Abstract
Invasion of human trophoblasts is promoted through activation of wingless (Wnt) signaling, suggesting a role of the pathway in placental development and morphogenesis. However, details on the process such as involvement of canonical and/or noncanonical Wnt signaling cascades as well as their target genes are largely unknown. Hence, signal transduction via canonical Wnt signaling or phosphatidylinositide 3-kinase (PI3K)/AKT and their cross talk as well as trophoblast-specific protease expression were investigated in trophoblastic SGHPL-5 cells and primary extravillous trophoblasts purified from first-trimester placentas. Western blot analyses revealed that the recombinant Wnt ligand Wnt-3A increased phosphorylation of AKT and the downstream kinase glycogen synthase kinase (GSK)-3β as well as accumulation of activated, nuclear β-catenin. In accordance, luciferase expression of a canonical Wnt/TCF reporter and cell migration in first-trimester villous explant cultures and of SGHPL-5 cells were stimulated. Chemical inhibition of PI3K abolished Wnt-dependent phosphorylation of AKT and GSK-3β and trophoblast motility but did not affect appearance of activated β-catenin or Wnt/TCF reporter activity. In contrast, inhibition of the canonical pathway through soluble Dickkopf-1 did not influence AKT and GSK-3β phosphorylation but reduced Wnt reporter activity, accumulation of active β-catenin, and cell migration. Both inhibitors decreased Wnt-3A-induced secretion of pro- and active matrix metalloproteinase-2 from SGHPL-5 cells and pure EVT. The data suggest that Wnt-3A may activate canonical Wnt signaling and PI3K/AKT through distinct receptors. The two signaling cascades act independently in trophoblasts; however, both pathways promote Wnt-dependent migration and the release of matrix metalloproteinase-2, which has been identified as novel Wnt target in invasive trophoblasts.
The remarkable process of invasive trophoblast differentiation is essential for placental development, fetal well-being, and pregnancy success. During early gestation, invasive trophoblasts develop from proliferative cell columns of anchoring villi to invade stroma and arterial vessels of the maternal decidua. Remodeling of the uterine arteries by these extravillous trophoblasts (EVT) is thought to initiate and maintain blood flow into the placenta by increasing vessel diameter and conductivity (1). Failures in the differentiation process are associated with different gestational diseases. Whereas severe forms of intrauterine growth retardation and preeclampsia are associated with limited invasion and incomplete transformation of spiral arteries, extensive trophoblast invasion was noticed for example in choriocarcinomas and placenta accreta (2, 3).
Numerous growth factors and signaling cascades of the fetal-maternal interface control timing and extent of trophoblast invasion (4-6). However, it is largely unknown whether changes in signaling may contribute to placental pathologies with extensive proliferation and/or invasion (7). Some of the few examples are alterations in epidermal growth factor (EGF) signaling components and angiogenic factors which could be detected in complete hydatidiform mole placentas (CHM) lacking embryonic tissue as well as in cases of placenta accreta (8, 9). Recent evidence suggested that wingless (Wnt) signaling could also be involved in the pathogenesis of CHM. Similar to observations in tumor cells, immunohistochemical analyses revealed abundant nuclear staining of β-catenin in invasive trophoblasts of CHM placentas (10). Because nuclear β-catenin is a key component of canonical Wnt signaling, aberrant activation of the particular pathway was suggested. Indeed, accumulating data indicate that Wnt signaling, which is critically involved in development and tumorigenesis (11), could also play a substantial role in placental development and trophoblast differentiation. In mice, gene ablation of the Wnt-dependent transcription factors lymphoid enhancer-binding factor (LEF)-1/T cell factor (TCF) and of the Wnt ligands Wnt-2 and Wnt-7b affected critical steps of extraembryonic development such as placental vascularization or chorion-allantoic fusion (12-14). Moreover, inhibition of canonical Wnt signaling impaired murine implantation, likely involving ligands such as Wnt-4, which is induced upon blastocyst attachment (15, 16).
Similarly, Wnt signaling pathways may control endometrial function, implantation, and placental development in humans. Endometrial cells express Wnt ligands and the soluble inhibitor Dickkopf-1 (Dkk1) in a menstrual cycle-dependent manner (17, 18). Dkk1 blocks canonical Wnt signaling upon interaction with lipoprotein receptor-related protein (LRP)-5 or -6, which form heterodimeric receptors with Frizzled (Fzd) family members. Human placentas were shown to express 14 of 19 Wnt ligands and eight of 10 Fzd receptors, respectively, suggesting that Wnt signaling may also control trophoblast function and differentiation (19). The expression pattern of distinct Wnt and Fzd varied with gestational age and between trophoblast subtypes, which might indicate cell-specific functions of Wnt ligands and involvement of different Wnt signaling pathways. So far, canonical Wnt signaling was shown to be critical for invasive trophoblast differentiation. Activation of the pathway by a recombinant Wnt ligand enhanced migration and invasion of SGHPL-5 cells and first-trimester cytotrophoblasts, which could be specifically inhibited by Dkk1 (10). On the other hand, Dkk1 could also play a positive role in implantation because blocking of the inhibitor reduced attachment and outgrowth of mouse ectoplacental cones (20).
Wnt signaling through the canonical pathway results in inactivation of the β-catenin destruction complex formed by proteins that include Axin, glycogen synthase kinase (GSK)-3β, and adenomatous polyposis coli (APC) (11). Inhibition of the complex/GSK-3β increases cytosolic levels and nuclear accumulation of β-catenin, which binds and activates LEF-1/TCF factors controlling proliferation- and invasion-associated genes. However, Wnt ligands, such as Wnt-3A, may also signal through noncanonical, β-catenin-independent pathways involving different receptors and downstream cascades such as Ca2+-dependent signaling or activation of ERK and AKT (21-23). Also, Wnt-3A was shown to initiate trophectoderm lineage differentiation (24).
To gain more insights into Wnt-regulated trophoblast motility, we therefore investigated Wnt-3A-mediated signaling cascades in SGHPL-5 cells and primary EVT. In particular, activation of phosphatidylinositide 3-kinase (PI3K)/AKT was studied because the pathway could potentially cross talk to canonical Wnt signaling through AKT-dependent inactivation of GSK-3β (25). To initiate identification of possible Wnt-3A-dependent target genes, the effects on matrix metalloproteinase (MMP)-2 expression and secretion were studied because the enzyme has been identified as one of the critical regulators of trophoblast invasiveness (26). The data suggest that Wnt-3A signaling promotes trophoblast motility and secretion of MMP-2 involving the canonical Wnt pathway as well as activation of PI3K-AKT.
Materials and Methods
Collection of placental tissues
Placental tissues of early (n = 80, between wk 7 and 8) pregnancy were obtained from legal abortions of uncomplicated pregnancies. Use of tissues was approved by the ethical committee of the Medical University of Vienna.
Cell culture of primary extravillous cytotrophoblasts
EVT of pooled first-trimester placentas (n = 70; pool size between seven and 10 placentas) were isolated by adapted enzymatic dispersion and Percoll (10–70%) density gradient centrifugation using a previously established protocol (27). Briefly, first-trimester placental tissue was washed with ice-cold PBS and Hanks’ balanced salt solution, villous tips were scraped with a scalpel blade and digested for 15 min in 0.125% trypsin (Life Technologies, Inc., Rockville, MD), 1% deoxyribonuclease I (Sigma Chemical Co., St. Louis, MO) in Mg/Ca-free Hanks’ balanced salt solution (Sigma). After percolation through a cell strainer (70 μm; Greiner, Kremsmünster, Australia), suspension was transferred on a Percoll gradient. Cell populations between the 35 and 50% layer were seeded on dishes coated with growth factor-reduced Matrigel (Becton Dickinson, Franklin Lakes, NJ). Cells were routinely checked on chamber slides 12 h after isolation using immunofluorescence with antibodies against cytokeratin 7 (clone OV-TL 12/30, 8.3 μg/ml; Dako, Glostrup, Denmark), vimentin (clone Vim 3B4, 1.2 μg/ml; Dako), α5β1 integrin (clone JBS5, 1:50; Chemicon, Temecula, CA), α1 integrin (clone FB12, 2 μg/ml; Chemicon), and α6 integrin (clone 4F10, 2 μg/ml; Chemicon). Isolated cells were positive for cytokeratin-7 (>97%) and largely negative for vimentin (<3%). The cytokeratin-7-positive cells expressed α5β1 integrin (100%), α1 integrin (100%), and α6 integrin (1%), suggesting that the trophoblast population consists mainly of EVT. Isolated cells were seeded in 24 wells at a density of 2.5 × 105 cells/cm2 and cultivated in 500 μl DMEM/F-12 with 10% fetal calf serum (FCS) (Biochrom, AG, Berlin, Germany).
Cultivation of SGHPL-5 cells
Cytotrophoblastic SGHPL-5 exhibiting features of invasive trophoblasts cells, such as HLA-G and cytokeratin-7 expression, were cultivated in a 1:1 mixture of DMEM/Ham’s F-12 supplemented with 10% FCS (GibcoBRL LifeTechnologies, Paisley, UK) as described (28).
First-trimester villous explant culture
Small pieces (1 × 1 mm) of villous tissue of different first-trimester placentas (n = 5) were dissected under the microscope and cultivated overnight in serum-free medium (DMEM/Ham’s F12 with 50 μg/ml gentamicin). To analyze effects of Wnt-3A on trophoblast migration, villous explants were seeded for 4 h on collagen I (serum-free medium), allowing for anchorage, and then stimulated with 100 ng/ml recombinant Wnt-3A (R&D Systems, Minneapolis, MN) in the absence or presence of recombinant Dkk1 (R&D Systems) or 10 μm LY294002 (PI3K inhibitor). Eight anchoring villi were analyzed per condition, and experiments were repeated with five different placentas. After 48 h, explant cultures were digitally photographed. For quantification, the area of outgrowth was measured using the imaging software CellP (Olympus, Hamburg, Germany).
Quantitative real-time PCR
SGHPL-5 cells or primary EVT (seeded in 24 wells, coated with growth factor-reduced Matrigel; Becton Dickinson) were incubated with 50 or 100 ng/ml Wnt-3A for 3–72 h. Total RNA was extracted by direct lysis in the culture dishes using TriFast Reagent (PeqLab, Erlangen, Germany) according to the manufacturer’s instructions. The RNA amount and integrity was evaluated using the Agilent Bioanalyzer 2100 (Agilent, Palo Alo, CA), and 2 μg RNA were reverse transcribed using 200 U RevertAid H Minus Moloney murine leukemia virus reverse transcriptase (Fermentas, St. Leon-Rot, Germany), 0.4 μl hexanucleotide mix (Roche, Mannheim, Germany), and 0.1 mm dNTP (Fermentas) in a final volume of 20 μl. Real-time PCR was performed on the ABI 5700 sequence detection system (Applied Biosystems, Foster City, CA) using TaqMan gene expression assays [TaqMan Universal PCR Master Mix, ×20 TaqMan gene expression assay mix for MMP-2, Hs00234422_m1, and TATA-box binding protein (TBP), TaqMan endogenous control] according to the manufacturer’s instructions. Calculation of signals was done as suggested in the PE Biosystems Sequence Detector User Bulletin and elsewhere (29). Briefly, threshold cycle (Ct) is defined as the first fluorescent signal reaching statistical significance above background. For each individual condition, ΔCt (the difference of CtMMP-2 and CtTBP) values are calculated representing normalization to the housekeeping gene. Subsequently, ΔΔCt values are built indicating normalization to controls. The amount of target normalized to an endogenous reference and relative to the unstimulated control is then given by 2−ΔΔCt.
Western blot analyses
For analyses of protein phosphorylation, SGHPL-5 cells and primary EVT were incubated overnight in serum-free medium and subsequently supplemented with 100 ng/ml Wnt-3A for 5, 15, 30, 60, and 120 min (SGHPL-5 cells) or for 5, 15, 30, and 60 min (primary EVT). In blocking studies, SGHPL-5 cells were preincubated for 1 h with 1 μg recombinant human Dkk1 or 10 μm LY294002 before addition of Wnt-3A. For MMP analyses, 400 μl supernatants of either SGHPL-5 cells or purified EVT, which had been stimulated for 48 and 6 h, respectively, with 100 ng/ml recombinant Wnt-3A were used. Supernatants of SGHPL-5 cells were concentrated 25-fold (to approximately 200 μg/ml) using Ultrafree-MC filter tubes (Millipore, Billerica, MA).
Nuclear/cytoplasmic extracts were prepared using NE-PERT nuclear and cytoplasmic extraction reagent according to the manufacturer’s instructions (Pierce, Rockford, IL). Western blot analyses were performed using standard protocols as recently done (10, 30). Briefly, equal amounts of protein lysate (10 μg) were separated on 10% SDS/PAA gels and transferred onto polyvinylidene difluoride membranes (Hybond-P; Amersham Pharmacia Biotech, Piscataway, NJ). After blocking, filters were incubated overnight (4 C) with rabbit antihuman antibodies against GSK-3β (1:1000; Cell Signaling Technology, Beverly, MA), phospho-GSK-3β (ser 9, 1:1000; Cell Signaling), AKT (1: 1000; Cell Signaling), phospho-AKT (Ser473, 1:1000; Cell Signaling), MMP-2 (1:1000; Cell Signaling), or mouse antihuman active β-catenin (ABC) (Millipore, Temecula, CA). After 1 h treatment (room temperature) with secondary antibodies (anti-mouse or antirabbit Ig horseradish peroxidase linked, Amersham; 1:50.000) signals were developed by using ECL Plus Western blotting detection system (Amersham). To analyze protein loading, filters were stripped as previously mentioned (10, 30) and incubated with rabbit antihuman glyceraldehyde-3-phosphate dehydrogenase (1:5000; Cell Signaling), mouse antihuman β-actin (1:50,000; Sigma), or mouse antihuman topoisomerase IIβ antibodies (normalization of nuclear extracts, 1:1000; BD Transduction Laboratories, Lexington, KY). PageRuler prestained protein ladder (Fermentas) was used as a molecular size marker. Quantification of signals on films was done by densitometric scanning using αEaserFC software (Alpha Innotech, San Leandro, CA).
Luciferase reporter assays
To analyze activity of the canonical Wnt pathway, SGHPL-5 cells were cotransfected with luciferase plasmids containing multimeric LEF/TCF cognate sequences (pTopFlash, Millipore, Billerica, MA) and CMV-β-Gal vectors as described recently (10). Briefly, cells cultivated in 24 wells were incubated with 1.5 μg luciferase reporter plasmid and 0.5 μg pCMV-β-Gal in 1.5 μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 7 h, medium was changed, and cells were stimulated with 100 ng/ml Wnt-3A for an additional 12 h. Luciferase activity was determined on a luminometer (Lumat LB 9507; EG&G Berthold, Bad Wildbad, Germany) using a luciferase assay system (Promega, Madison, WI). Activity of β-galactosidase was quantitated on a photometer by determining the conversion of the chromogenic substrate chlorophenol red-β-d-galactopyranoside (Roche Diagnostics, Vienna, Austria) at 570 nm as described (10). For each sample, luciferase and β-Gal assays were performed in duplicate and mean values were calculated. To correct for variations in transfection efficiency luciferase activities were normalized to β-Gal values. To investigate the effect of Wnt stimulation on MMP-2 transcription, SGHPL-5 cells were also transfected with luciferase reporter plasmids harboring either the wild-type proximal (1.9 kb) MMP-2 promoter or a mutated 5′-flanking region (31). In the latter, two TCF cognate sequences at −861 and −804 mediating Wnt-dependent transcription in effector T cells had been mutated (31).
Migration assays
To analyze migration, SGHPL-5 cells were seeded in DMEM/Ham’s F-12 plus 10% FCS on uncoated Transwell chambers (BD Biosciences, Bedford, MA; 50,000 cells per well). After attachment, cells were incubated with 100 ng/ml Wnt-3A in both chambers, and 24 h later, noninvaded cells on the upper side of the inserts were removed by a cotton swab. Cells on the lower surface were fixed in ice-cold methanol and stained with 1 μg/ml 4′,6-diamidine-2′-phenylindole dihydrochloride (Roche Diagnostics) and covered with Fluoromount G (Soubio, Birmingham, AL). For evaluation, cells were counted using Olympus Cell Imaging software. In blocking studies, cells were preincubated for 1 h with 1 μg recombinant human Dkk1 or 10 μm LY294002 before supplementation of Wnt-3A.
Statistical analyses
Statistical analyses were performed with Student’s t test or ANOVA using SPSS 14 (SPSS Inc., Chicago, IL). A P value < 0.05 was considered statistically significant.
Results
Wnt activates AKT in SGHPL-5 cells independently of canonical Wnt signaling
To analyze whether Wnt stimulation may influence AKT activity of trophoblastic SGHPL-5 cells, Western blot analyses were performed (Fig. 1). Incubation with recombinant Wnt-3A increased phosphorylation of AKT at Ser473 with peak levels at 5 and 15 min (Fig. 1A). Similarly, Wnt-dependent phosphorylation of GSK-3β at Ser9 was noticed, potentially resulting in inactivation of GSK-3β through AKT signaling (25). Interestingly, Dkk1 failed to inhibit Wnt-induced phosphorylation of AKT or GSK-3β, suggesting that a noncanonical Wnt receptor/pathway may affect the particular kinases. In contrast, in the presence of the PI3K inhibitor LY294002, Wnt-dependent phosphorylation of AKT and GSK-3β were largely abolished, indicating regulation through the PI3K pathway. Densitometric scanning of films revealed that compared with controls (100%), Wnt-stimulated Ser473 phosphorylation of AKT increased to 230 and 215% at 5 and 15 min, respectively, (Fig. 1B). Similarly, phosphorylation of GSK-3β at Ser9 was significantly increased at 15, 30, and 60 min of Wnt-3A stimulation (Fig. 1C). LY294002 significantly decreased AKT and GSK-3β phosphorylation under the different experimental conditions, whereas Dkk1 was not effective. Wnt-3A-dependent activation of AKT was also observed in primary EVT (Fig. 1D).
FIG. 1.
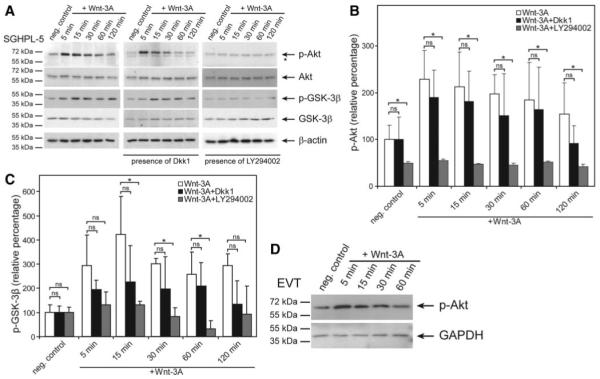
Western blot analyses showing Wnt-3A-induced AKT and GSK-3β phosphorylation. Stimulation of primary EVT and trophoblastic SGHPL-5 cells (in the absence or presence of inhibitors), preparation of protein lysates and Western blot analyses were performed as described in Materials and Methods. Marker bands (kDa) are depicted on the left. β-Actin (42 kDa) was used as a loading control. Representative examples of n = 3 are shown. A, Phosphorylation (p-) of AKT and GSK-3β in the absence or presence of Dkk1 or LY294002. Specific signals of p-Ser473-AKT (60 kDa), total AKT (60 kDa), p-Ser9-GSK-3β (46 kDa), and total GSK-3β (46 kDA) are depicted by arrows. *, Nonspecific signal. B and C, Quantification of p-Ser437-AKT and p-Ser9-GSK-3β signals, respectively, after densitometric scanning of films (n = 3). Mean values of unstimulated controls (n.c.) were arbitrarily set at 100%; error bars indicate sd. ns, Not significant; *, P < 0.05. D, Wnt-3A-dependent activation of AKT in primary EVT. A representative example is shown. GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
Inhibition of PI3K/AKT signaling does not affect canonical Wnt signaling in trophoblasts
Subsequently, the putative influence of PI3K inhibition on canonical Wnt signaling was investigated using Western blot analyses of ABC and Wnt/TCF reporter luciferase assays (Fig. 2). Incubation of SGHPL-5 cells with Wnt-3A resulted in nuclear accumulation of ABC with peak levels at 6 h (Fig. 2A). Pretreatment of cultures with Dkk1 strongly suppressed appearance of ABC. In contrast, inhibition of PI3K with LY294002 did not substantially affect nuclear ABC levels. In accordance, Dkk1 abolished Wnt-3A-induced luciferase activity of the canonical Wnt reporter pTopFlash reporter, whereas LY294002 had no influence on Wnt-dependent luciferase expression (Fig. 2B). Similarly, accumulation of ABC was observed in lysates of Wnt-3A-stimulated, primary EVT, which could not be suppressed upon LY294002 treatment (Fig. 2C). Hence, the data suggest that AKT-dependent phosphorylation of GSK-3β through Wnt may not be critical for canonical Wnt signaling in invasive trophoblasts.
FIG. 2.
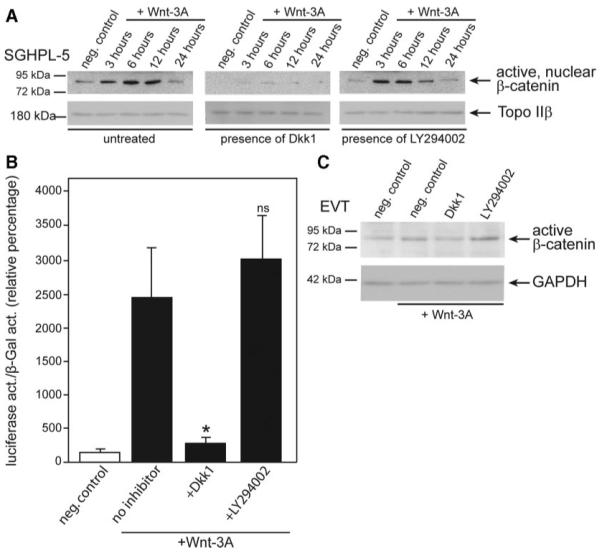
Analyses of the putative cross talk between PI3K/AKT and canonical Wnt signaling in SGHPL-5 cells and primary EVT. Wnt-3A stimulation of cultures, preparation of extracts, Western blotting, and pTopFlash luciferase assays were performed as described in Materials and Methods. A, Western blot demonstrating Wnt-3A-dependent nuclear accumulation of active, nuclear β-catenin (ABC, 92 kDa, indicated by arrow) in the absence or presence of Dkk1 or LY294002. The nuclear protein topoisomerase IIβ (TopoIIβ) (180 kDa) was used as a loading control. A representative example is shown. B, Luciferase activity of the canonical Wnt reporter after treatment with Dkk1 or LY294002. Mean values ± sd of four experiments performed in duplicates are shown. Normalized value of unstimulated control was arbitrarily set at 100%. ns, Not significant; *, P < 0.05. C, Western blot showing accumulation of ABC in lysates of primary EVT after Wnt-3A treatment (6 h) and preincubation with Dkk1 or LY294002. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 37 kDa) was used as a loading control. neg., Negative control; act., activity.
Inhibition of canonical Wnt or PI3K/AKT signaling decreases trophoblast migration
To delineate the role of canonical Wnt and PI3K/AKT signaling in Wnt-dependent trophoblast migration, Wnt-3A-stimulated SGHPL-5 cells and first-trimester villous explant cultures were treated with Dkk1 or LY294002 (Fig. 3). As previously noticed (10), Dkk1 significantly decreased Wnt-3A-induced migration through Transwells (Fig. 3A). Similarly, inhibition of PI3K/AKT strongly reduced Wnt-3A-induced cell migration, suggesting that both signaling pathways contribute to Wnt-dependent trophoblast motility. Accordingly, Wnt-3A-induced migration in primary explant cultures was diminished in the presence of either Dkk1 or the PI3K inhibitor (Fig. 3B). Compared with controls (100%), the area of outgrowth was increased to 250% in the presence of Wnt-3A (Fig. 3C), whereas Dkk1 and LY294002 abolished the effect.
FIG. 3.
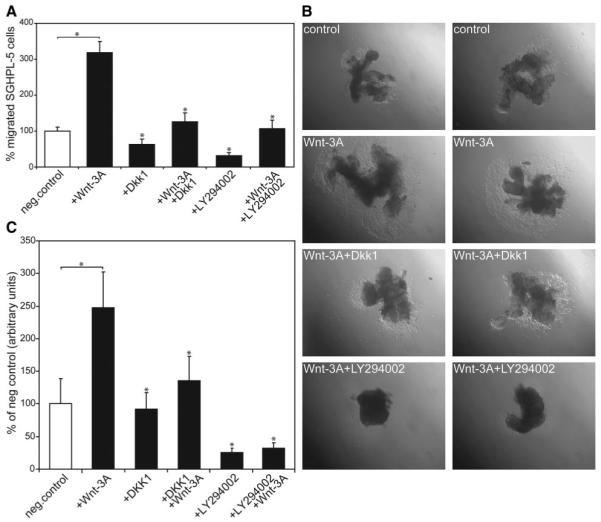
Wnt-3A-induced migration of SGHPL-5 cells and in first-trimester villous explant cultures in the absence or presence of Dkk1 or LY294002. Transwell migration assays and outgrowth of primary EVT on collagen I were performed as described in Materials and Methods. A, Wnt-3A-mediated SGHPL-5 cell migration in the absence or presence of Dkk1 or LY294002. Bars represent mean values of each three different experiments performed in duplicate; error bars indicate sd. Mean value of untreated cultures (negative control) was arbitrarily set to 100%. *, P < 0.05 compared with Wnt-stimulated migration. B, Wnt-3A-mediated EVT migration in villous explant cultures. Two representative samples per condition were photographed (40-fold magnification). C, Quantification of the area of outgrowth in Wnt-3A-stimulated villous explant cultures. Bars represent mean values of each of 20 different explant cultures prepared from five different first-trimester placentas; error bars indicate sd. Mean value of untreated cultures (negative control) was arbitrarily set to 100%. *, P < 0.05 compared with Wnt-stimulated outgrowth. neg., Negative.
Inhibition of canonical Wnt or PI3K/AKT signaling impairs MMP-2 secretion
To gain first insights into the mechanism of Wnt-induced migration and invasion, one of the critical proteases involved in trophoblast motility, MMP-2, was investigated by Western blotting and gelatin zymography (Fig. 4). After stimulation with Wnt-3A, increased concentrations of the pro-form (72 kDa) of MMP-2 were detectable by Western blotting in supernatants of SGHPL-5 cells and primary EVT (Fig. 4A). Quantification of Western blot data of SGHPL-5 cells revealed that Wnt-3A increased MMP-2 to 290% compared with controls (100%), whereas inhibition of canonical or PI3K/AKT signaling reduced Wnt-dependent secretion to 165 and 35%, respectively (Fig. 4B). Similarly, Wnt-3A stimulated accumulation of pro-MMP-2 to 430%, and inhibition through Dkk1 or LY294002 was noticed in supernatants of purified EVT (Fig. 4B). Accordingly, Wnt-3A-stimulated secretion and Dkk1/LY294002-dependent inhibition of MMP-2 could also be detected by gelatin zymography in both culture systems (Fig. 4C). The active form of MMP-2 was undetectable in SGHPL-5 supernatants. In contrast, the 64 kDa active form could be observed in EVT supernatants. Its soluble amount was coregulated with the 72 kDa pro-form, suggesting that processing of the enzyme may not be affected by Wnt-3A and/or inhibitor treatment.
FIG. 4.
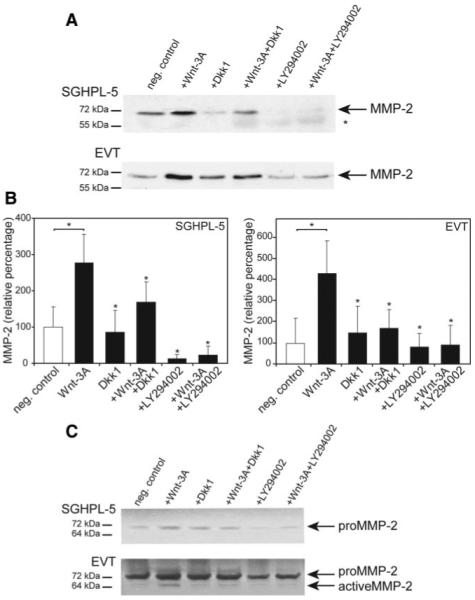
Western blot analyses and gelatin zymography of soluble MMP-2 in supernatants of trophoblast cultures. Wnt-3A stimulation, inhibitor treatment, Western blotting, zymography, and densitometric scanning were performed as described in Materials and Methods. Representative examples are shown. A, Western blot of supernatants of SGHPL-5 cells and primary EVT. The pro-form of MMP-2 is indicated by arrows. *, Nonspecific signals. B, Quantification of MMP-2 Western blot data obtained from either SGHPL-5 cells or primary EVT. Bars represent mean values of three different experiments; error bars indicate sd. Mean value of untreated cultures (negative control) was arbitrarily set to 100%. *, P < 0.05 compared with Wnt-stimulated condition. C, Gelatin zymography of supernatants isolated from Wnt-3A/inhibitor-treated SGHPL-5 cells and primary EVT. Pro- and active forms of MMP-2 are depicted. neg., Negative.
Wnt-3A-dependent secretion of MMP-2 does not involve changes in mRNA expression
Recent evidence in another cellular system suggested that Wnt-induced MMP-2 expression may involve direct transcriptional activation through TCF (31). To investigate whether a similar mechanism could be operational in trophoblasts, MMP-2 mRNA expression was analyzed after time- and dose-dependent stimulation with Wnt-3A (Fig. 5). Quantitative real-time PCR analyses revealed that incubation with Wnt-3A did not significantly change MMP-2 mRNA expression, either in SGHPL-5 cells or EVT. Hence, the data suggest that post-transcriptional effects of Wnt-3A lead to elevated MMP-2 concentrations in trophoblast supernatants.
FIG. 5.
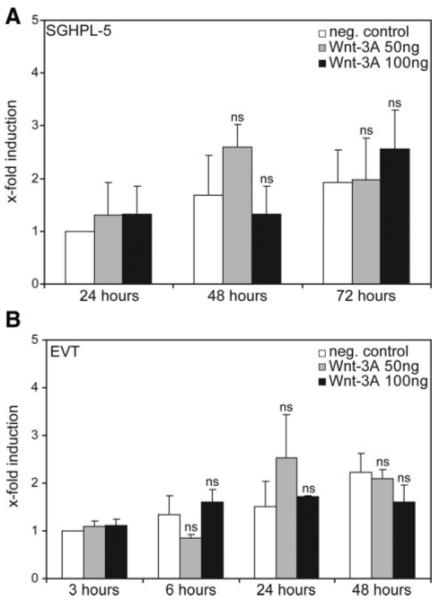
Quantitative real-time PCR of MMP-2 mRNA expression in cultured SGHPL-5 cells and EVT. Stimulation, RNA isolation, and quantitative real-time PCR were performed as described in Materials and Methods. For comparison of different experiments, values of controls at 3 h (EVT) and 24 h (SGHPL-5) were arbitrarily set at 1. Bars indicate mean values ± sd of three PCR (three different cell preparations) performed in duplicate. *, P < 0.05; ns, not significant compared with the controls of the respective time point. A, SGHPL-5 cells; B, primary EVT. neg., Negative.
Discussion
Accumulating data in the literature suggest that signaling through PI3K/AKT is critically involved in trophoblast invasion and migration. A variety of important growth factors of the fetal-maternal interface such as EGF (32), IGF-II (33), or hepatocyte growth factor (34) but also hormones like chorionic gonadotropin (35) stimulate AKT activation, thereby increasing trophoblast motility. Hence, the putative central role of the signal transduction pathway in trophoblast motility prompted us to also investigate the influence of Wnt on AKT activation. Wnt-3A was recently shown to increase trophoblast invasion and migration accompanied by EVT-specific expression of Wnt/β-catenin-dependent TCFs (10), but details on the molecular mechanism remained elusive.
Western blot analyses revealed that Wnt-3A increased AKT phosphorylation, and inhibition of PI3K/AKT signaling abolished Wnt-dependent migration, emphasizing the critical role of AKT in trophoblast motility. Because endometrium and trophoblast express a variety of Wnt ligands and Fzd receptors (17, 19), we assume that both tissues may contribute to Wnt-induced signaling and migration. Besides the role of AKT, results of this and a previous study (10) demonstrated that the canonical Wnt pathway is also critically involved because Dkk1 inhibited invasion and migration of first-trimester cytotrophoblasts and SGHPL-5 cells as well as in villous explant cultures. Hence, we conclude that similar to other growth factors (6), Wnt ligands promote trophoblast motility through activation of several signaling pathways. Whereas structure and function of the canonical LRP-5/6-Fzd receptor has been intensively investigated, less is known about noncanonical Wnt receptors (11, 36). Along those lines, Wnt receptors required for Wnt-3A-dependent AKT activation have not been characterized. Here, we show that activation of AKT cannot occur through the canonical Wnt receptor because Dkk1 failed to inhibit phosphorylation of AKT and its downstream target GSK-3β. This would be in agreement with a previous observation showing that Wnt-3A-dependent AKT activation does not involve β-catenin/TCF (22). Therefore, Wnt-induced phosphorylation of AKT could be triggered through Fzd receptors without requiring LRP-5/6 as observed in other noncanonical Wnt pathways (36). On the other hand, various alternative Wnt receptors have been identified (37). For example, phosphorylation of AKT could eventually be achieved through receptor tyrosine kinases such as Ryk or Ror, which were recently shown to be involved in Wnt-dependent neuronal processes (37). Indeed, mRNA expression of Ryk was noticed in DNA gene chip analyses of SGHPL-5 cells primary EVTs (data not shown).
In addition, the potential cross talk between canonical Wnt and AKT signaling in trophoblasts was investigated because AKT may inactivate GSK-3β through phosphorylation at Ser9 and thereby increase stability of β-catenin and TCF-dependent transcription. However, the role of AKT in modulating the canonical Wnt pathway varies between cell types. Whereas dominant-negative and -positive AKT were shown to inhibit and activate, respectively, canonical Wnt signaling in 293T and P19CL6 cells (25, 38), AKT did not affect the pathway in other cellular systems (25, 39, 40). Similarly, comparison of the effects of constitutively active or wild-type GSK-3β in LβT2 cultures suggested that phospho-inhibition of GSK-3β at Ser9 is not critical for Wnt/TCF signaling in these cells (41). In agreement with the later observations, inhibition of AKT failed to repress accumulation of ABC or TCF reporter activity in the trophoblast model systems, although AKT-dependent phosphorylation of GSK-3β upon Wnt stimulation had been detected. Hence, we conclude that Wnt-induced modification of GSK-3β through AKT is not critical for the canonical Wnt pathway in trophoblasts. The differing results of the Wnt-AKT cross talk might be explained by differences between kinetic mechanisms of cell types. Besides phosphorylation of GSK-3β, recruitment of Axin to the LRP-5/6-Fzd receptor is thought to be sufficient for inhibition of the β-catenin destruction complex and to induce canonical Wnt signaling (11). It could well be that membrane recruitment of Axin occurs faster than AKT-mediated phosphorylation/inactivation of GSK-3β. Inhibition of the enzyme through AKT would then be of minor relevance for signaling through the canonical Wnt pathway.
Depending on the cell type, Wnt ligands were shown to activate MMP expression involving canonical or noncanonical pathways (31, 42). With respect to trophoblast invasiveness, Wnt-dependent production of gelatinases is of particular interest, because these enzymes are thought to play critical roles (4). Indeed, both signaling pathways, canonical Wnt and AKT, promoted Wnt-3A-dependent accumulation of MMP-2 in trophoblast supernatants. Therefore, we assume that induction of the protease could be one of the critical mechanisms promoting Wnt-dependent trophoblast motility. AKT-dependent MMP secretion seems to emerge as a common theme in trophoblast invasion because factors such as chorionic gonadotropin or EGF were also shown to increase levels of MMP-2 and MMP-9, respectively, through activation of the particular kinase (35, 43). Mechanisms controlling MMP-2 release through canonical Wnt or AKT signaling have not been elucidated in detail. Although MMP-2 was previously identified as a direct target of TCF/β-catenin in effector T cells (31), Wnt-dependent induction of the MMP-2 mRNA could not be detected in trophoblasts. Accordingly, a MMP-2 reporter harboring wild-type TCF binding sites could not be stimulated with Wnt-3A, and another plasmid containing mutated TCF cognate sequences did not show reduced luciferase expression in SGHPL-5 cells (data not shown). Therefore, we conclude that post-transcriptional mechanisms are responsible for Wnt-induced elevation of pro-MMP-2 in trophoblast supernatants. Increased MMP-2 levels could, for example, be achieved by AKT-dependent inhibition of proteasomal degradation (44). However, if Wnt may act through such a mechanism needs further investigation.
In conclusion, the results suggest that Wnt ligands can promote trophoblast motility through activation of distinct pathways, i.e. canonical Wnt and AKT signaling (Fig. 6). Both cascades promote Wnt-dependent secretion of MMP-2, which has been identified as a novel Wnt target in invasive trophoblasts.
FIG. 6.
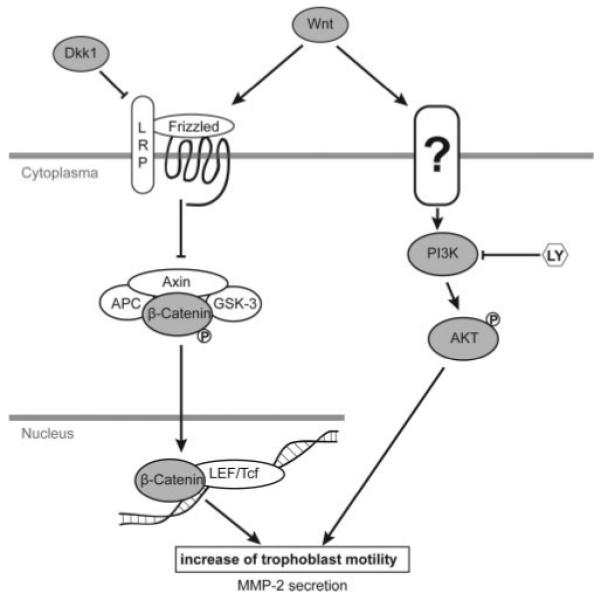
Current model system showing Wnt-dependent pathways involved in trophoblast migration. The potential cross talk between Wnt and AKT is not critical in trophoblasts. The Wnt receptor promoting AKT phosphorylation in a LRP-5/6-independent manner remains currently unknown. Wnt-3A-dependent activation of both signaling pathways results in elevated trophoblast migration and increased MMP-2 secretion. APC, Adenomatous polyposis coli; LY, LY294002.
Acknowledgments
We thank G. Whitley and C. Hughes for providing SGHPL-5 cells and MMP-2 promoter luciferase constructs, respectively. We are grateful to G. Puller for preparation of graphics.
S.S., P.H., and C.L. are supported by Grant P-17894-B14 of the Austrian Science Funds, Vienna, Austria.
Abbreviations
- ABC
Active β-catenin
- CHM
complete hydatidiform mole
- Ct
cycle threshold
- Dkk1
Dickkopf-1
- EGF
epidermal growth factor
- EVT
extravillous trophoblast
- FCS
fetal calf serum
- Fzd
Frizzled
- GSK
glycogen synthase kinase
- LEF
lymphoid enhancer-binding factor
- LRP
lipoprotein receptor-related protein
- MMP
matrix metalloproteinase
- PI3K
phosphatidylinositide 3-kinase
- TCF
T cell factor
- Wnt
wingless
Footnotes
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteropla-cental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 3.Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13:591–599. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- 4.Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion: a review. Placenta. 2000;21(Suppl A):S55–S60. doi: 10.1053/plac.2000.0521. [DOI] [PubMed] [Google Scholar]
- 5.Lala PK, Hamilton GS. Growth factors, proteases and protease inhibitors in the maternal-fetal dialogue. Placenta. 1996;17:545–555. doi: 10.1016/s0143-4004(96)80071-3. [DOI] [PubMed] [Google Scholar]
- 6.Pollheimer J, Knöfler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005;26(Suppl A):S21–S30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Lash GE, Quenby S, Burton GJ, Nakashima A, Kamat BR, Ray J, Bulmer JN. Gestational diseases: a workshop report. Placenta. 2008;29S:92–94. doi: 10.1016/j.placenta.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Balaram P, John M, Enose S, Symaladevi PK. Demonstration of TGF-α-EGFR and EGF-EGFR autocrine loops and their relation to proliferation in complete hydatidiform moles (CHM) Int J Gynecol Cancer. 2001;11:397–402. doi: 10.1046/j.1525-1438.2001.01040.x. [DOI] [PubMed] [Google Scholar]
- 9.Tseng JJ, Chou MM. Differential expression of growth-, angiogenesis- and invasion-related factors in the development of placenta accreta. Taiwan J Obstet Gynecol. 2006;45:100–106. doi: 10.1016/S1028-4559(09)60205-9. [DOI] [PubMed] [Google Scholar]
- 10.Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knöfler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168:1134–1147. doi: 10.2353/ajpath.2006.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 12.Galceran J, Fariñas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1−/−Tcf1−/− mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 14.Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237:324–332. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/β-catenin signaling is required for implantation. Proc Natl Acad Sci USA. 2005;102:8579–8584. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- 18.Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab. 2006;91:1453–1461. doi: 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- 19.Sonderegger S, Husslein H, Leisser C, Knöfler M. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta. 2007;28(Suppl A):S97–S102. doi: 10.1016/j.placenta.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng S, Li J, Miao C, Jia L, Hu Z, Zhao P, Li J, Zhang Y, Chen Q, Duan E. Dickkopf-1 secreted by decidual cells promotes trophoblast cell invasion during murine placentation. Reproduction. 2008;135:367–375. doi: 10.1530/REP-07-0191. [DOI] [PubMed] [Google Scholar]
- 21.Kühl M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci. 2004;9:967–974. doi: 10.2741/1307. [DOI] [PubMed] [Google Scholar]
- 22.Kim SE, Lee WJ, Choi KY. The PI3 kinase-Akt pathway mediates Wnt3a-induced proliferation. Cell Signal. 2007;19:511–518. doi: 10.1016/j.cellsig.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. Both ERK and Wnt/β-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci. 2005;118:313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- 24.He S, Pant D, Schiffmacher A, Meece A, Keefer CL. Lymphoid enhancer factor 1-mediated Wnt signaling promotes the initiation of trophoblast lineage differentiation in mouse embryonic stem cells. Stem Cells. 2008;26:842–849. doi: 10.1634/stemcells.2007-0356. [DOI] [PubMed] [Google Scholar]
- 25.Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 26.Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarrade A, Lai Kuen R, Malassiné A, Tricottet V, Blain P, Vidaud M, Evain-Brion D. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001;81:1199–1211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- 28.Choy MY, St Whitley G, Manyonda IT. Efficient, rapid and reliable establishment of human trophoblast cell lines using poly-l-ornithine. Early Pregnancy. 2000;4:124–143. [PubMed] [Google Scholar]
- 29.Huber AV, Saleh L, Prast J, Haslinger P, Knöfler M. Human chorionic gonadotrophin attenuates NF-κB activation and cytokine expression of endometriotic stromal cells. Mol Hum Reprod. 2007;13:595–604. doi: 10.1093/molehr/gam032. [DOI] [PubMed] [Google Scholar]
- 30.Knöfler M, Saleh L, Bauer S, Galos B, Rotheneder H, Husslein P, Helmer H. Transcriptional regulation of the human chorionic gonadotropin β gene during villous trophoblast differentiation. Endocrinology. 2004;145:1685–1694. doi: 10.1210/en.2003-0954. [DOI] [PubMed] [Google Scholar]
- 31.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu Q, Yang M, Tsang BK, Gruslin A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod. 2004;10:677–684. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- 33.Qiu Q, Basak A, Mbikay M, Tsang BK, Gruslin A. Role of pro-IGF-II processing by proprotein convertase 4 in human placental development. Proc Natl Acad Sci USA. 2005;102:11047–11052. doi: 10.1073/pnas.0502357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cartwright JE, Tse WK, Whitley GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002;279:219–226. doi: 10.1006/excr.2002.5616. [DOI] [PubMed] [Google Scholar]
- 35.Prast J, Saleh L, Husslein H, Sonderegger S, Helmer H, Knöfler M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. 2008;149:979–987. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 37.Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008;50:229–243. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 39.Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 40.Yuan H, Mao J, Li L, Wu D. Suppression of glycogen synthase kinase activity is not sufficient for leukemia enhancer factor-1 activation. J Biol Chem. 1999;274:30419–30423. doi: 10.1074/jbc.274.43.30419. [DOI] [PubMed] [Google Scholar]
- 41.Gardner S, Maudsley S, Millar RP, Pawson AJ. Nuclear stabilization of β-catenin and inactivation of glycogen synthase kinase-3β by gonadotropin-releasing hormone: targeting Wnt signaling in the pituitary gonadotrope. Mol Endocrinol. 2007;21:3028–3038. doi: 10.1210/me.2007-0268. [DOI] [PubMed] [Google Scholar]
- 42.O’Connell MP, French AD, Leotlela PD, Weeraratna AT. Assaying Wnt5A-mediated invasion in melanoma cells. Methods Mol Biol. 2008;468:243–253. doi: 10.1007/978-1-59745-249-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- 44.Park BK, Zeng X, Glazer RI. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 2001;61:7647–7653. [PubMed] [Google Scholar]


