EW. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIBRACH CL, FEIGENBAUM SL, BASS KE, CUI TY, VERASTAS N, SADOVSKY Y, QUIGLEY JP, FRENCH DL, FISHER SJ. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269:17125–17131. [PubMed] [Google Scholar]
- LIU J, CHAKRABORTY C, GRAHAM CH, BARBIN YP, DIXON SJ, LALA PK. Noncatalytic domain of upa stimulates human extravillous trophoblast migration by using phospholipase c, phosphatidylinositol 3-kinase and mitogen-activated protein kinase. Exp Cell Res. 2003;286:138–151. doi: 10.1016/s0014-4827(03)00089-2. [DOI] [PubMed] [Google Scholar]
- LOGAN SK, HANSELL EJ, DAMSKY CH, WERB Z. T-antigen inhibits metalloproteinase expression and invasion in human placental cells transformed with temperature-sensitive simian virus 40. Matrix Biol. 1996;15:81–89. doi: 10.1016/s0945-053x(96)90149-3. [DOI] [PubMed] [Google Scholar]
- LOREGGER T, POLLHEIMER J, KNÖFLER M. Regulatory transcription factors controlling function and differentiation of human trophoblast—a review. Placenta. 2003;24(Suppl A):S104–S110. doi: 10.1053/plac.2002.0929. [DOI] [PubMed] [Google Scholar]
- MACPHEE DJ, MOSTACHFI H, HAN R, LYE SJ, POST M, CANIGGIA I. Focal adhesion kinase is a key mediator of human trophoblast development. Lab Invest. 2001;81:1469–1483. doi: 10.1038/labinvest.3780362. [DOI] [PubMed] [Google Scholar]
- MANNING BD, CANTLEY LC. Akt/pkb signaling: Navigating down-stream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANY A, HUBEL CA, FISHER SJ, ROBERTS JM, ZHOU Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol. 2000;156:321–331. doi: 10.1016/S0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFADYEN IR, PRICE AB, GEIRSSON RT. The relation of birthweight to histological appearances in vessels of the placental bed. Br J Obstet Gynaecol. 1986;93:476–481. [PubMed] [Google Scholar]
- MCKINNON T, CHAKRABORTY C, GLEESON LM, CHIDIAC P, LALA PK. Stimulation of human extravillous trophoblast migration by igf-ii is mediated by igf type 2 receptor involving inhibitory g protein(s) and phosphorylation of mapk. J Clin Endocrinol Metab. 2001;86:3665–3674. doi: 10.1210/jcem.86.8.7711. [DOI] [PubMed] [Google Scholar]
- MEINHARDT G, HUSSLEIN P, KNÖFLER M. Tissue-specific and ubiquitous basic helix-loop-helix transcription factors in human placental trophoblasts. Placenta. 2005;26:527–39. doi: 10.1016/j.placenta.2004.09.005. [DOI] [PubMed] [Google Scholar]
- MEISSER A, CAMEO P, ISLAMI D, CAMPANA A, BISCHOF P. Effects of interleukin-6 (il-6) on cytotrophoblastic cells. Mol Hum Reprod. 1999;5:1055–1058. doi: 10.1093/molehr/5.11.1055. [DOI] [PubMed] [Google Scholar]
- MOHAMED OA, JONNAERT M, LABELLE-DUMAIS C, KURODA K, CLARKE HJ, DUFORT D. Uterine wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci USA. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONKLEY SJ, DELANEY SJ, PENNISI DJ, CHRISTIANSEN JH, WAINWRIGHT BJ. Targeted disruption of the wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- MOON KC, PARK JS, NORWITZ ER, KIM DI, OH KJ, PARK CW, JUN JK, SYN HC. Expression of extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinase in the invasive trophoblasts at the human placental bed. Placenta. 2008;29:391–395. doi: 10.1016/j.placenta.2008.02.001. [DOI] [PubMed] [Google Scholar]
- NICOLA C, CHIRPAC A, LALA PK, CHAKRABORTY C. Roles of rho guanosine 5′-triphosphatase a, rho kinases, and extracellular signal regulated kinase (1/2) in prostaglandin e2-mediated migration of first-trimester human extravillous trophoblast. Endocrinology. 2008a;149:1243–1251. doi: 10.1210/en.2007-1136. [DOI] [PubMed] [Google Scholar]
- NICOLA C, LALA PK, CHAKRABORTY C. Prostaglandin e2-mediated migration of human trophoblast requires rac1 and cdc42. Biol Reprod. 2008b;78:976–982. doi: 10.1095/biolreprod.107.065433. [DOI] [PubMed] [Google Scholar]
- PAIVA P, SALAMONSEN LA, MANUELPILLAI U, WALKER C, TAPIA A, WALLACE EM, DIMITRIADIS E. Interleukin-11 promotes migration, but not proliferation, of human trophoblast cells, implying a role in placentation. Endocrinology. 2007;148:5566–5572. doi: 10.1210/en.2007-0517. [DOI] [PubMed] [Google Scholar]
- PARIA BC, MA W, TAN J, RAJA S, DAS SK, DEY SK, HOGAN BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARR BA, CORNISH VA, CYBULSKY MI, MCMAHON AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237:324–332. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- PAVAN L, TARRADE A, HERMOUET A, DELOUIS C, TITEUX M, VIDAUD M, THEROND P, EVAIN-BRION D, FOURNIER T. Human invasive trophoblasts transformed with simian virus 40 provide a new tool to study the role of ppargamma in cell invasion process. Carcinogenesis. 2003;24:1325–1336. doi: 10.1093/carcin/bgg074. [DOI] [PubMed] [Google Scholar]
- PEIFFER I, BELHOMME D, BARBET R, HAYDONT V, ZHOU YP, FORTUNEL NO, LI M, HATZFELD A, FABIANI JN, HATZFELD JA. Simultaneous differentiation of endothelial and trophoblastic cells derived from human embryonic stem cells. Stem Cells Dev. 2007;16:393–402. doi: 10.1089/scd.2006.0013. [DOI] [PubMed] [Google Scholar]
- PIJNENBORG R, ANTHONY J, DAVEY DA, REES A, TILTMAN A, VERCRUYSSE L, VAN ASSCHE A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- PIJNENBORG R, BLAND JM, ROBERTSON WB, BROSENS I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- PIJNENBORG R, DIXON G, ROBERTSON WB, BROSENS I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- POEHLMANN TG, FITZGERALD JS, MEISSNER A, WENGENMAYER T, SCHLEUSSNER E, FRIEDRICH K, MARKERT UR. Trophoblast invasion: Tuning through lif, signalling via stat3. Placenta. 2005;26(Suppl A):S37–S41. doi: 10.1016/j.placenta.2005.01.007. [DOI] [PubMed] [Google Scholar]
- POLLHEIMER J, HUSSLEIN P, KNÖFLER M. Invasive trophoblasts generate regulatory collagen xviii cleavage products. Placenta. 2005a;26(Suppl A):S42–S45. doi: 10.1016/j.placenta.2004.12.005. [DOI] [PubMed] [Google Scholar]
- POLLHEIMER J, KNÖFLER M. Signalling pathways regulating the invasive differentiation of human trophoblasts: A review. Placenta. 2005b;26(Suppl A):S21–S30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- POLLHEIMER J, LOREGGER T, SONDEREGGER S, SALEH L, BAUER S, BILBAN M, CZERWENKA K, HUSSLEIN P, KNÖFLER M. Activation of the canonical wingless/t-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168:1134–1147. doi: 10.2353/ajpath.2006.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRAKOBPHOL A, GENBACEV O, GORMLEY M, KAPIDZIC M, FISHER SJ. A role for the l-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol. 2006;298:107–117. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- PRAST J, SALEH L, HUSSLEIN H, SONDEREGGER S, HELMER H, KNÖFLER M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and akt signaling. Endocrinology. 2008;149:979–987. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULLIKUTH AK, CATLING AD. Scaffold mediated regulation of mapk signaling and cytoskeletal dynamics: A perspective. Cell Signal. 2007;19:1621–1632. doi: 10.1016/j.cellsig.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIU Q, BASAK A, MBIKAY M, TSANG BK, GRUSLIN A. Role of pro-igf-ii processing by proprotein convertase 4 in human placental development. Proc Natl Acad Sci USA. 2005;102:11047–11052. doi: 10.1073/pnas.0502357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIU Q, YANG M, TSANG BK, GRUSLIN A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod. 2004a;10:677–684. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- QIU Q, YANG M, TSANG BK, GRUSLIN A. Egf-induced trophoblast secretion of mmp-9 and timp-1 involves activation of both pi3k and mapk signalling pathways. Reproduction. 2004b;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- QUINN LM, LATHAM SE, KALIONIS B. Homeobox gene hb24, a regulator of haematopoiesis, is a candidate for regulating differentiation of the extra-embryonic trophoblast cell lineage. Reprod Fertil Dev. 1997;9:617–623. doi: 10.1071/r97025. [DOI] [PubMed] [Google Scholar]
- QUINN LM, LATHAM SE, KALIONIS B. A distal-less class homeobox gene, dlx4, is a candidate for regulating epithelial-mesenchymal cell interactions in the human placenta. Placenta. 1998;19:87–93. doi: 10.1016/s0143-4004(98)90103-5. [DOI] [PubMed] [Google Scholar]
- RED-HORSE K, KAPIDZIC M, ZHOU Y, FENG KT, SINGH H, FISHER SJ. Ephb4 regulates chemokine-evoked trophoblast responses: A mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132:4097–106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
- RILEY P, ANSON-CARTWRIGHT L, CROSS JC. The hand1 bhlh transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- SCHAIFF WT, BARAK Y, SADOVSKY Y. The pleiotropic function of ppar gamma in the placenta. Mol Cell Endocrinol. 2006;249:10–5. doi: 10.1016/j.mce.2006.02.009. [DOI] [PubMed] [Google Scholar]
- SCHATZ F, LOCKWOOD CJ. Progestin regulation of plasminogen activator inhibitor type 1 in primary cultures of endometrial stromal and decidual cells. J Clin Endocrinol Metab. 1993;77:621–625. doi: 10.1210/jcem.77.3.8370684. [DOI] [PubMed] [Google Scholar]
- SCHLAEPFER DD, HAUCK CR, SIEG DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- SCHLAEPFER DD, MITRA SK. Multiple connections link fak to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- SHIELDS SK, NICOLA C, CHAKRABORTY C. Rho guanosine 5′-triphosphatases differentially regulate insulin-like growth factor i (igf-i) receptor-dependent and -independent actions of igf-ii on human trophoblast migration. Endocrinology. 2007;148:4906–4917. doi: 10.1210/en.2007-0476. [DOI] [PubMed] [Google Scholar]
- SHIOKAWA S, IWASHITA M, AKIMOTO Y, NAGAMATSU S, SAKAI K, HANASHI H, KABIR-SALMANI M, NAKAMURA Y, UEHATA M, YOSHIMURA Y. Small guanosine triphospatase rhoa and rho-associated kinase as regulators of trophoblast migration. J Clin Endocrinol Metab. 2002;87:5808–5816. doi: 10.1210/jc.2002-020376. [DOI] [PubMed] [Google Scholar]
- SONDEREGGER S, HUSSLEIN H, LEISSER C, KNÖFLER M. Complex expression pattern of wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta. 2007;28(Suppl A):S97–S102. doi: 10.1016/j.placenta.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARRADE A, KUEN RLAI, MALASSINE A, TRICOTTET V, BLAIN P, VIDAUD M, EVAIN-BRION D. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001a;81:1199–1211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- TARRADE A, SCHOONJANS K, PAVAN L, AUWERX J, ROCHETTE-EGLY C, EVAIN-BRION D, FOURNIER T. Ppargamma/rxralpha heterodimers control human trophoblast invasion. J Clin Endocrinol Metab. 2001b;86:5017–5024. doi: 10.1210/jcem.86.10.7924. [DOI] [PubMed] [Google Scholar]
- THUMKEO D, KEEL J, ISHIZAKI T, HIROSE M, NONOMURA K, OSHIMA H, OSHIMA M, TAKETO MM, NARUMIYA S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TJOA ML, LEVINE RJ, KARUMANCHI SA. Angiogenic factors and preeclampsia. Front Biosci. 2007;12:2395–2402. doi: 10.2741/2241. [DOI] [PubMed] [Google Scholar]
- TULAC S, NAYAK NR, KAO LC, VAN WAES M, HUANG J, LOBO S, GERMEYER A, LESSEY BA, TAYLOR RN, SUCHANEK E, et al. Identification, characterization, and regulation of the canonical wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- TULAC S, OVERGAARD MT, HAMILTON AE, JUMBE NL, SUCHANEK E, GIUDICE LC. Dickkopf-1, an inhibitor of wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab. 2006;91:1453–1461. doi: 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- VARANOU A, WITHINGTON SL, LAKASING L, WILLIAMSON C, BURTON GJ, HEMBERGER M. The importance of cysteine cathepsin proteases for placental development. J Mol Med. 2006;84:305–317. doi: 10.1007/s00109-005-0032-2. [DOI] [PubMed] [Google Scholar]
- VENKATESHA S, TOPORSIAN M, LAM C, HANAI J, MAMMOTO T, KIM YM, BDOLAH Y, LIM KH, YUAN HT, LIBERMANN TA, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- VICOVAC L, JONES CJ, APLIN JD. Trophoblast differentiation during formation of anchoring villi in a model of the early human placenta in vitro. Placenta. 1995;16:41–56. doi: 10.1016/0143-4004(95)90080-2. [DOI] [PubMed] [Google Scholar]
- WELLS M. The pathology of gestational trophoblastic disease: Recent advances. Pathology. 2007;39:88–96. doi: 10.1080/00313020601137367. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO E, ITO T, ABE A, SIDO F, INO K, ITAKURA A, MIZUTANI S, DOVAT S, NOMURA S, KIKKAWA F. Ikaros is expressed in human extravillous trophoblasts and involved in their migration and invasion. Mol Hum Reprod. 2005;11:825–831. doi: 10.1093/molehr/gah239. [DOI] [PubMed] [Google Scholar]
- YANG M, LEI ZM, CH VRAO. The central role of human chorionic gonadotropin in the formation of human placental syncytium. Endocrinology. 2003a;144:1108–1120. doi: 10.1210/en.2002-220922. [DOI] [PubMed] [Google Scholar]
- YANG ZZ, TSCHOPP O, HEMMINGS-MIESZCZAK M, FENG J, BRODBECK D, PERENTES E, HEMMINGS BA. Protein kinase b alpha/akt1 regulates placental development and fetal growth. J Biol Chem. 2003b;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- ZDRAVKOVIC M, ABOAGYE-MATHIESEN G, GUIMOND MJ, HAGER H, EBBESEN P, LALA PK. Susceptibility of mhc class i expressing extravillous trophoblast cell lines to killing by natural killer cells. Placenta. 1999;20:431–440. doi: 10.1053/plac.1999.0393. [DOI] [PubMed] [Google Scholar]
- ZHOU Y, FISHER SJ, JANATPOUR M, GENBACEV O, DEJANA E, WHEELOCK M, DAMSKY CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYBINA TG, KAUFMANN P, FRANK HG, FREED J, KADYROV M, BIESTERFELD S. Genome multiplication of extravillous trophoblast cells in human placenta in the course of differentiation and invasion into endometrium and myometrium. I. Dynamics of polyploidization. Tsitologiia. 2002;44:1058–1067. [PubMed] [Google Scholar]