Abstract
The cytokine tumour necrosis factor α (TNF) is a well known member of the TNF superfamily consisting of at least 18 ligands and 29 different receptors involved in numerous cellular processes. TNF signals through two distinct receptors TNFR1 and TNFR2 thereby controlling expression of cytokines, immune receptors, proteases, growth factors and cell cycle genes which in turn regulate inflammation, survival, apoptosis, cell migration, proliferation and differentiation. Since expression of TNF was discovered in amnion and placenta many studies demonstrated the presence of the cytokine and its receptors in the diverse human reproductive tissues. Whereas TNF has been implicated in ovulation, corpus luteum formation and luteolysis, this review focuses on the functions of TNF in human placental, endometrial and decidual cell types of normal tissues and also discusses its role in endometrial and gestational diseases. Physiological levels of the cytokine could be important for balancing cell fusion and apoptotic shedding of villous trophoblasts and to limit trophoblast invasion into maternal decidua. Regulation of the TNF/TNFR system by steroid hormones also suggests a role in uterine function including menstrual cycle-dependent destruction and regeneration of endometrial tissue. Aberrant levels of TNF, however, are associated with diverse reproductive diseases such as amniotic infections, recurrent spontaneous abortions, preeclampsia, preterm labour or endometriosis. Hence, concentrations, receptor distribution and length of stimulation determine whether TNF has beneficial or adverse effects on female reproduction and pregnancy.
Keywords: Placenta, Trophoblast, Endometrium, TNF
1. Human tumour necrosis factor
Tumour necrosis factor (TNF, cachexin or cachectin and formerly known as tumour necrosis factor-α) is a pleiotropic inflammatory cytokine. It was first isolated by Carswell et al. in 1975 in an attempt to identify cytotoxic factors responsible for necrosis of the sarcoma Meth A [1].
1.1. Structure and general function
The human TNF gene was cloned in 1984 and maps within the major histocompatibility complex to Chromosome 6p21.3 [2]. It spans about 3 kb and consist of 4 exons whereas the last exon codes for more than 80% of the secreted protein [3]. At this time its homology to another factor cytotoxic to tumour cells, TNF-β (also termed lymphotoxin) was noticed [2]. Whereas TNF is mainly produced in monocytes and/or macrophages, TNF-β is a product of lymphoid cells, but binds to the same surface receptor as TNF [4]. Both proteins have similar biological activities [3] but investigation of their structures revealed that TNF-β is a glycoprotein that has no cysteine residues whereas TNF contains one disulphide bond [5]. In vitro site mutagenesis of these cysteine residues demonstrated that the disulfide bond is important for the biological function of TNF [6]. Throughout the years, however, it became clear that both proteins belong to a superfamily of soluble TNF ligands comprising at least 18 different members controlling diverse cellular functions such as apoptosis, inflammation, sepsis and development of the immune system [7].
The TNF protein is a homotrimer primarily produced as a 212 amino acid type II transmembrane protein [8,9]. Three monomers associate around a 3-fold axis to form a compact bell-shaped trimer. This structure is typical for members of the TNF family but comparison to known protein structures also showed structural homology to several viral coat proteins [10]. TNF can act in its membrane-bound form through cell-to-cell contact or after cleavage from the cell membrane as a soluble 51 kDa homotrimer (sTNF). Cleavage is carried out by the metalloproteinase TNF alpha converting enzyme (TACE, also called ADAM17) [11]. The homotrimeric sTNF dissociates at concentrations below the nanomolar range, thereby losing its bioactivity. The cytokine is predominantly produced upon activation of myeloid cells, e.g. macrophages, but also by endothelial cells, fibroblasts and neuronal tissue.
TNF exhibits its biological properties upon binding to its cognate membrane receptors TNFR1 (TNFRSF1A, CD120a, p55) and TNFR2 (TNFRSF1B, CD120b, p75) which are members of the TNF receptor superfamily [12]. This superfamily consists of at least 29 trans-membrane proteins which are activated through the different TNF superfamily ligands and signal through six different members of a family of intracellular mediators termed TNFR associated factors (TRAFs). A hallmark of the TNFR superfamily is cysteine-rich regions in their extracellular domain including 6 highly conserved cysteine residues [13]. TNFR1 and TNFR2 contain each four cysteine-rich repeats [14]. Like the TNF ligands, the receptors also form a trimeric structure. It was long believed, that the ligand recruits three receptor monomers into the final 3:3 complex [12] being the key event for initiation of signal transduction. However, recent evidence indicated that a distal cysteine-rich domain which is called PLAD (pre-ligand binding assembly domain) keeps TNFR1 and TNFR2 in a pre-assembled oligomeric status avoiding causeless auto-activation [15]. Upon ligand binding the receptor undergoes a conformational change towards a higher-order receptor complex achieving signal competence [16]. TNFR1 is constitutively expressed in most tissues and seems to be the key mediator of TNF signalling. In contrast, TNFR2 is strongly regulated and predominantly found in immune cells indicating that this receptor plays a major role in the lymphoid system [17]. The extracellular domains of both receptors can also be cleaved from the membrane resulting in the production of soluble TNF (sTNF) receptors. The secreted proteins eventually neutralize TNF, even though their binding affinities are much lower than those of the membrane receptors [18]. Whereas TNFR2 is cleaved by TACE [19], the proteolytic enzyme releasing sTNFR1 is still unknown. Shedding of sTNFR1 seems to be physiologically important since mutations leading to cleavage resistance are related to dominantly inherited auto-inflammatory syndromes (TNFR1-associated periodic syndromes) [20].
TNF has a wide spectrum of bioactivities and most cells show at least some response sensitivity to TNF (Fig. 1). In general, the cytokine displays a functional duality being involved in tissue regeneration and destruction [16]. Under physiological conditions, TNF is involved in immune surveillance and defence, in cellular homeostasis, protection against certain neurological insults as well as in the control of cell survival, proliferation, migration and differentiation [21]. Owing to its strong pro-inflammatory and immuno-stimulatory activities, TNF is associated with a number of pathological events. The cytokine is involved in the progression of many autoimmune diseases, e.g. rheumatoid arthritis and inflammatory bowel diseases [22,23]. Hence, usage of sTNF receptors and TNF-neutralising antibodies became important therapeutic strategies for these disorders [24].
Fig. 1.

The diverse biological effects of TNF. Beneficial vs. adverse effects depend on local TNF concentrations, the expression pattern of TNF receptors and the abundance of inhibitors such as sTNFRs.
In general, TNF concentrations seem to determine whether the cytokine exerts beneficial or harmful effects. High doses of sTNF in response to lipopolysaccharides and other bacterial toxins play a key role in the development of septic shock [25]. Low concentrations over a long period of this particular cytokine are often associated with cachexia (i.e. weakness, loss of weight and muscle atrophy) which can be found in tumour patients [26]. All the different well described roles of TNF indicate that there must be a complex interaction pattern between TNF concentration, tissue and cell type, TNF receptor distribution and duration of TNF stimulation leading to a specific physiological or pathological reaction.
1.2. TNF-dependent signalling pathways
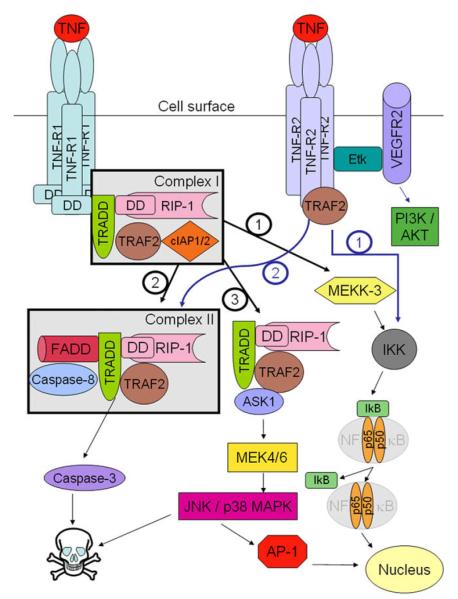
TNF signalling is mediated through TNFR1 and TNFR2 (Fig. 2). Although both receptors contain a highly homologous, cysteinerich extracellular domain, their intracellular regions do not show sequence homology [27]. The two receptors are differentially expressed on cells and overlapping as well as distinct signal transduction was observed. However, general differences between TNFR1- and TNFR2-dependent signalling were noticed. Activation of TNFR1 basically leads to pro-inflammatory as well as programmed cell death pathways, both associated with tissue injury. Signalling through TNFR2 can induce apoptosis but also support survival promoting tissue repair and angiogenesis [28].
Fig. 2.
TNF-dependent signalling pathways mediated through TNFR1 and TNFR2. Signalling of TNF through TNFR1 either results in formation of complex I and activation of NFκB (pathway 1) promoting inflammation, survival and differentiation or provokes cell death through complex II and activation of caspases (pathway 2). Recruitment of ASK-1 (pathway 3) induces JNK and AP-1 which in turn may initiate apoptosis or survival. TNFR2-dependent signalling may also promote cell death or survival through pathways 1 and 2, respectively, but also activate PI3K/AKT suppressing apoptosis and increasing promoting proliferation, migration and survival.
1.2.1. TNFR1-dependent signalling
The cytoplasmic domain of the unstimulated receptor is bound by silencer of death domain (SODD) preventing constitutive signalling of TNFR1 [29]. Upon TNF stimulation, SODD is released and TNFR-associated death domain protein (TRADD) binds to the intracellular domain followed by recruitment of the serine/threonine kinase receptor interacting protein-1 (RIP-1) [30], TRAF-2, as well as of cellular inhibitor of apoptosis proteins cIAP1 and cIAP2. This protein complex, termed complex I, is considered to activate the NFκB pathway via MAP (mitogen activated protein) kinase kinase kinase-3 (MEKK-3) leading to phosphorylation of the inhibitor of κB kinase (IKK) [31] which in turn phosphorylates the inhibitor of κB(IκB). This phosphorylation step causes ubiquitination-dependent degradation of IκB allowing NFκB to enter the nucleus and to initiate gene transcription. NFκB is a well described key regulatory transcription factor regulating numerous processes such as inflammation, development, oncogenesis or cellular stress. 200 physiological stimuli are known to activate NFκB. These include bacterial and viral products, cellular receptors and ligands, mitogens and growth factors and physical and biochemical stress inducers. The active transcription factor controls a plethora of genes such cytokines, chemokines, stress response genes, regulators of apoptosis, immune receptors and adhesion molecules, but also growth and cell cycle proteins. The NFκB pathway can also be activated through TRAF-2 [32].
The second signalling pathway elicits cell death. By a largely unknown mechanism receptor complex I is internalised and a complex consisting of TRADD, RIP-1 and TRAF-2 is released from TNFR1 [33]. Subsequently, Fas-associated DD protein (FADD) binding to TRADD and pro-caspase-8 are recruited to form complex II. This signalling step results in activation of caspase-8 through two distinct pathways [34] and finally activate caspase-3 initiating apoptosis.
In a third signalling pathway another protein is recruited to the TRADD-RIP-TRAF-2 complex, the apoptosis-signalling kinase-1 (ASK-1) which is a member of the MEKK family. This protein complex is thought to activate the MAP kinase kinases MEK-4 and MEK-6 [35]. These enzymes phosphorylate and activate c-Jun N-terminal kinases (JNKs) and p38 MAPKs. JNKs phosphorylate c-Jun, a subunit of the transcription factor activating protein-1 (AP-1). Similar to NFκB, AP-1 is considered to promote inflammation and cell survival. However, TNF signalling through ASK-1 provokes cell death since overexpression of dominant-negative ASK-1 or knock-down inhibits TNF-induced apoptosis [35,36].
Besides pathways modulating cell survival TNF was also shown to promote growth through activation of extracellular regulated kinases (ERK) as well as AKT [37]. In conclusion, TNF acting through TNFR1 induces numerous signalling pathways provoking a variety of cellular effects. The complexity and cross-talk of TNF signalling pathways still have to be elucidated.
1.2.2. TNFR2-dependent signalling
Compared to TNFR1-dependent signalling, TNFR2-mediated pathways are less well understood. TNFR2 has no death domain but can mediate signalling via TRAFs sharing signalling effects with TNFR1 such as activation of NFκB and JNKs. The intracellular domain of TNFR2 can directly interact with TRAF-2 resulting in further recruitment of RIP and FADD and activation of caspases. However, depending on the cell type TNFR2 can promote proliferation or apoptosis and the protein has also been implicated in models of cerebral malaria and microvascular cell damage [38]. TNFR2 was also shown to activate the endothelial/epithelial tyrosine kinase (Etk) which activates the PI3K-Akt pathway via vascular endothelial growth factor (VEGF) finally modulating cell adhesion, proliferation, migration and survival [39].
2. Expression pattern of TNF and TNF receptors in gestational tissues
Since TNF was first detected in amniotic fluid and placental supernatants during normal human pregnancy [40], numerous studies have investigated the expression pattern of the particular cytokine as well as its receptors, TNFR1 and TNFR2, in different gestational tissues. In general, many cell types of human endometrium, decidua and placenta were shown to express TNF and its receptors suggesting that multiple autocrine and paracrine interactions may occur.
2.1. Expression of TNF
TNF mRNA and protein are expressed in endometrium and in myometrial smooth muscle cells of the uterus based on in situ hybridisation studies and immunohistochemical analyses [41-43]. Various cell types of the endometrium, i.e. fibroblasts, macrophages [44], glandular epithelial cells [42] and vascular cells [45], were all described to express the particular cytokine. In detail, TNF protein content in the glands is negative to weak in the early proliferative phase with increasing levels during the proliferative phase and a maximum in the late proliferative phase. The expression remains high throughout the secretory phase with a staining intensity slightly lower than in the late proliferative phase. Changes in localisation were also noticed since during secretory phase TNF is predominantly expressed in the apical part of the glandular epithelium. Towards the end of the cycle, TNF mRNA decreases whereas TNF-α protein remains high in endometrial glands. The expression intensity is similar in basal and functional layers of the endometrium. Weak production in stromal cells was suggested throughout the whole cycle [46] whereas others noticed continuous expression from mid-proliferative to late secretory phase and in decidua during the first trimester of pregnancy [45]. Upon decidualisation TNF mRNA has been detected in stromal cells and macrophages [44] as well as in uterine NK cells, T-cells [47] and endothelial cells [48]. Several authors also noticed basal secretion of TNF protein from decidual tissue or isolated decidual stromal cells in vitro [49-51].
Placental expression of TNF changes during pregnancy [41] suggesting a specific function in developmental differentiation processes. After in vitro fertilisation varying amounts of TNF have been detected in supernatants of human embryos which however was not related to pregnancy outcome [52]. During the first third of gestation TNF mRNA seems to be predominantly expressed in all cell types of the trophoblastic lineage including villous cytotrophoblasts and syncytiotrophoblasts as well as in proliferating cytotrophoblasts of cell islands and invasive trophoblasts [43,53]. As pregnancy proceeds mRNA expression switches from the trophoblastic cell population to a stronger signal within villous stromal cells [54]. Immunohistochemical analyses detected TNF protein predominantly in cell columns [55]. The signal intensity is maintained during invasion and even gains intensity when the extravillous trophoblasts (EVT) displace the endothelial cells of spiral arteries [55]. However, at later stages of pregnancy TNF protein expression decreases in invasive cells and trophoblast giant cells lack any TNF expression [55].
2.2. Expression of TNF receptors
Expression of the two TNF receptors was detected in endometrial epithelial cells throughout the whole menstrual cycle with higher expression in the basalis layer as compared to the functionalis [56]. Others reported increasing levels of TNFR1 during the menstrual cycle with peak levels in the late secretory phase [57]. TNFR2 varies during the cycle and highest expression was observed in the late proliferative and late secretory phase [57]. Another study failed to detect TNFR2 protein in endometrial glands of human decidua, but observed partial expression in decidual NK cells, T-cells, macrophages, stromal and endothelial cells [49]. Indeed, numerous studies have shown that decidual cell cultures respond to TNF treatment suggesting that different decidual cell types express TNF receptors.
In the placenta, first trimester trophoblasts of cell columns were shown to exhibit strong signals of TNFR1 mRNA whereas villous cytotrophoblasts and syncytiotrophoblasts show a non-uniform distribution of the receptor mRNA. In contrast to TNF mRNA, TNFR1 mRNA is highly expressed in first trimester villous stromal cells [58]. In term placentas, TNFR1 mRNA remains consistently strong within the villous stroma and endothelial cells but has also been detected in syncytiotrophoblasts although at lower levels [58]. Placental distribution of TNFR2 mRNA is similar to TNFR1 mRNA but signals are weaker and increase in villous stromal cells of late pregnancy [58]. In vitro expression of TNFR1 mRNA and to smaller extents TNFR2 mRNA could also be observed in cultured first trimester trophoblasts [59].
TNFR1 protein was shown to be widely expressed in villous cytotrophoblasts, cell columns and invasive trophoblasts of early gestation [59]. In villous mesenchymal cells production is weak whereas strong signals were obtained in syncytiotrophoblasts during all gestational ages [58,60]. Regarding placental TNFR2 protein expression inconsistent data are found in the literature. Whereas TNFR2 polypeptide was noticed in first trimester cytotrophoblasts and syncytium with decreasing levels in these cell types towards the end of pregnancy [58], others failed to detect the protein in any of the first trimester trophoblast subtypes [49,59]. With respect to syncytial expression TNFR2 polypeptide could be detected in most but not all studies [49,58-60]. Interestingly, there might be differences in the intracellular distribution and staining pattern between the two receptors. While syncytial TNFR1 protein showed a perinuclear localisation, the staining of TNFR2 demonstrated a more granular pattern in the cytoplasm [60]. In vitro, however, surface as well perinuclear staining of both receptors could be detected in cultured third trimester trophoblast [61]. In addition, these cells also rapidly release sTNFR1 and sTNR2 into the culture medium [62] providing an explanation for elevated concentrations of soluble TNF receptors in the urine of pregnant women [63].
3. Function of the TNF–TNF receptor system in gestational tissues
Pleiotrophic functions of TNF on diverse reproductive cell types were described. Whereas the focus of this review is to elucidate the role of the cytokine in placenta and endometrium with respect to physiology and disease, we would like to refer to other reviews summarising effects of TNF on ovarian function. Expression of TNF and its receptors has been detected on oocytes, granulosa cells and interstitial cells suggesting autocrine as well as paracrine interactions. The cytokine was shown to inhibit steroidogenesis in undifferentiated ovaries whereas it stimulates progesterone synthesis in differentiated ovaries [64,65]. However, TNF also has been implicated in regression of the corpus luteum since luteal TNF was shown increase upon the decline in progesterone secretion. TNF-dependent regression could be mediated through induced prostaglandin synthesis and/or apoptotic processes [66]. In summary, it was concluded that TNF is critically involved in homeostasis of ovarian function, ovulation, corpus luteum formation and luteolysis.
3.1. Physiological and pathological role of TNF in the placenta
TNF provokes a variety of biological effects on placental and endometrial cell types such as apoptosis, inhibition of trophoblast cell fusion and invasion or epithelial shedding likely involving both types of TNF receptors. However, details on TNF-mediated signalling such as downstream adaptor proteins or phosphorylation steps in these cell types are largely unknown.
3.1.1. Apoptosis
Numerous studies investigated the role of TNF in programmed cell death of placental trophoblasts. In summary, the data suggest that local TNF production could be critically involved in the physiological balance of trophoblast turnover and renewal. Cytotoxic effects of TNF alone or in combination with interferon γ (INFγ) on cultured third trimester villous cytotrophoblasts were described [61,67]. Utilisation of either TNFR1- or TNFR2-specific TNF proteins suggested that apoptosis is predominantly induced trough TNFR1 [61]. This was confirmed by utilisation of antibodies which mimic TNF ligand and hence induce signalling in a receptor subtype-specific manner [61]. Alternatively, recent evidence suggested that TNF may also induce trophoblast death by antagonising the caspase-inhibitory action of XIAP (X-linked inhibitor of apoptosis) through elevation of the pro-apoptotic protein XAF1 (XIAP associated factor 1) [68]. TNF-dependent cytotoxicity can be dose-dependently blocked by supplementation of epidermal growth factor (EGF) which is thought to the interrupt an early step of the apoptotic response [69]. Trophoblast survival upon EGF treatment could also be partly mediated through EGF-dependent fibronectin secretion [70]. Similar to EGF other abundant growth factors of the fetal–maternal interface such as bFGF, IGF-1, and PDGF were also shown to partially inhibit TNF-induced apoptosis of villous cytotrophoblasts [71]. In contrast to other cell types, TNF-induced programmed cell death did not involve production of reactive oxygen species or reactive nitrogen intermediates [72].
Similar to third trimester cells, the cytokine induced TUNEL-positivity and elevation of caspase-3 enzyme activity in purified cytotrophoblasts of first trimester placentae suggesting that the TNFα-dependent apoptotic cascade is also executed in a portion of early trophoblasts [59]. Loss of syncytiotrophoblasts during placental inflammation may also involve a TNF-dependent process. Using an in vitro co-culture model activated maternal monocytes were shown to induced syncytial apoptosis through TNF [73]. Focal damage of the epithelium could provide a mechanism for maternal leukocyte infiltration into the fetal stroma. In contrast to trypsinisolated cytotrophoblasts, TNF failed to induce apoptosis in invasive trophoblasts differentiating from whole villous explant cultures of early pregnancy [74]. This suggests that trophoblasts which have not been damaged by the isolation procedure may be less sensitive to TNF-induced cell death. Alternatively, matrix-components and/or growth factors present in the organ cultures may protect from TNF-dependent cytotoxicity. Along those lines, others showed that TNF provoked apoptosis of the chorionic trophoblast cell lines TCL-1 [75]. However, TNFα did not induce death when these cells were cultured on different matrices including fibronectin, collagen 1, collagen 4 or laminin but provoked integrin switching [75]. Hence, it was concluded that TNF may also play a role in EVT differentiation.
Another physiological function of trophoblast-derived TNF could be apoptosis of vascular smooth muscle cells (vSMC) surrounding spiral arteries of the maternal decidua [76]. Removal of those cells is though to be part of a biological process enlarging the diameter of spiral arteries to increase blood flow into the placenta and the developing fetus. Trophoblast cells and trophoblast-conditioned media were shown to induce SMC cell death suggesting that soluble mediators are involved [77]. Indeed, a role of the TNF superfamily members FAS ligand and TRAIL which are produced in placenta and trophoblast has already been demonstrated [77,78]. However, potential involvement of TNF in this process still has to be determined.
3.1.2. Syncytialisation and hormone production
Placental hormones such as human chorion gonadotrophin (hCG) are crucial for maintenance of gestation and successful pregnancy outcome. In placental tissue, the major source of hCG is the multinucleated syncytiotrophoblast layer and hCG subunit transcription, mRNA expression and secretion strongly increase during in vitro cell fusion of primary trophoblasts [79-81]. Since production of hCG could be stimulated by placental as well as decidual growth factors, the role of cytokines including TNF was studied by different investigators.
The first evidence for a critical role of TNF in hormone production was obtained from studies in choriocarcinoma cell lines. TNF was shown to increase hCG secretion of JAR cells, whereas the cytokine reduced hCG secretion from term placenta [82]. Similarly, others also observed TNF-dependent expression of hCG in JAR and JEG cells [83,84]. In contrast, cytokine treatment of primary trophoblasts isolated from term pregnancies decreased hCG secretion up to 75% [85]. Most recently, the role of TNF in trophoblast syncytialisation and hCG expression was analysed in more detail using trophoblast of term placentae and first trimester villous explant cultures. Whereas TNF did not affect expression of the α-subunit of hCG in the purified term trophoblasts, the cytokine suppressed promoter activity of the β5-hCG gene, β-hCG mRNA levels as well as secretion of total hCG [86]. Diminished hCG production and release was attributed to the inhibitory effects of TNF on trophoblast syncytialisation. Along those lines, it was suggested that the cytokine may also negatively affect cell fusion during early pregnancy since TNF reduced endogenous and secreted β-hCG levels as well as the syncytium recovery rate of denuded first trimester villous explant cultures [86].
In conclusion, the data suggest that TNF-dependent hCG expression and secretion could be beneficial for particular functions of choriocarcinoma cell such as proliferation and survival. Indeed, downregulation of β-hCG in these cells was shown to suppress cell growth and to increase apoptosis [87]. In contrast, TNF inhibits syncytialisation and hCG expression in primary trophoblasts suggesting that elevated concentrations of the cytokine could have adverse functions on placental development and pregnancy outcome. The suppressive effects of the cytokine could play a role in the pathogenesis of different gestational diseases, for example in recurrent spontaneous abortions (see Section 3.1.4.2).
3.1.3. Trophoblast proliferation and invasion
The extravillous differentiation program, i.e. formation of anchoring villi trough adhesion and proliferation and generation of different EVT subtypes invading different compartments of the maternal decidua, is an essential process of placental development. The invasive trophoblasts migrate into decidual stroma as well as into the spiral arteries thereby displacing maternal endothelial cells. This process is thought to be associated with enlargement of vessel diameter resulting in increased blood and oxygen supply to the developing placenta and fetus. The mechanisms that initiate proliferation of cell islands during early pregnancy and at distinct attachment sites of anchoring villi are largely unknown but cytokines could potentially be involved.
First evidence that TNF could modulate trophoblast growth was obtained in blastocysts and isolated trophoblasts from rodents [88]. In general, TNF is considered to negatively affect blastocyst since elevated TNF concentrations were shown to decrease proliferation and to increase apoptosis predominantly through TNFR1-dependent signalling [88-91]. This, however, could be mainly due to the adverse effects of the cytokine on the inner cell mass (ICM) of blastocysts rather than on trophoblast [92,93]. In mouse blastocysts, TNF did not alter trophoblast proliferation but increased numbers of multinucleated trophoblast cells [93]. Similarly, the cytokine did not affect cell growth of isolated first trimester trophoblasts, villous explant cultures or of the trophoblast cell line HTR-8/SVneo [59,74,94]. In contrast, TNF may play a role in the autocrine control of choriocarcinoma cell proliferation since recombinant TNF increased proliferation of JEG and JAR cells which could be specifically inhibited by TNFR1 antibodies [43]. Along those lines, analyses of trophoblast cell lines derived from TNFR1 or TNFR2 knock-out mice indicated that inhibition of proliferation is predominantly achieved through TNFR1 signalling [95].
With respect to invasive trophoblast differentiation accumulating evidence suggests that TNF may exert negative effects on cell survival and migration and hence could play a role in gestational diseases with failed trophoblast invasion. Although TNF alone may not be a sufficient inducer of EVT apoptosis [74], the presence of both elevated numbers of macrophages, as it occurs in the decidua of preeclamptic women, and TNF provoked programmed cell death of a hybridoma trophoblast cell model [96]. Several investigators, however, demonstrated that TNF specifically inhibits trophoblast migration and invasion. Although TNF did not affect adhesion, the cytokine was shown to decrease in vitro motility of JEG-3 and HTR-8SVneo cells [97]. In the latter and in first trimester villous explant cultures TNF was shown to decrease migration mainly through upregulation of plasminogen activator inhibitor-1 (PAI-1) since PAI-1-inhibitory antibodies largely abolished the suppressive effect of the cytokine [74,94]. PAI-1 is known to specifically block the pro-invasive enzyme urokinase plasminogen activator-1 (uPA) playing a critical role in trophoblast invasion [98]. TNF-dependent induction of PAI-1 likely involves NFκB-dependent signalling [94]. Similarly, activated macrophages secreting TNF were shown to limit HTR-8/SVneo cell invasiveness through TNF-dependent production of PAI-1 and inhibition of uPA activity [99].
Despite its negative effects on trophoblast migration and invasion, TNF was shown to stimulate matrix metalloproteinase-9 (MMP-9) expression in first trimester trophoblasts, explant cultures and decidual cells [74,100,101]. Since MMP-9 is thought to be one of the key enzymes in trophoblast invasion, TNF-dependent induction of the protease could be a mechanism to balance adverse effects of exceeding cytokine levels. This may also apply to other MMPs regulated by TNF in trophoblasts [102,103]. On the other hand, elevated expression of MMP-9 was noticed in invasive trophoblasts of pregnancies complicated with trisomy 21, in particular in cases with increased apoptosis and poor pregnancy outcome [104]. Also, elevated production of MMP-9 was observed in EVT and adjacent decidual cells of preeclamptic women [100]. These data suggest that aberrant TNF levels could also impair critical steps of trophoblast adhesion and invasion by increasing MMP-9 expression and abnormal degradation of decidual/trophoblast-derived extracellular matrix (ECM) proteins.
3.1.4. TNF and pregnancy diseases
3.1.4.1. Balance of Th1 and Th2 cytokines
The balance of pro- and anti-inflammatory cytokines is critical for implantation, placental development and pregnancy outcome. Expression of inflammation-associated T helper 1 (Th1) cytokines such as TNF could be favourable during the pre-implantation and implantation period. For example, TNF was shown to induce shedding of MUC1 associated with embryo implantation [105].
Pregnancy, however, is associated with a decrease of Th1 and induction of Th2 cytokines. The pregnant uterus is well known as an immune privileged organ since the predominant expression of anti-inflammatory Th2 mediators are likely important to reduce aberrant inflammation and allograft rejection of the fetus. Break down of the immune privilege at the fetal–maternal interface and changes in the Th1/Th2 cytokine profile could have different adverse consequences including recurrent spontaneous abortion (RSA), preterm rupture of membranes, preterm labour or preeclampsia [106,107]. The concept of the Th1/Th2 shift in pregnancy originally proposed by Wegmann et al. [108], however, may represent a simplistic view since accumulating evidence suggests a role of several Th1 cytokines in pregnancy [109]. Similarly, the role of uterine NK cells comprising 70% of all decidual immune cells does not follow the Th1/Th2 paradigm. Nowadays, uNK cells are thought play a substantial role in physiological and pathological pregnancies. They secrete angiogenic growth factors, Th1 (including TNF) and Th2 cytokines and interact with unusual MHC proteins such as HLA-E, -G, -F expressed on extravillous trophoblasts suggesting regulatory functions on trophoblast invasion and vascularisation [107,110]. Indeed, a reduced uNK cell proportion in decidua basalis of pregnancies with fetal growth restriction and a positive correlation between extent of trophoblast invasion and number of uNK cells was observed [111].
Nevertheless, exaggerated secretion of Th1 cytokines such as TNF or IL-1 as occurs upon amniotic infection is known to cause detrimental effects on intra-uterine tissues such as abortion and preterm labour. Similar to other pro-inflammatory cytokines, TNF is secreted from a variety of gestational cells upon stimulation with bacteria or lipopolysaccharides in vitro including placental fragments, amnion, and chorio-decidua [112,113] whereas trophoblast might be less active [114]. LPS-induced secretion of TNF from chorio-decidua could for example induce apoptosis in myometrial cells through TNFR1 [115]. Besides infections with bacteria and viruses, elevated Th1 immune response may also take place when there is limited secretion of soluble MHC proteins such as HLA-G from trophoblast, insufficient influx of NK cells into the decidua, hypersecretion of inflammatory cytokines due to genetic polymorphisms and others [116]. Although the inflammatory response provoked through TNF is regarded as the adverse effect of the cytokine on pregnancy, one may also interpret it in the context of immunosurveillance [117]. Initiation of a Th1 response represents a conserved mechanism allowing for preservation of our species. Restricting infection at the early beginning, even at the expense of fetal survival, guarantees well being of the mother. This evolutionary strategy still applies to women living in countries without access to anti-infective drugs.
3.1.4.2. Recurrent spontaneous abortion
Many factors are thought to be involved in recurrent spontaneous abortion (RSA). Beside chromosomal and structural abnormalities, inflammation processes are one of the main triggers for miscarriage. Infections with pathogens that target the placenta and elicit inflammatory responses can cause abortion by enhancing the levels of damaging cytokines including TNF and INFγ. Determination of cytokines including the detrimental (TNF, INFγ) and beneficial ones (TGFβ, IL-6) revealed unchanged Th2 but increased Th1 cytokine levels in women with RSA supporting the hypothesis that the latter are crucially involved. An increase in TNF and INF concentrations ranging from 40% to 70% were observed in the abortion group compared to controls [118]. In agreement with the adverse role of TNF, reduced amounts of soluble TNF receptors were also described in women with RSA which could be restored to normal levels under progesterone substitution [119]. Treatment with TNF inhibitors also seems to increase live birth rates among women with RSA [120]. In general, TNF levels are significantly higher in women with RSA revealing TNF as “the bad guy”. In many studies, however, TNF is not the only mediator and very often additional triggers are required to finally culminate in an abortive pathology.
TNF, however, may also play a role in preventing the development of offsprings with structural anomalies: under teratogenic stress less embryos with malformations were observed in TNF +/+ as compared to TNF −/− mice [121]
3.1.4.3. Preterm labour
At least 25% of all preterm births occur in pregnant women with microbial infection of the amniotic cavity which normally forms a sterile environment for the fetal development and growth [122]. Nevertheless, more than 60% of preterm deliveries remain unexplained without signs of amniotic infection.
Even in the absence of infection pro-inflammatory cytokines, such as IL-1β and TNF are thought to play crucial roles is in preterm as well as term delivery by transforming the uterus from a quiescent to an active state. The cytokines stimulate uterine activity via production of uterine activation proteins (UAPs) of which prostaglandins, in particular PGF2α and its receptor, MMPs, vEGF, and oxytocin receptor are likely of main importance [123]. TNF, for example, increases in vitro PG production by stimulating endometrial and trophoblastic cyclooxygenase-2 (COX-2) expression [124,125] and by decreasing PG 15-hydroxy dehydrogenase [126] which coverts PGs into inactive metabolites. Cytokine-dependent elevation of PG levels provokes uterine contractions and activates MMPs such as MMP-2 and MMP-9 [127] that degrade the extracellular matrix of the chorio-amniotic membranes. Another critical protein stimulated by TNF could be cortisol since it increases placental cortisol releasing hormone (CRH) production implicated in preterm labour [128]. TNF-dependent elevation of cortisol is achieved by inhibition of placental 11β-hydroxysteroid dehydrogenase which converts cortisol into its inactive derivate cortisone [129]. In vivo, experiments with rhesus monkeys showed that intra-amniotic infusions of TNF induced a variable degree of uterine activity among individual animals stimulating either preterm labour or an uterine contraction pattern of moderate intensity while IL-1β substitution resulted in preterm labour in all cases [130]. Hence, similar to other pregnancy complications the combination and extent of aberrant cytokine levels is critical for severeness of the disease.
3.1.4.4. Preeclampsia and IUGR
Despite a tremendous number of studies the etiology of preeclampsia is still under discussion. Several hints suggest the involvement of immunological reactions leading to the typical mild to severe clinical signs including increased blood pressure and proteinuria that can result in malnutrition of the fetus. Indeed, in the last two decades elevated serum levels of TNF [131-135], and sTNF receptors [134,136-138] as well as increased mRNA/protein expression of TNF/TNFRs were noticed in leukocytes [139] and placenta [140] of preeclamptic women. For example serum concentrations of TNF were shown to be increased from 0.93 pg/ml (healthy women) to 1.39 pg/ml in preeclamptic women [131]. Another group found 210 pg/ml of the cytokine in sera of preeclamptic women as compared to 65 pg/ml in controls [135]. Similarly, sTNF receptors were found to be elevated in the serum of severe preeclamptic patients during the second (140 ng/ml vs. 116 ng/ml) and third (182 ng/ml vs. 142 ng/ml) trimester of pregnancy [138]. Two other groups failed to detect elevated levels of TNF in villous tissue suggesting that other sources than the placenta may also contribute to increased cytokine concentrations in preeclampsia [141,142]. Several authors published a change in the Th1/Th2 profile with elevated Th1 cytokine levels such as TNF and reduced Th2 cytokines including IL-10 and IL-4 [143,144]. Interestingly, despite proteinuria urinary concentrations of TNF are lower in preeclamptic patients, suggesting that decreased renal clearance of the cytokine could contribute to the inflammatory response [131]. Using circulating TNF as a marker controversial data were published concerning the predictability of preeclampsia prior to clinical manifestation. Most authors, however, failed to detect a correlation between TNF levels and the later onset of preeclampsia [145-148]. Hence, the increase of TNF in sera of preeclamptic women might be a consequence rather than a cause of the disease. However, TNF could be a marker for the severity of preeclampsia since a correlation of plasma levels to different stages of the disease could be observed [149].
Preeclampsia is an endothelial disorder and TNF plays a significant role in changing the balance between oxidant and antioxidant, the pattern of prostaglandin production and expression of adhesion molecules in blood vessels [139,147]. Indeed, increased protein expression of soluble adhesion molecules such as sVCAM-1, sP-selectin, sE-selectin in the plasma of preeclamptic women could be a feature of endothelial cell activation and/or damage [150,151]. Placental TNF could be involved in these processes since hypoxia/re-oxygenation of placental tissues in vitro increased secretion of the cytokine and activation of endothelial cells in a TNF-dependent manner [54]. Although increased apoptosis was also noticed in preeclamptic placentae [152], TNF may not be a primary cause[153]. Stimulation of normal and preeclamptic trophoblasts failed to demonstrate increased programmed cell death but resulted in higher sensitivity of the latter to TNF-induced secretion of tissue factor [154].
With respect to the placental bed of preeclamptic women elevated TNF expression was observed in foam cells of non-invaded spiral arteries suggesting a role in the development of atherotic lesions [55]. Activated macrophages also likely represent a source of elevated TNF in preeclamptic decidua which may negatively affect trophoblast migration through upregulation of protease inhibitors such as PAI-1 (see Section 3.1.3).
To delineate genetic factors in preeclampsia polymorphisms of the promoter region of the TNF gene were investigated. Regarding the C-850T polymorphism the T allele was significantly reduced in preeclampsia suggesting a protective role in a finish population[155]. In a Caucasian population another polymorphism, G-308A, showed significant differences only in preeclamptic women with intra-uterine growth restriction (IUGR) [156]. However, other studies failed to detect an association of C-850T and G-308A mutations with preeclampsia in Maya-Mestizo women [157] or of several polymorphisms with familiar preeclampsia in a Dutch population [158] suggesting ethnic differences. Similarly, a mutation in the TNF promoter region which is associated with increased transcription of the cytokine did not correlate with preeclampsia [159].
In IUGR elevated levels of TNF were only observed in women with placental dysfunction but not in those with normal placental perfusion indicating that increased cytokine concentrations could be a phenomenon of a specific subset of IUGRs [160]. IUGR placentae may also have the capacity to release more TNF than normal placentae: using a perfusion model system IUGR placentae were shown to secrete higher amounts of the cytokine upon angiotensin II stimulation [161]. Hence, elevated TNF could potentially promote endothelial cell activation which is also being discussed for IUGR [162]. Furthermore, in vitro studies with villous cytotrophoblasts from IUGR pregnancies demonstrated a higher TNF-induced apoptotic rate when compared with uncomplicated pregnancies [163].
3.2. Physiological and pathological role of TNF in the endometrium
The endometrium is a unique part of the female body where permanent remodelling processes occur during the reproductive stage. The alternating expression of TNF throughout the different endometrial cell types under non-pregnant and pregnant conditions suggests an important role for this particular cytokine. Recently, interactions of chemokines and cytokines in uterine cell types during reproductive physiology and pathology were discussed. The authors pointed out that cytokines such as TNF are important regulators of RANTES, MCP-1 and IL-8 which are produced in a time- and co-ordinated manner in the endometrium suggesting specific roles in angiogenesis, apoptosis, proliferation, differentiation and leukocyte trafficking [164]. Similar to the placenta, inflammation-dependent expression of TNF as well as TNF-dependent induction of cytokines in endometrial cells and chorio-decidua involves signalling through the NFκB pathway [165,166].
3.2.1. Menstrual cycle and decidualisation
As mentioned in Section 2.1 the concentration of TNF varies with the different phases of the menstrual cycle with a constitutive high expression level throughout the secretory phase. It is likely that the abundance of TNF is regulated by levels of steroid hormones. In ovariectomised mice TNF levels dropped whereas administration of oestradiol and progesterone resulted in re-appearance of the cytokine [167]. In humans, however, rise of TNF levels occur when steroid hormones are low. Hence, TNF expression might also be controlled by withdrawal of the hormones. Indeed, depletion of oestrogen in nude mice transplanted with human endometrial carcinoma cells resulted in increased TNF concentrations [168]. However, steroid hormones failed to induce TNF in cultured endometrial epithelial cells [168], suggesting differences in hormone-dependent regulation of TNF between mice and humans or between in vivo and in vitro conditions.
One particular function of TNF could be direct as well as indirect induction of apoptosis in endometrial epithelial and stromal cells associated with menstruation. In the absence of implantation TNF could play a role in shedding of endometrial tissue. Indeed, TNF treatment of endometrial epithelial cells provoked growth arrest and apoptosis as well as loss of epithelial cell–cell contacts and vascular integrity [169]. Also, combined treatment of endometrial stromal cells with TNF and INFγ was shown to increase the apoptotic receptor Fas as well as Fas ligand-dependent apoptosis [170]. TNF may also control other cytokines potentially involved in local inflammation, menstruation and bleeding. In vitro, the cytokine increases production of IL-11 and IL-15 in both endometrial stromal and epithelial cells [171] and promotes expression of macrophage migration inhibitory factor (MIF) [172]. Targets of TNF associated with menstruation are enzymes required for matrix remodelling such as MMPs [173]. The assumption that TNF is involved in endometrial shedding and alteration of vessel function is further substantiated by the fact that increased levels were found in the menstrual effluent of women with menorrhagia [174].
On the other hand, a function of TNF in endometrial regeneration was suggested since the cytokine was also shown to promote expression of soluble HB-EGF acting as mitogenic factor on endometrial stromal cells [175]. Production of the latter is also associated with decidualisation suggesting that the growth factor could be required to attenuate TNF-dependent apoptosis in these cells [176]. Elevated concentrations of the cytokine might indeed negatively affect the decidualisation process: TNF was shown to impair cAMP- and steroid hormone-dependent expression of prolactin secretion from endometrial stromal cells [177].
As mentioned in Section 3.1.3 TNF produced in decidual cells may exert regulatory effects on trophoblast implantation and invasion. Besides expression in macrophages, stromal and epithelial cells, secretion of the cytokine was also noticed from decidual CD56brightCD16− NK cells and CD3+ T-cells [47]. The choriocarcinoma cell line JAR can stimulate TNF release from uterine NK cells [178]. Trophoblast-dependent expression of the cytokine in these cells may negatively affect trophoblast motility and hence represent a mechanism to limit the extent of invasion. A critical, trophoblast-derived molecule in this process could be soluble HLA-G since the protein was shown to induce TNF in uterine mononuclear cells [179]. However, soluble HLA-G was also shown to suppress TNF secretion from uNK cells suggesting differences between diverse uterine immune cells or experimental settings [180].
One of the key functions of TNF in the placental bed might be induction of lymphangiogenesis during early pregnancy which could be important for fluid balance and trafficking of immune cells. Appearance of lymphatic vessels is sparse in normal cycling endometrium but occurs upon trophoblast implantation and invasion as was shown in vivo by transplanting placental villi into Scid mice [181]. In vitro, TNF-neutralising antibodies impaired lymphatic cell migration induced by trophoblast-conditioned medium suggesting a role of TNF in this process [181].
3.2.2. TNF and endometrial diseases
3.2.2.1. Endometriosis
Endometriosis is defined as the presence of endometrial tissue at extrauterine locations, most commonly on the peritoneum and ovaries. The chronic disease occurs in about 10% of women in reproductive age and in up to 50% of women with infertility [182]. The hallmarks of endometriosis are a low-grade inflammation, increased concentrations of activated macrophages and their secreted cytokines, growth and angiogenic factors. Increased levels of TNF were firstly detected in peritoneal fluids of women with endometrioses compared to women with normal pelvic anatomy [183]. Subsequently, several authors showed increased numbers of peritoneal, activated macrophages, elevated levels of TNF in serum and in peritoneal fluid/macrophages as well as a positive correlation between TNF concentration and the severity of endometriosis [184-188]. During menstruation eutopic, endometrial expression of TNF mRNA was significantly higher in women with endometriosis supporting the idea that increased endometrial cytokine secretion may also contribute to an inflammatory microenvironment favouring the development of the disease [189]. TACE was also found to be elevated in endometrioses which could explain elevated levels of soluble TNF [182]. Among the large number of target molecules, TNF was shown to induce IL-1, IL-6 or IL-8 expression in endometriotic cells via the NFκB pathway [166,190,191].
However, TNF may also directly affect endometriotic cells. In vitro assays showed that the adherence of endometrial stromal cells to mesothelial cells was significantly increased by pre-treatment of the latter with TNF suggesting that the cytokine may facilitate pelvic adhesion and formation of ectopic lesions [192]. Others, however, failed to detect TNF-dependent changes in adhesion of endometrial epithelial cells [193]. Interestingly, TNF was shown to increase proliferation of eutopic and ectopic endometriotic cells whereas the cytokine inhibited growth of endometrial cells from healthy women [194]. Upon inhibition of different TNF-dependent signalling pathways in endometriotic epithelial cells decreased expression and secretion of markers for epithelial–mesenchymal transition, inflammation and disease progression were noticed [195]. Moreover, abnormal angiogenic activity in endometriosis might be influenced by TNF. The cytokine was shown to increase vEGF secretion from neutrophils providing an explanation for elevated, peritoneal vEGF concentrations in endometriosis patients [196].
Inhibition of Th1 cytokines such as TNF could be beneficial in the treatment of endometriosis. Indeed, different substances which are being tested or commonly used in patients such as hCG, GnRH analogues or danazol were shown to suppress endometriotic TNF production or expression of TNF-dependent genes in vitro [166,191,197]. However, inhibition of TNF alone may not be sufficiently effective in vivo. Whereas antibodies neutralising TNF activity were shown to decrease numbers and size of endometriotic lesions in a baboon model of induced endometriosis [198], infliximab, commonly used for treatment of Crohn’s disease and rheumatoid arthritis, failed to relieve pain in endometriotic women [199]. Also, treatment with infliximab may cause menstrual disorders such as menorrhagia [200].
3.2.2.2. Fertility problems
Multiple reasons are described in the context of fertility problems. In nearly 25–30% males are responsible due to reduced sperm motility, morphology or quantity whereas in 50% female infertility is the underlying cause. TNF can reduce sperm motility [201] and promoter mutations within the TNF gene which are associated with elevated TNF levels were detected in infertile males [202]. In women, anatomic factors, polycystic ovary syndrome (PCOS), ovulatory dysfunction, fallopian tube occlusion, endometriosis, or others are thought to be responsible for unwanted childlessness. Similar to endometriosis, tubal obstruction and PCOS are associated with peritoneal inflammatory cytokines including TNF, IFN and IL-1 [203]. Increased TNF was also detected in peritoneal fluid of nulligravid and nulliparous women compared to women with two ore more pregnancies/deliveries suggesting that the presence of TNF is associated with primary infertility [183]. In a logistic regression model, serum TNF levels also had a significant and negative impact on the likelihood of pregnancy [204].
4. Conclusions
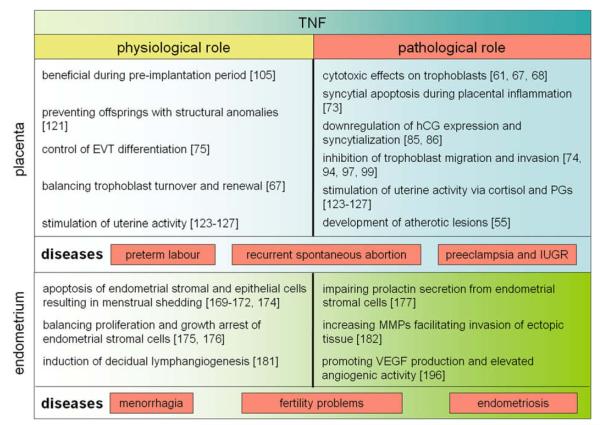
The vast abundance of TNF and its membrane-bound and soluble receptors in endometrium, decidua and placenta tissue suggest a role of the cytokine in reproductive tissues. TNF concentrations, receptor distribution and duration of TNF stimulation may determine whether the cytokine has beneficial or adverse effects on reproductive cell types. A summary of its presumptive functions under physiological and pathological conditions is depicted (Fig. 3). Similar to other organs, TNF may control destruction and renewal of rapidly regenerating endometrial and villous trophoblast epithelia. Besides regulation of tissue homeostasis local TNF might be important during the pre-implantation period, for decidual lymphangiogenesis, to promote labour at term, as well as to limit the extent of trophoblast invasiveness. Exaggerated TNF response, however, may have contributing negative effects on reproduction and pregnancy including menorrhagia, endometriosis, chorioamnionitis, miscarriage, preterm labour, preeclampsia and IUGR. When aberrantly activated, TNF may have pleiotrophic effects on placenta and endometrium. In the uterus, the cytokine may inhibit decidualisation, promote epithelial apoptosis, favour pelvic implantation, angiogenesis and proliferation of endometriotic tissue and amplify the TH1 response by increasing inflammatory cytokines. In the placenta, TNF may impair trophoblast cell fusion and hormone production and provoke increased apoptosis, prostaglandin and cortisol production.
Fig. 3.
An overview of the diverse functions of TNF in endometrium and placenta under physiological and pathological conditions.
Despite our increasing knowledge on the diverse functions TNF in normal and pathological reproduction, much remains to be learned about TNF-dependent signalling cascades in the diverse gestational tissues and its interactions with other Th1 cytokines. Moreover, we assume that in vitro stimulation of placental and endometrial cell types with TNF predominantly provokes detrimental effects which might differ from local effects of the cytokine in vivo, particularly at low doses. This assumption is approved by investigations in other cellular systems. For example, TNF acts predominantly anti-angiogenic on in vitro cultured endothelial cells, whereas the cytokine has pro-angiogenic activities in vivo [205]. Therefore, continuous research and improvement of model systems are required to gain more insights into the complex functions of TNF in physiological and pathological placenta and endometrium.
Acknowledgements
Research in the laboratory of M. Knöfler is supported by grant Nr. 12487 of the Jubilaümsfonds of the Austrian National Bank, by grant Nr. P-17894-B14 of the Austrian Science Funds and by a grant (Nr. APP00323OFF) of the Herzfeldeŕsche Familienstiftung.
Abbreviations
- ASK-1
apoptosis-signalling kinase-1
- AP-1
activator protein-1
- bFGF
basic fibroblast growth factor
- cIAP
cellular inhibitor of apoptosis protein
- COX-2
cyclooxygenase-2
- CRH
cortisol releasing hormone
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EMT
epithelial-mesenchymal transition
- ERK
extracellular regulated kinases
- Etk
endothelial/epithelial kinase
- EVT
extravillous trophoblast
- GnRH
gonadotropin-releasing hormone
- HB-EGF
heparin-binding EGF-like growth factor
- hCG
human chorion gonadotrophin
- HLA
human leukocyte antigen
- ICM
inner cell mass
- IGF-1
insulin-like growth factor 1
- IkB
inhibitor of kB
- INFγ
interferon-γ
- IUGR
intra-uterine growth restriction
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAP
mitogen activated protein
- MCP-1
monocyte-chemotactic protein-1
- MEKK
MAP kinase kinase kinase
- MHC
major histocompatibility complex
- MIF
migration inhibitory factor
- MMP
matrix metallo proteinase
- NFκB
nuclear factor kappa B
- NK cells
natural killer cells
- PAI-1
plasminogen activator inhibitor 1
- PCOS
polycystic ovary syndrome
- PDGF
platelet-derived growth factor
- PG
prostaglandin
- PI3K
phosphoinositid-3-kinase
- RANTES
regulated upon activation normal T-cell expressed and secreted
- RIP-1
serine/threonine kinase receptor interacting protein-1
- SNP
single nucleotide polymorphism
- SODD
silencer of death domain
- STB
syncytiotrophoblast
- sTNF
soluble tumour necrosis factor alpha
- TACE
TNF-converting enzyme
- TGF
transforming growth factor
- Th1
T helper
- Th1
T helper 2
- TNF
tumour necrosis factor alpha
- TNFR1
tumour necrosis factor receptor 1
- TNFR2
tumour necrosis factor receptor 2
- TRADD
TNFR-associated death domain
- TRAF2
TNF receptor-associated factor 2
- TRAIL
TNF-related apoptosis-inducing ligand
- TUNEL
TdT-mediated dUTP-biotin nick end labelling
- UAP
uterine activation protein
- uPA
urokinase-type plasminogen activator
- VCAM
vascular cell adhesion molecule
- vCTB
villous cytotrophoblast
- vEGF
vascular endothelial growth factor
- vSMC
vascular smooth muscle cells
- XAF1
XIAP associated factor 1
- XIAP
X-linked inhibitor of apoptosis.
References
- [1].Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312:724–9. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- [3].Nedwin GE, Naylor SL, Sakaguchi AY, Smith D, Jarrett-Nedwin J, Pennica D, et al. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985;13:6361–73. doi: 10.1093/nar/13.17.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Old LJ. Tumor necrosis factor (TNF) Science. 1985;230:630–2. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- [5].Aggarwal BB, Aiyer RA, Pennica D, Gray PW, Goeddel DV. Human tumour necrosis factors: structure and receptor interactions. Ciba Found Symp. 1987;131:39–51. doi: 10.1002/9780470513521.ch4. [DOI] [PubMed] [Google Scholar]
- [6].Narachi MA, Davis JM, Hsu YR, Arakawa T. Role of single disulfide in recombinant human tumor necrosis factor-alpha. J Biol Chem. 1987;262:13107–10. [PubMed] [Google Scholar]
- [7].Zhang G. Tumor necrosis factor family ligand-receptor binding. Curr Opin Struct Biol. 2004;14:154–60. doi: 10.1016/j.sbi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [8].Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- [9].Tang P, Hung MC, Klostergaard J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry. 1996;35:8216–25. doi: 10.1021/bi952182t. [DOI] [PubMed] [Google Scholar]
- [10].Eck MJ, Sprang SR. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J Biol Chem. 1989;264:17595–605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- [11].Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- [12].Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- [13].Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–62. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- [14].Naismith JH, Sprang SR. Modularity in the TNF-receptor family. Trends Biochem Sci. 1998;23:74–9. doi: 10.1016/s0968-0004(97)01164-x. [DOI] [PubMed] [Google Scholar]
- [15].Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–4. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- [16].Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- [17].Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- [18].Wallach D, Engelmann H, Nophar Y, Aderka D, Kemper O, Hornik V, et al. Soluble and cell surface receptors for tumor necrosis factor. Agents Actions Suppl. 1991;35:51–7. [PubMed] [Google Scholar]
- [19].Solomon KA, Pesti N, Wu G, Newton RC. Cutting edge: a dominant negative form of TNF-alpha converting enzyme inhibits proTNF and TNFRII secretion. J Immunol. 1999;163:4105–8. [PubMed] [Google Scholar]
- [20].McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited auto-inflammatory syndromes. Cell. 1999;97:133–44. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- [21].Sriram K, O’Callaghan JP. Divergent roles for tumor necrosis factor-alpha in the brain. J Neuroimmune Pharmacol. 2007;2:140–53. doi: 10.1007/s11481-007-9070-6. [DOI] [PubMed] [Google Scholar]
- [22].Taylor PC, Peters AM, Paleolog E, Chapman PT, Elliott MJ, McCloskey R, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [23].Blam ME, Stein RB, Lichtenstein GR. Integrating anti-tumor necrosis factor therapy in inflammatory bowel disease: current and future perspectives. Am J Gastroenterol. 2001;96:1977–97. doi: 10.1111/j.1572-0241.2001.03931.x. [DOI] [PubMed] [Google Scholar]
- [24].Feldmann M, Maini RN. Lasker clinical medical research award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245–50. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- [25].Mannel DN, Echtenacher B. TNF in the inflammatory response. Chem Immunol. 2000;74:141–61. [PubMed] [Google Scholar]
- [26].Beutler B, Greenwald D, Hulmes JD, Chang M, Pan YC, Mathison J, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–4. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- [27].Ledgerwood EC, Pober JS, Bradley JR. Recent advances in the molecular basis of TNF signal transduction. Lab Invest. 1999;79:1041–50. [PubMed] [Google Scholar]
- [28].Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- [29].Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543–6. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- [30].Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–96. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- [31].Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, et al. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620–4. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- [32].Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Z. The alpha and beta subunits of IkappaB kinase (IKK) mediate TRAF2-dependent IKK recruitment to tumor necrosis factor (TNF) receptor 1 in response to TNF. Mol Cell Biol. 2001;21:3986–94. doi: 10.1128/MCB.21.12.3986-3994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schutze S, Machleidt T, Adam D, Schwandner R, Wiegmann K, Kruse ML, et al. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J Biol Chem. 1999;274:10203–12. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- [34].Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- [35].Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–4. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- [36].Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–8. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rivas MA, Carnevale RP, Proietti CJ, Rosemblit C, Beguelin W, Salatino M, et al. TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res. 2008;314:509–29. doi: 10.1016/j.yexcr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- [38].MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–92. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- [39].Zhang R, Xu Y, Ekman N, Wu Z, Wu J, Alitalo K, et al. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J Biol Chem. 2003;278:51267–76. doi: 10.1074/jbc.M310678200. [DOI] [PubMed] [Google Scholar]
- [40].Jaattela M, Kuusela P, Saksela E. Demonstration of tumor necrosis factor in human amniotic fluids and supernatants of placental and decidual tissues. Lab Invest. 1988;58:48–52. [PubMed] [Google Scholar]
- [41].Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139:327–35. [PMC free article] [PubMed] [Google Scholar]
- [42].Hunt JS, Chen HL, Hu XL, Tabibzadeh S. Tumor necrosis factor-alpha messenger ribonucleic acid and protein in human endometrium. Biol Reprod. 1992;47:141–7. doi: 10.1095/biolreprod47.1.141. [DOI] [PubMed] [Google Scholar]
- [43].Yang Y, Yelavarthi KK, Chen HL, Pace JL, Terranova PF, Hunt JS. Molecular, biochemical, and functional characteristics of tumor necrosis factor-alpha produced by human placental cytotrophoblastic cells. J Immunol. 1993;150:5614–24. [PubMed] [Google Scholar]
- [44].Vince G, Shorter S, Starkey P, Humphreys J, Clover L, Wilkins T, et al. Localization of tumour necrosis factor production in cells at the materno/fetal interface in human pregnancy. Clin Exp Immunol. 1992;88:174–80. doi: 10.1111/j.1365-2249.1992.tb03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Philippeaux MM, Piguet PF. Expression of tumor necrosis factor-alpha and its mRNA in the endometrial mucosa during the menstrual cycle. Am J Pathol. 1993;143:480–6. [PMC free article] [PubMed] [Google Scholar]
- [46].von Wolff M, Classen-Linke I, Heid D, Krusche CA, Beier-Hellwig K, Karl C, et al. Tumour necrosis factor-alpha (TNF-alpha) in human endometrium and uterine secretion: an evaluation by immunohistochemistry, ELISA and semiquantitative RT-PCR. Mol Hum Reprod. 1999;5:146–52. doi: 10.1093/molehr/5.2.146. [DOI] [PubMed] [Google Scholar]
- [47].Jokhi PP, King A, Sharkey AM, Smith SK, Loke YW. Screening for cytokine messenger ribonucleic acids in purified human decidual lymphocyte populations by the reverse-transcriptase polymerase chain reaction. J Immunol. 1994;153:4427–35. [PubMed] [Google Scholar]
- [48].Tabibzadeh S. Ubiquitous expression of TNF-alpha/cachectin immunoreactivity in human endometrium. Am J Reprod Immunol. 1991;26:1–4. doi: 10.1111/j.1600-0897.1991.tb00692.x. [DOI] [PubMed] [Google Scholar]
- [49].Jokhi PP, King A, Loke YW. Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface. Cytokine. 1997;9:126–37. doi: 10.1006/cyto.1996.0146. [DOI] [PubMed] [Google Scholar]
- [50].Gustafsson C, Hummerdal P, Matthiesen L, Berg G, Ekerfelt C, Ernerudh J. Cytokine secretion in decidual mononuclear cells from term human pregnancy with or without labour: ELISPOT detection of IFN-gamma, IL-4, IL-10, TGF-beta and TNF-alpha. J Reprod Immunol. 2006;71:41–56. doi: 10.1016/j.jri.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [51].Bergqvist A, Nejaty H, Froysa B, Bruse C, Carlberg M, Sjoblom P, et al. Production of interleukins 1beta, 6 and 8 and tumor necrosis factor alpha in separated and cultured endometrial and endometriotic stromal and epithelial cells. Gynecol Obstet Invest. 2000;50:1–6. doi: 10.1159/000010269. [DOI] [PubMed] [Google Scholar]
- [52].Witkin SS, Liu HC, Davis OK, Rosenwaks Z. Tumor necrosis factor is present in maternal sera and embryo culture fluids during in vitro fertilization. J Reprod Immunol. 1991;19:85–93. doi: 10.1016/0165-0378(91)90008-e. [DOI] [PubMed] [Google Scholar]
- [53].King A, Jokhi PP, Smith SK, Sharkey AM, Loke YW. Screening for cytokine mRNA in human villous and extravillous trophoblasts using the reverse-transcriptase polymerase chain reaction (RT-PCR) Cytokine. 1995;7:364–71. doi: 10.1006/cyto.1995.0046. [DOI] [PubMed] [Google Scholar]
- [54].Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164:1049–61. doi: 10.1016/s0002-9440(10)63192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pijnenborg R, McLaughlin PJ, Vercruysse L, Hanssens M, Johnson PM, Keith JC, Jr, et al. Immunolocalization of tumour necrosis factor-alpha (TNF-alpha) in the placental bed of normotensive and hypertensive human pregnancies. Placenta. 1998;19:231–9. doi: 10.1016/s0143-4004(98)90054-6. [DOI] [PubMed] [Google Scholar]
- [56].Tabibzadeh S, Zupi E, Babaknia A, Liu R, Marconi D, Romanini C. Site and menstrual cycle-dependent expression of proteins of the tumour necrosis factor (TNF) receptor family, and BCL-2 oncoprotein and phase-specific production of TNF alpha in human endometrium. Hum Reprod. 1995;10:277–86. doi: 10.1093/oxfordjournals.humrep.a135928. [DOI] [PubMed] [Google Scholar]
- [57].Hunt JS, Chen HL, Miller L. Tumor necrosis factors: pivotal components of pregnancy? Biol Reprod. 1996;54:554–62. doi: 10.1095/biolreprod54.3.554. [DOI] [PubMed] [Google Scholar]
- [58].Yelavarthi KK, Hunt JS. Analysis of p60 and p80 tumor necrosis factor-alpha receptor messenger RNA and protein in human placentas. Am J Pathol. 1993;143:1131–41. [PMC free article] [PubMed] [Google Scholar]
- [59].Knofler M, Mosl B, Bauer S, Griesinger G, Husslein P. TNF-alpha/TNFRI in primary and immortalized first trimester cytotrophoblasts. Placenta. 2000;21:525–35. doi: 10.1053/plac.1999.0501. [DOI] [PubMed] [Google Scholar]
- [60].Austgulen R, Espevik T, Mecsei R, Scott H. Expression of receptors for tumor necrosis factor in human placenta at term. Acta Obstet Gynecol Scand. 1992;71:417–24. doi: 10.3109/00016349209021090. [DOI] [PubMed] [Google Scholar]
- [61].Yui J, Hemmings D, Garcia-Lloret M, Guilbert LJ. Expression of the human p55 and p75 tumor necrosis factor receptors in primary villous trophoblasts and their role in cytotoxic signal transduction. Biol Reprod. 1996;55:400–9. doi: 10.1095/biolreprod55.2.400. [DOI] [PubMed] [Google Scholar]
- [62].Knofler M, Stenzel M, Husslein P. Shedding of tumour necrosis factor receptors from purified villous term trophoblasts and cytotrophoblastic BeWo cells. Hum Reprod. 1998;13:2308–16. doi: 10.1093/humrep/13.8.2308. [DOI] [PubMed] [Google Scholar]
- [63].Austgulen R, Liabakk NB, Brockhaus M, Espevik T. Soluble TNF receptors in amniotic fluid and in urine from pregnant women. J Reprod Immunol. 1992;22:105–16. doi: 10.1016/0165-0378(92)90009-s. [DOI] [PubMed] [Google Scholar]
- [64].Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215:135–41. doi: 10.1016/j.mce.2003.11.022. [DOI] [PubMed] [Google Scholar]
- [65].Wuttke W, Jarry H, Pitzel L, Knoke I, Spiess S. Luteotrophic and luteolytic actions of ovarian peptides. Hum Reprod. 1993;8(Suppl 2):141–6. doi: 10.1093/humrep/8.suppl_2.141. [DOI] [PubMed] [Google Scholar]
- [66].Terranova PF, Rice VM. Review: cytokine involvement in ovarian processes. Am J Reprod Immunol. 1997;37:50–63. doi: 10.1111/j.1600-0897.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- [67].Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15:819–35. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- [68].Straszewski-Chavez SL, Visintin IP, Karassina N, Los G, Liston P, Halaban R, et al. XAF1 mediates tumor necrosis factor-alpha-induced apoptosis and X-linked inhibitor of apoptosis cleavage by acting through the mitochondrial pathway. J Biol Chem. 2007;282:13059–72. doi: 10.1074/jbc.M609038200. [DOI] [PubMed] [Google Scholar]
- [69].Garcia-Lloret MI, Yui J, Winkler-Lowen B, Guilbert LJ. Epidermal growth factor inhibits cytokine-induced apoptosis of primary human trophoblasts. J Cell Physiol. 1996;167:324–32. doi: 10.1002/(SICI)1097-4652(199605)167:2<324::AID-JCP17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [70].Pijnenborg R, Luyten C, Vercruysse L, Keith JC, Jr, Van Assche FA. Cytotoxic effects of tumour necrosis factor (TNF)-alpha and interferon-gamma on cultured human trophoblast are modulated by fibronectin. Mol Hum Reprod. 2000;6:635–41. doi: 10.1093/molehr/6.7.635. [DOI] [PubMed] [Google Scholar]
- [71].Smith S, Francis R, Guilbert L, Baker PN. Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta. 2002;23:322–30. doi: 10.1053/plac.2001.0783. [DOI] [PubMed] [Google Scholar]
- [72].Smith SC, Guilbert LJ, Yui J, Baker PN, Davidge ST. The role of reactive nitrogen/oxygen intermediates in cytokine-induced trophoblast apoptosis. Placenta. 1999;20:309–15. doi: 10.1053/plac.1998.0383. [DOI] [PubMed] [Google Scholar]
- [73].Garcia-Lloret MI, Winkler-Lowen B, Guilbert LJ. Monocytes adhering by LFA-1 to placental syncytiotrophoblasts induce local apoptosis via release of TNF-alpha. A model for hematogenous initiation of placental inflammations. J Leukoc Biol. 2000;68:903–8. [PubMed] [Google Scholar]
- [74].Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–22. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- [75].Fukushima K, Miyamoto S, Komatsu H, Tsukimori K, Kobayashi H, Seki H, et al. TNFalpha-induced apoptosis and integrin switching in human extravillous trophoblast cell line. Biol Reprod. 2003;68:1771–8. doi: 10.1095/biolreprod.102.010314. [DOI] [PubMed] [Google Scholar]
- [76].Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler Thromb Vasc Biol. 2003;23:1553–8. doi: 10.1161/01.ATV.0000086961.44581.B7. [DOI] [PubMed] [Google Scholar]
- [77].Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Aplin JD, et al. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism. Am J Pathol. 2006;169:1863–74. doi: 10.2353/ajpath.2006.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Keogh RJ, Harris LK, Freeman A, Baker PN, Aplin JD, Whitley GS, et al. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–41. doi: 10.1161/01.RES.0000261352.81736.37. [DOI] [PubMed] [Google Scholar]
- [79].Knofler M, Saleh L, Bauer S, Galos B, Rotheneder H, Husslein P, et al. Transcriptional regulation of the human chorionic gonadotropin beta gene during villous trophoblast differentiation. Endocrinology. 2004;145:1685–94. doi: 10.1210/en.2003-0954. [DOI] [PubMed] [Google Scholar]
- [80].Knofler M, Saleh L, Bauer S, Vasicek R, Griesinger G, Strohmer H, et al. Promoter elements and transcription factors involved in differentiation-dependent human chorionic gonadotrophin-alpha messenger ribonucleic acid expression of term villous trophoblasts. Endocrinology. 2000;141:3737–48. doi: 10.1210/endo.141.10.7713. [DOI] [PubMed] [Google Scholar]
- [81].Ringler GE, Kao LC, Miller WL, Strauss JF., 3rd Effects of 8-bromo-cAMP on expression of endocrine functions by cultured human trophoblast cells. Regulation of specific mRNAs. Mol Cell Endocrinol. 1989;61:13–21. doi: 10.1016/0303-7207(89)90185-8. [DOI] [PubMed] [Google Scholar]
- [82].Marth C, Brattia M, Muller-Holzner E, Mayer I, Zech J, Tabarelli M, et al. Modulation of secretion of human chorionic gonadotropin by biologic response modifiers on term placenta and choriocarcinoma cells. Mol Biother. 1989;1:140–4. [PubMed] [Google Scholar]
- [83].Pedersen AM, Fulton SK, Porter L, Francis GL. Tumor necrosis factor-alpha affects in vitro hormone production by JEG-3 choriocarcinoma cell cultures. J Reprod Immunol. 1995;29:69–80. doi: 10.1016/0165-0378(95)00931-a. [DOI] [PubMed] [Google Scholar]
- [84].Yanushpolsky EH, Ozturk M, Polgar K, Berkowitz RS, Hill JA. The effects of cytokines on human chorionic gonadotropin (hCG) production by a trophoblast cell line. J Reprod Immunol. 1993;25:235–47. doi: 10.1016/0165-0378(93)90066-q. [DOI] [PubMed] [Google Scholar]
- [85].Monzon-Bordonaba F, Vadillo-Ortega F, Feinberg RF. Modulation of trophoblast function by tumor necrosis factor-alpha: a role in pregnancy establishment and maintenance? Am J Obstet Gynecol. 2002;187:1574–80. doi: 10.1067/mob.2002.128028. [DOI] [PubMed] [Google Scholar]
- [86].Leisser C, Saleh L, Haider S, Husslein H, Sonderegger S, Knofler M. Tumour necrosis factor-alpha impairs chorionic gonadotrophin beta-subunit expression and cell fusion of human villous cytotrophoblast. Mol Hum Reprod. 2006;12:601–9. doi: 10.1093/molehr/gal066. [DOI] [PubMed] [Google Scholar]
- [87].Hamada AL, Nakabayashi K, Sato A, Kiyoshi K, Takamatsu Y, Laoag-Fernandez JB, et al. Transfection of antisense chorionic gonadotropin beta gene into choriocarcinoma cells suppresses the cell proliferation and induces apoptosis. J Clin Endocrinol Metab. 2005;90:4873–9. doi: 10.1210/jc.2004-2458. [DOI] [PubMed] [Google Scholar]
- [88].Hunt JS, Atherton RA, Pace JL. Differential responses of rat trophoblast cells and embryonic fibroblasts to cytokines that regulate proliferation and class I MHC antigen expression. J Immunol. 1990;145:184–9. [PubMed] [Google Scholar]
- [89].Pampfer S, Vanderheyden I, Vesela J, De Hertogh R. Neutralization of tumor necrosis factor alpha (TNF alpha) action on cell proliferation in rat blastocysts by antisense oligodeoxyribonucleotides directed against TNF alpha p60 receptor. Biol Reprod. 1995;52:1316–26. doi: 10.1095/biolreprod52.6.1316. [DOI] [PubMed] [Google Scholar]
- [90].Kawamura K, Kawamura N, Kumagai J, Fukuda J, Tanaka T. Tumor necrosis factor regulation of apoptosis in mouse preimplantation embryos and its antagonism by transforming growth factor alpha/phosphatidylionsitol 3-kinase signaling system. Biol Reprod. 2007;76:611–8. doi: 10.1095/biolreprod.106.058008. [DOI] [PubMed] [Google Scholar]
- [91].Pampfer S, Moulaert B, Vanderheyden I, Wuu YD, De Hertogh R. Effect of tumour necrosis factor alpha on rat blastocyst growth and glucose metabolism. J Reprod Fertil. 1994;101:199–206. doi: 10.1530/jrf.0.1010199. [DOI] [PubMed] [Google Scholar]
- [92].Drake BL, Head JR. Murine trophoblast cells are not killed by tumor necrosis factor-alpha. J Reprod Immunol. 1990;17:93–9. doi: 10.1016/0165-0378(90)90043-6. [DOI] [PubMed] [Google Scholar]
- [93].Whiteside EJ, Boucaut KJ, Teh A, Garcia-Aragon J, Harvey MB, Herington AC. Elevated concentration of TNF-alpha induces trophoblast differentiation in mouse blastocyst outgrowths. Cell Tissue Res. 2003;314:275–80. doi: 10.1007/s00441-003-0791-4. [DOI] [PubMed] [Google Scholar]
- [94].Huber AV, Saleh L, Bauer S, Husslein P, Knofler M. TNFalpha-mediated induction of PAI-1 restricts invasion of HTR-8/SVneo trophoblast cells. Placenta. 2006;27:127–36. doi: 10.1016/j.placenta.2005.02.012. [DOI] [PubMed] [Google Scholar]
- [95].Rasmussen CA, Pace JL, Banerjee S, Phillips TA, Hunt JS. Trophoblastic cell lines generated from tumour necrosis factor receptor-deficient mice reveal specific functions for the two tumour necrosis factor receptors. Placenta. 1999;20:213–22. doi: 10.1053/plac.1998.0356. [DOI] [PubMed] [Google Scholar]
- [96].Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–52. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- [97].Todt JC, Yang Y, Lei J, Lauria MR, Sorokin Y, Cotton DB, et al. Effects of tumor necrosis factor-alpha on human trophoblast cell adhesion and motility. Am J Reprod Immunol. 1996;36:65–71. doi: 10.1111/j.1600-0897.1996.tb00141.x. [DOI] [PubMed] [Google Scholar]
- [98].Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–87. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- [99].Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod. 2005;73:237–43. doi: 10.1095/biolreprod.104.038000. [DOI] [PubMed] [Google Scholar]
- [100].Lockwood CJ, Oner C, Uz YH, Kayisli UA, Huang SJ, Buchwalder LF, et al. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78:1064–72. doi: 10.1095/biolreprod.107.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Meisser A, Chardonnens D, Campana A, Bischof P. Effects of tumour necrosis factor-alpha, interleukin-1 alpha, macrophage colony stimulating factor and transforming growth factor beta on trophoblastic matrix metalloproteinases. Mol Hum Reprod. 1999;5:252–60. doi: 10.1093/molehr/5.3.252. [DOI] [PubMed] [Google Scholar]
- [102].Hiden U, Glitzner E, Ivanisevic M, Djelmis J, Wadsack C, Lang U, et al. MT1-MMP expression in first-trimester placental tissue is upregulated in type 1 diabetes as a result of elevated insulin and tumor necrosis factor-alpha levels. Diabetes. 2008;57:150–7. doi: 10.2337/db07-0903. [DOI] [PubMed] [Google Scholar]
- [103].Hiden U, Wadsack C, Prutsch N, Gauster M, Weiss U, Frank HG, et al. The first trimester human trophoblast cell line ACH-3P: a novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations – TNF-alpha stimulates MMP15 expression. BMC Dev Biol. 2007;7:137. doi: 10.1186/1471-213X-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wright A, Zhou Y, Weier JF, Caceres E, Kapidzic M, Tabata T, et al. Trisomy 21 is associated with variable defects in cytotrophoblast differentiation along the invasive pathway. Am J Med Genet A. 2004;130A:354–64. doi: 10.1002/ajmg.a.30254. [DOI] [PubMed] [Google Scholar]
- [105].Thathiah A, Brayman M, Dharmaraj N, Julian JJ, Lagow EL, Carson DD. Tumor necrosis factor alpha stimulates MUC1 synthesis and ectodomain release in a human uterine epithelial cell line. Endocrinology. 2004;145:4192–203. doi: 10.1210/en.2004-0399. [DOI] [PubMed] [Google Scholar]
- [106].Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–9. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- [107].Sargent IL, Borzychowski AM, Redman CW. NK cells and pre-eclampsia. J Reprod Immunol. 2007;76:40–4. doi: 10.1016/j.jri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [108].Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- [109].Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007;29:95–113. doi: 10.1007/s00281-007-0069-0. [DOI] [PubMed] [Google Scholar]
- [110].Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–94. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- [111].Eide IP, Rolfseng T, Isaksen CV, Mecsei R, Roald B, Lydersen S, et al. Serious foetal growth restriction is associated with reduced proportions of natural killer cells in decidua basalis. Virchows Arch. 2006;448:269–76. doi: 10.1007/s00428-005-0107-z. [DOI] [PubMed] [Google Scholar]
- [112].Paradowska E, Blach-Olszewska Z, Gejdel E. Constitutive and induced cytokine production by human placenta and amniotic membrane at term. Placenta. 1997;18:441–6. doi: 10.1016/s0143-4004(97)80045-8. [DOI] [PubMed] [Google Scholar]
- [113].Sato TA, Keelan JA, Mitchell MD. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J Immunol. 2003;170:158–66. doi: 10.4049/jimmunol.170.1.158. [DOI] [PubMed] [Google Scholar]
- [114].Griesinger G, Saleh L, Bauer S, Husslein P, Knofler M. Production of pro- and anti-inflammatory cytokines of human placental trophoblasts in response to pathogenic bacteria. J Soc Gynecol Investig. 2001;8:334–40. [PubMed] [Google Scholar]
- [115].Leroy MJ, Dallot E, Czerkiewicz I, Schmitz T, Breuiller-Fouche M. Inflammation of choriodecidua induces tumor necrosis factor alpha-mediated apoptosis of human myometrial cells. Biol Reprod. 2007;76:769–76. doi: 10.1095/biolreprod.106.058057. [DOI] [PubMed] [Google Scholar]
- [116].Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med. 2006;11:302–8. doi: 10.1016/j.siny.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [117].Wilczynski JR. Th1/Th2 cytokines balance–yin and yang of reproductive immunology. Eur J Obstet Gynecol Reprod Biol. 2005;122:136–43. doi: 10.1016/j.ejogrb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- [118].Shaarawy M, Nagui AR. Enhanced expression of cytokines may play a fundamental role in the mechanisms of immunologically mediated recurrent spontaneous abortion. Acta Obstet Gynecol Scand. 1997;76:205–11. doi: 10.3109/00016349709048142. [DOI] [PubMed] [Google Scholar]
- [119].Chernyshov VP, Vodyanik MA, Pisareva SP. Lack of soluble TNF-receptors in women with recurrent spontaneous abortion and possibility for its correction. Am J Reprod Immunol. 2005;54:284–91. doi: 10.1111/j.1600-0897.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- [120].Winger EE, Reed JL. Treatment with tumor necrosis factor inhibitors and intravenous immunoglobulin improves live birth rates in women with recurrent spontaneous abortion. Am J Reprod Immunol. 2008;60:8–16. doi: 10.1111/j.1600-0897.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- [121].Toder V, Fein A, Carp H, Torchinsky A. TNF-alpha in pregnancy loss and embryo maldevelopment: a mediator of detrimental stimuli or a protector of the fetoplacental unit? J Assist Reprod Genet. 2003;20:73–81. doi: 10.1023/A:1021740108284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- [123].Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008 doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [124].Kniss DA, Zimmerman PD, Garver CL, Fertel RH. Interleukin-1 receptor antagonist blocks interleukin-1-induced expression of cyclooxygenase-2 in endometrium. Am J Obstet Gynecol. 1997;177:559–67. doi: 10.1016/s0002-9378(97)70146-7. [DOI] [PubMed] [Google Scholar]
- [125].Imseis HM, Zimmerman PD, Samuels P, Kniss DA. Tumour necrosis factor-alpha induces cyclo-oxygenase-2 gene expression in first trimester trophoblasts: suppression by glucocorticoids and NSAIDs. Placenta. 1997;18:521–6. doi: 10.1016/0143-4004(77)90005-4. [DOI] [PubMed] [Google Scholar]
- [126].Pomini F, Caruso A, Challis JR. Interleukin-10 modifies the effects of interleukin-1beta and tumor necrosis factor-alpha on the activity and expression of prostaglandin H synthase-2 and the NAD+-dependent 15-hydrox-yprostaglandin dehydrogenase in cultured term human villous trophoblast and chorion trophoblast cells. J Clin Endocrinol Metab. 1999;84:4645–51. doi: 10.1210/jcem.84.12.6188. [DOI] [PubMed] [Google Scholar]
- [127].Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod. 2001;7:1187–93. doi: 10.1093/molehr/7.12.1187. [DOI] [PubMed] [Google Scholar]
- [128].Dudley DJ. Immunoendocrinology of preterm labor: the link between corticotropin-releasing hormone and inflammation. Am J Obstet Gynecol. 1999;180:S251–6. doi: 10.1016/s0002-9378(99)70711-8. [DOI] [PubMed] [Google Scholar]
- [129].Kossintseva I, Wong S, Johnstone E, Guilbert L, Olson DM, Mitchell BF. Proinflammatory cytokines inhibit human placental 11beta-hydroxysteroid dehydrogenase type 2 activity through Ca2+ and cAMP pathways. Am J Physiol Endocrinol Metab. 2006;290:E282–8. doi: 10.1152/ajpendo.00328.2005. [DOI] [PubMed] [Google Scholar]
- [130].Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- [131].Cackovic M, Buhimschi CS, Zhao G, Funai EF, Norwitz ER, Kuczynski E, et al. Fractional excretion of tumor necrosis factor-alpha in women with severe preeclampsia. Obstet Gynecol. 2008;112:93–100. doi: 10.1097/AOG.0b013e31817c4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- [133].Sharma A, Satyam A, SharmaLeptin JB. IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- [134].Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- [135].Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–7. [discussion: 7–9] [PubMed] [Google Scholar]
- [136].Visser W, Beckmann I, Knook MA, Wallenburg HC. Soluble tumor necrosis factor receptor II and soluble cell adhesion molecule 1 as markers of tumor necrosis factor-alpha release in preeclampsia. Acta Obstet Gynecol Scand. 2002;81:713–9. [PubMed] [Google Scholar]
- [137].Kupferminc MJ, Peaceman AM, Aderka D, Wallach D, Socol ML. Soluble tumor necrosis factor receptors and interleukin-6 levels in patients with severe preeclampsia. Obstet Gynecol. 1996;88:420–7. doi: 10.1016/0029-7844(96)00179-2. [DOI] [PubMed] [Google Scholar]
- [138].Schipper EJ, Bolte AC, Schalkwijk CG, Van Geijn HP, Dekker GA. TNF-receptor levels in preeclampsia-results of a longitudinal study in high-risk women. J Matern Fetal Neonatal Med. 2005;18:283–7. doi: 10.1080/14767050500246466. [DOI] [PubMed] [Google Scholar]
- [139].Chen G, Wilson R, Wang SH, Zheng HZ, Walker JJ, McKillop JH. Tumour necrosis factor-alpha (TNF-alpha) gene polymorphism and expression in preeclampsia. Clin Exp Immunol. 1996;104:154–9. doi: 10.1046/j.1365-2249.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32:157–69. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- [141].Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–12. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- [142].Hayashi M, Ueda Y, Yamaguchi T, Sohma R, Shibazaki M, Ohkura T, et al. Tumor necrosis factor-alpha in the placenta is not elevated in pre-eclamptic patients despite its elevation in peripheral blood. Am J Reprod Immunol. 2005;53:113–9. doi: 10.1111/j.1600-0897.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- [143].Dong M, He J, Wang Z, Xie X, Wang H. Placental imbalance of Th1- and Th2-type cytokines in preeclampsia. Acta Obstet Gynecol Scand. 2005;84:788–93. doi: 10.1111/j.0001-6349.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- [144].Jonsson Y, Ruber M, Matthiesen L, Berg G, Nieminen K, Sharma S, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- [145].Hamai Y, Fujii T, Yamashita T, Nishina H, Kozuma S, Mikami Y, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38:89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- [146].Heikkinen J, Mottonen M, Pulkki K, Lassila O, Alanen A. Cytokine levels in midtrimester amniotic fluid in normal pregnancy and in the prediction of pre-eclampsia. Scand J Immunol. 2001;53:310–4. doi: 10.1046/j.1365-3083.2001.00872.x. [DOI] [PubMed] [Google Scholar]
- [147].Meekins JW, McLaughlin PJ, West DC, McFadyen IR, Johnson PM. Endothelial cell activation by tumour necrosis factor-alpha (TNF-alpha) and the development of pre-eclampsia. Clin Exp Immunol. 1994;98:110–4. doi: 10.1111/j.1365-2249.1994.tb06615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Serin IS, Ozcelik B, Basbug M, Kilic H, Okur D, Erez R. Predictive value of tumor necrosis factor alpha (TNF-alpha) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2002;100:143–5. doi: 10.1016/s0301-2115(01)00484-5. [DOI] [PubMed] [Google Scholar]
- [149].Peracoli JC, Rudge MV, Peracoli MT. Tumor necrosis factor-alpha in gestation and puerperium of women with gestational hypertension and pre-eclampsia. Am J Reprod Immunol. 2007;57:177–85. doi: 10.1111/j.1600-0897.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- [150].Chaiworapongsa T, Romero R, Yoshimatsu J, Espinoza J, Kim YM, Park K, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;12:19–27. doi: 10.1080/jmf.12.1.19.27. [DOI] [PubMed] [Google Scholar]
- [151].Coata G, Pennacchi L, Bini V, Liotta L, Di Renzo GC. Soluble adhesion molecules: marker of pre-eclampsia and intrauterine growth restriction. J Matern Fetal Neonatal Med. 2002;12:28–34. doi: 10.1080/jmf.12.1.28.34. [DOI] [PubMed] [Google Scholar]
- [152].Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184:1249–50. doi: 10.1067/mob.2001.112906. [DOI] [PubMed] [Google Scholar]
- [153].Kharfi A, Bureau M, Giguere Y, Moutquin JM, Forest JC. Dissociation between increased apoptosis and expression of the tumor necrosis factor-alpha system in term placental villi with preeclampsia. Clin Biochem. 2006;39:646–51. doi: 10.1016/j.clinbiochem.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [154].Kwaan HC, Wang J, Boggio L, Weiss I, Grobman WA. The thrombogenic effect of an inflammatory cytokine on trophoblasts from women with preeclampsia. Am J Obstet Gynecol. 2004;191:2142–7. doi: 10.1016/j.ajog.2004.05.026. [DOI] [PubMed] [Google Scholar]
- [155].Heiskanen J, Romppanen EL, Hiltunen M, Iivonen S, Mannermaa A, Punnonen K, et al. Polymorphism in the tumor necrosis factor-alpha gene in women with preeclampsia. J Assist Reprod Genet. 2002;19:220–3. doi: 10.1023/A:1015306818507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Molvarec A, Jermendy A, Nagy B, Kovacs M, Varkonyi T, Hupuczi P, et al. Association between tumor necrosis factor (TNF)-alpha G-308A gene polymorphism and preeclampsia complicated by severe fetal growth restriction. Clin Chim Acta. 2008;392:52–7. doi: 10.1016/j.cca.2008.03.009. [DOI] [PubMed] [Google Scholar]
- [157].Canto-Cetina T, Canizales-Quinteros S, de la Chesnaye E, Coral-Vazquez R, Mendez JP, Canto P. Analysis of C-850T and G-308A polymorphisms of the tumor necrosis factor-alpha gene in maya-mestizo women with preeclampsia. Hypertens Pregnancy. 2007;26:283–91. doi: 10.1080/10641950701372732. [DOI] [PubMed] [Google Scholar]
- [158].Lachmeijer AM, Crusius JB, Pals G, Dekker GA, Arngrimsson R, ten Kate LP. Polymorphisms in the tumor necrosis factor and lymphotoxin-alpha gene region and preeclampsia. Obstet Gynecol. 2001;98:612–9. doi: 10.1016/s0029-7844(01)01487-9. [DOI] [PubMed] [Google Scholar]
- [159].Dizon-Townson DS, Major H, Ward K. A promoter mutation in the tumor necrosis factor alpha gene is not associated with preeclampsia. J Reprod Immunol. 1998;38:55–61. doi: 10.1016/s0165-0378(98)00008-4. [DOI] [PubMed] [Google Scholar]
- [160].Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2003;82:1099–102. doi: 10.1046/j.1600-0412.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- [161].Holcberg G, Huleihel M, Sapir O, Katz M, Tsadkin M, Furman B, et al. Increased production of tumor necrosis factor-alpha TNF-alpha by IUGR human placentae. Eur J Obstet Gynecol Reprod Biol. 2001;94:69–72. doi: 10.1016/s0301-2115(00)00321-3. [DOI] [PubMed] [Google Scholar]
- [162].Johnson MR, Anim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? Bjog. 2002;109:836–9. doi: 10.1111/j.1471-0528.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- [163].Kilani RT, Mackova M, Davidge ST, Winkler-Lowen B, Demianczuk N, Guilbert LJ. Endogenous tumor necrosis factor alpha mediates enhanced apoptosis of cultured villous trophoblasts from intrauterine growth-restricted placentae. Reproduction. 2007;133:257–64. doi: 10.1530/REP-06-0080. [DOI] [PubMed] [Google Scholar]
- [164].Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–21. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- [165].Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668–73. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- [166].Huber AV, Saleh L, Prast J, Haslinger P, Knofler M. Human chorionic gonadotrophin attenuates NF-kappaB activation and cytokine expression of endometriotic stromal cells. Mol Hum Reprod. 2007;13:595–604. doi: 10.1093/molehr/gam032. [DOI] [PubMed] [Google Scholar]
- [167].Roby KF, Hunt JS. Mouse endometrial tumor necrosis factor-alpha messenger ribonucleic acid and protein: localization and regulation by estradiol and progesterone. Endocrinology. 1994;135:2780–9. doi: 10.1210/endo.135.6.7988471. [DOI] [PubMed] [Google Scholar]
- [168].Tabibzadeh S, Satyaswaroop PG, von Wolff M, Strowitzki T. Regulation of TNF-alpha mRNA expression in endometrial cells by TNF-alpha and by oestrogen withdrawal. Mol Hum Reprod. 1999;5:1141–9. doi: 10.1093/molehr/5.12.1141. [DOI] [PubMed] [Google Scholar]
- [169].Tabibzadeh S. The signals and molecular pathways involved in human menstruation, a unique process of tissue destruction and remodelling. Mol Hum Reprod. 1996;2:77–92. doi: 10.1093/molehr/2.2.77. [DOI] [PubMed] [Google Scholar]
- [170].Fluhr H, Krenzer S, Stein GM, Stork B, Deperschmidt M, Wallwiener D, et al. Interferon-gamma and tumor necrosis factor-alpha sensitize primarily resistant human endometrial stromal cells to fas-mediated apoptosis. J Cell Sci. 2007;120:4126–33. doi: 10.1242/jcs.009761. [DOI] [PubMed] [Google Scholar]
- [171].Roberts M, Luo X, Chegini N. Differential regulation of interleukins IL-13 and IL-15 by ovarian steroids, TNF-alpha and TGF-beta in human endometrial epithelial and stromal cells. Mol Hum Reprod. 2005;11:751–60. doi: 10.1093/molehr/gah233. [DOI] [PubMed] [Google Scholar]
- [172].Cao WG, Morin M, Sengers V, Metz C, Roger T, Maheux R, et al. Tumour necrosis factor-alpha up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-kappaB. Hum Reprod. 2006;21:421–8. doi: 10.1093/humrep/dei315. [DOI] [PubMed] [Google Scholar]
- [173].Singer CF, Marbaix E, Lemoine P, Courtoy PJ, Eeckhout Y. Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. Eur J Biochem. 1999;259:40–5. doi: 10.1046/j.1432-1327.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- [174].Malik S, Day K, Perrault I, Charnock-Jones DS, Smith SK. Reduced levels of VEGF-A and MMP-2 and MMP-9 activity and increased TNF-alpha in menstrual endometrium and effluent in women with menorrhagia. Hum Reprod. 2006;21:2158–66. doi: 10.1093/humrep/del089. [DOI] [PubMed] [Google Scholar]
- [175].Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, et al. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002;87:5769–77. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chobotova K, Karpovich N, Carver J, Manek S, Gullick WJ, Barlow DH, et al. Heparin-binding epidermal growth factor and its receptors mediate decidualization and potentiate survival of human endometrial stromal cells. J Clin Endocrinol Metab. 2005;90:913–9. doi: 10.1210/jc.2004-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Inoue T, Kanzaki H, Iwai M, Imai K, Narukawa S, Higuchi T, et al. Tumour necrosis factor alpha inhibits in-vitro decidualization of human endometrial stromal cells. Hum Reprod. 1994;9:2411–7. doi: 10.1093/oxfordjournals.humrep.a138460. [DOI] [PubMed] [Google Scholar]
- [178].Rieger L, Kammerer U, Hofmann J, Sutterlin M, Dietl J. Choriocarcinoma cells modulate the cytokine production of decidual large granular lymphocytes in coculture. Am J Reprod Immunol. 2001;46:137–43. doi: 10.1111/j.8755-8920.2001.460204.x. [DOI] [PubMed] [Google Scholar]
- [179].van der Meer A, Lukassen HG, van Cranenbroek B, Weiss EH, Braat DD, van Lierop MJ, et al. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod. 2007;13:123–33. doi: 10.1093/molehr/gal100. [DOI] [PubMed] [Google Scholar]
- [180].Rieger L, Hofmeister V, Probe C, Dietl J, Weiss EH, Steck T, et al. Th1- and Th2-like cytokine production by first trimester decidual large granular lymphocytes is influenced by HLA-G and HLA-E. Mol Hum Reprod. 2002;8:255–61. doi: 10.1093/molehr/8.3.255. [DOI] [PubMed] [Google Scholar]
- [181].Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–52. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Gottschalk C, Malberg K, Arndt M, Schmitt J, Roessner A, Schultze D, et al. Matrix metalloproteinases and TACE play a role in the pathogenesis of endometriosis. Adv Exp Med Biol. 2000;477:483–6. doi: 10.1007/0-306-46826-3_49. [DOI] [PubMed] [Google Scholar]
- [183].Eisermann J, Gast MJ, Pineda J, Odem RR, Collins JL. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil Steril. 1988;50:573–9. doi: 10.1016/s0015-0282(16)60185-1. [DOI] [PubMed] [Google Scholar]
- [184].Cho SH, Oh YJ, Nam A, Kim HY, Park JH, Kim JH, et al. Evaluation of serum and urinary angiogenic factors in patients with endometriosis. Am J Reprod Immunol. 2007;58:497–504. doi: 10.1111/j.1600-0897.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- [185].Keenan JA, Chen TT, Chadwell NL, Torry DS, Caudle MR. IL-1 beta, TNF-alpha, and IL-2 in peritoneal fluid and macrophage-conditioned media of women with endometriosis. Am J Reprod Immunol. 1995;34:381–5. doi: 10.1111/j.1600-0897.1995.tb00968.x. [DOI] [PubMed] [Google Scholar]
- [186].Richter ON, Dorn C, Rosing B, Flaskamp C, Ulrich U. Tumor necrosis factor alpha secretion by peritoneal macrophages in patients with endometriosis. Arch Gynecol Obstet. 2005;271:143–7. doi: 10.1007/s00404-003-0591-9. [DOI] [PubMed] [Google Scholar]
- [187].Steff AM, Gagne D, Page M, Rioux A, Hugo P, Gosselin D. Serum concentrations of insulin-like growth factor-1, soluble tumor necrosis factor receptor-1 and angiogenin in endometriosis patients. Am J Reprod Immunol. 2004;51:166–73. doi: 10.1046/j.8755-8920.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- [188].Harada T, Enatsu A, Mitsunari M, Nagano Y, Ito M, Tsudo T, et al. Role of cytokines in progression of endometriosis. Gynecol Obstet Invest. 1999;47(Suppl. 1):34–9. doi: 10.1159/000052857. [discussion: 9–40] [DOI] [PubMed] [Google Scholar]
- [189].Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, et al. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 2006;85:1667–75. doi: 10.1016/j.fertnstert.2005.11.060. [DOI] [PubMed] [Google Scholar]
- [190].Yamauchi N, Harada T, Taniguchi F, Yoshida S, Iwabe T, Terakawa N. Tumor necrosis factor-alpha induced the release of interleukin-6 from endometriotic stromal cells by the nuclear factor-kappaB and mitogen-activated protein kinase pathways. Fertil Steril. 2004;82(Suppl. 3):1023–8. doi: 10.1016/j.fertnstert.2004.02.134. [DOI] [PubMed] [Google Scholar]
- [191].Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S, et al. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab. 2003;88:730–5. doi: 10.1210/jc.2002-020666. [DOI] [PubMed] [Google Scholar]
- [192].Zhang RJ, Wild RA, Ojago JM. Effect of tumor necrosis factor-alpha on adhesion of human endometrial stromal cells to peritoneal mesothelial cells: an in vitro system. Fertil Steril. 1993;59:1196–201. doi: 10.1016/s0015-0282(16)55976-7. [DOI] [PubMed] [Google Scholar]
- [193].Debrock S, De Strooper B, Vander Perre S, Hill JA, D’Hooghe TM. Tumour necrosis factor-alpha, interleukin-6 and interleukin-8 do not promote adhesion of human endometrial epithelial cells to mesothelial cells in a quantitative in vitro model. Hum Reprod. 2006;21:605–9. doi: 10.1093/humrep/dei375. [DOI] [PubMed] [Google Scholar]
- [194].Braun DP, Ding J, Dmowski WP. Peritoneal fluid-mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil Steril. 2002;78:727–32. doi: 10.1016/s0015-0282(02)03318-6. [DOI] [PubMed] [Google Scholar]
- [195].Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, et al. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol Pharmacol. 2008;73:1394–404. doi: 10.1124/mol.107.042176. [DOI] [PubMed] [Google Scholar]
- [196].Na YJ, Yang SH, Baek DW, Lee DH, Kim KH, Choi YM, et al. Effects of peritoneal fluid from endometriosis patients on the release of vascular endothelial growth factor by neutrophils and monocytes. Hum Reprod. 2006;21:1846–55. doi: 10.1093/humrep/del077. [DOI] [PubMed] [Google Scholar]
- [197].Mori H, Nakagawa M, Itoh N, Wada K, Tamaya T. Danazol suppresses the production of interleukin-1 beta and tumor necrosis factor by human monocytes. Am J Reprod Immunol. 1990;24:45–50. doi: 10.1111/j.1600-0897.1990.tb01037.x. [DOI] [PubMed] [Google Scholar]
- [198].Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, et al. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006;21:1856–62. doi: 10.1093/humrep/del044. [DOI] [PubMed] [Google Scholar]
- [199].Koninckx PR, Craessaerts M, Timmerman D, Cornillie F, Kennedy S. Anti-TNF-alpha treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Hum Reprod. 2008;23:2017–23. doi: 10.1093/humrep/den177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Scheinfeld N. Menorrhagia and severe menstrual pain related to the use of adalimumab in a psoriatic. J Dermatolog Treat. 2008;19:188–9. doi: 10.1080/09546630801955143. [DOI] [PubMed] [Google Scholar]
- [201].Perdichizzi A, Nicoletti F, La Vignera S, Barone N, D’Agata R, Vicari E, et al. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J Clin Immunol. 2007;27:152–62. doi: 10.1007/s10875-007-9071-5. [DOI] [PubMed] [Google Scholar]
- [202].Tronchon V, Vialard F, El Sirkasi M, Dechaud H, Rollet J, Albert M, et al. Tumor necrosis factor-alpha -308 polymorphism in infertile men with altered sperm production or motility. Hum Reprod. 2008 doi: 10.1093/humrep/den277. [DOI] [PubMed] [Google Scholar]
- [203].Iborra A, Palacio JR, Martinez P. Oxidative stress and autoimmune response in the infertile woman. Chem Immunol Allergy. 2005;88:150–62. doi: 10.1159/000087832. [DOI] [PubMed] [Google Scholar]
- [204].Murakami T, Okamura C, Matsuzaki S, Terada Y, Yokomizo R, Noda T, et al. Prediction of pregnancy in infertile women with endometriosis. Gynecol Obstet Invest. 2002;53(Suppl. 1):26–32. doi: 10.1159/000049421. [DOI] [PubMed] [Google Scholar]
- [205].Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]