Abstract
Chorionic gonadotropin (CG) is indispensable for human pregnancy because it controls implantation, decidualization, and placental development. However, its particular role in the differentiation process of invasive trophoblasts has not been fully unraveled. Here we demonstrate that the hormone promotes trophoblast invasion and migration in different trophoblast model systems. RT-PCR and Western blot analyses revealed expression of the LH/CG receptor in trophoblast cell lines and different trophoblast primary cultures. In vitro, CG increased migration and invasion of trophoblastic SGHPL-5 cells through uncoated and Matrigel-coated transwells, respectively. The hormone also increased migration of first-trimester villous explant cultures on collagen I. Proliferation of the trophoblast cell line and villous explant cultures measured by cumulative cell numbers and in situ 5-bromo-2′-deoxyuridine labeling, respectively, was unaffected by CG. Addition of the hormone activated ERK-1/2 and AKT in SGHPL-5 cells and pure, extravillous trophoblasts. Inhibition of MAPK kinase/ERK and phosphatidylinositide 3-kinase/AKT blocked phosphorylation of the kinases and attenuated CG-dependent invasion of SGHPL-5 cells. Similarly, the inhibitors decreased hormone-stimulated migration in villous explant cultures. Western blot analyses and gelatin zymography suggested that CG increased matrix metalloproteinase (MMP)-2 protein levels and activity in both culture systems. Inhibition of ERK or AKT diminished CG-induced MMP-2 expression. In summary, the data demonstrate that CG promotes trophoblast invasion and migration through activation of ERK and AKT signaling involving their downstream effector MMP-2. Because the increase of CG during the first trimester of pregnancy correlates with rising trophoblast motility, the hormone could be a critical regulator of the early invasion process.
Human Chorionic Gonadotropin (CG), a member of the family of glycoprotein hormones, is composed of a specific β-subunit and α-subunit common to FSH, LH, and TSH (1). CG is indispensable for successful progression of pregnancy. By binding to the LH/CG receptor, the classical role of the hormone is to maintain production of steroid hormones in the corpus luteum until the seventh week of gestation before the luteoplacental shift in progesterone production takes place (2). However, throughout the years expression of G protein-coupled LH/CG receptor has been discovered in diverse nongonadal cell types such as placenta, uterus, breast, skin, and others, suggesting that hormone may govern a plethora of molecular processes associated with reproduction (3). Indeed, diverse effects of CG on fetal and maternal cells were detected including enhancement of trophoblast differentiation and decidualization, inhibition of myometrial contractility, and promotion of uterine angiogenesis (4–8). This has led to the idea that use of the hormone could be a therapeutic approach for treating different diseases associated with these tissues. Actually, beneficial effects of CG were observed when tested in patients with miscarriages, preterm labor, breast cancer, or endometriosis (3, 9–11), which could be partly explained by the anti-inflammatory actions of the hormone (12, 13).
CG expression is restricted to the villous epithelium of the placenta (14); its activation during trophoblast cell fusion involves cAMP-dependent transcription factors (15, 16). The hormone is thought to play a key role in the syncytialization process because growth factors, such as TGF-α, leukemia inhibitory factor, or epithelial growth factor (EGF), require LH/CG receptor to promote trophoblast differentiation (17). Maternal serum concentrations of CG increase rapidly after implantation, peak at 9–10 wk gestation and persist at low levels throughout the remainder of pregnancy (18). Interestingly, abundant CG levels parallel the invasive activity of trophoblasts, which is highest around 10–12 wk gestation. Migratory trophoblasts are known to produce LH/CG receptor and CG expression has also been recently detected in the invasive cell population (19, 20). Hence, besides paracrine effects on surrounding cell types of the placental bed, autocrine control of trophoblast invasion would be conceivable. However, the function of CG in this process has not been satisfactorily answered (21). Using an in vitro amnion invasion assay, first-trimester cytotrophoblasts were shown to invade less efficiently upon treatment with CG (22). In contrast, choriocarcinoma cells and HTR-8/SVneo cells showed increased invasiveness and migration in the presence of CG (23, 24). Although this could be a particular feature of tumorigenic/immortalized trophoblasts, the correlation of high CG serum levels and elevated invasion in ectopic pregnancies may support the assumption that the hormone promotes trophoblast invasion (25, 26). On the other hand, different CG preparations may contain EGF as a contaminant which in vitro considerably affects trophoblast function (27). In summary, the differences in trophoblast model systems and experimental settings so far did not allow to definitively delineate the role of CG in trophoblast invasion.
In this report, we used differentiating villous explant cultures as well as trophoblastic SGHPL-5 cells exhibiting features of extravillous trophoblasts to study the role of CG in trophoblast invasion and migration. The data suggest that EGF-free CG promotes these processes through activation of cascades, which are though to play a central role in invasive trophoblast differentiation, i.e. ERK and AKT signaling (28).
Materials and Methods
Collection of placental tissues
Placental tissues of early pregnancy (n = 17, between 7 and 10 wk) and late (n = 3 between 36 and 40 wk) were obtained from legal abortions and cesarean sections of uncomplicated pregnancies, respectively. Use of tissues was approved by the local ethical committee and required informed consent of patients.
Cell culture of primary cytotrophoblasts and fibroblasts
Cytotrophoblasts of term (n = 3) and first-trimester (n = 3) placentae were isolated by enzymatic dispersion, Percoll (5–70%) density gradient centrifugation, and immunopurification as described (15, 29, 30). Cell preparations were cultivated on gelatin-coated (1%) dishes and were routinely checked at the time of isolation (24 h) using immunofluorescence with cytokeratin 7 (clone OV-TL 12/30, 8.3 μg/ml; Dako, Glostrup, Denmark) and vimentin antibodies (clone Vim 3B4, 1.2 μg/ml; Dako) to detect trophoblasts (>99%) and stromal cells (<1%), respectively. First-trimester villous fibroblast (n = 3) were isolated from gradients of trypsinized placental material (between 25 and 35% Percoll) and passaged two times in DMEM supplemented with 10% fetal calf serum (FCS). Villous fibroblasts were characterized by vimentin immunocytochemistry (100%), and a contamination with trophoblasts was excluded by cytokeratin 7 staining. Decidual fibroblasts (n = 3) were isolated from decidual tissue attached to first-trimester placentae using enzymatic digestion with 2 mg/ml collagenase I (484 IU/ml; Life Technologies, Inc., Paisley, UK) and 0.5 mg/ml DNase I (Sigma, St. Louis, MO) as described (31). Isolated cells were seeded in DMEM/F-12 supplemented with 10% FCS (Biochrom AG, Berlin, Germany). Fibroblasts were characterized after first passage by immunofluorescence and were positive for vimentin (100%) and pan-keratin (1%) but negative for CD45 and CD56.
First-trimester villous explant culture
Small pieces (2 × 2 mm) of villous tissue of different first-trimester placentae (n = 14) were dissected under the microscope and cultivated on collagen I-coated dishes in serum-free medium (DMEM/Ham’s F12 with 50 μg/ml gentamicin) as described elsewhere (32, 33). For analyses of LH/CG receptor expression, pure extravillous trophoblasts, which had migrated from anchoring sites, were mechanically separated from villous material after 72 h and RNA/protein were extracted as previously mentioned (34, 35).
Stimulation of pure extravillous trophoblast (EVT)
Approximately 50 dissected villi per placenta were attached on plastics coated with a thin layer of collagen I using coverslips. After formation of EVTs (72 h), villi were mechanically removed from the dishes using tweezers as mentioned (34, 35). The residual migratory EVT (100% cytokeratin 7 positive) were incubated with serum-free medium the absence or presence of hormone. For analyses of ERK and AKT phosphorylation, pure EVT of each three different placentae were incubated with 50 IU/ml CG for 10 and 15 min. Pure, urinary CG (C-0434; Sigma) used in this study was previously shown to lack contaminating EGF (27). Subsequently each EVT pool was lysed to obtain cellular protein extracts. For detection of secreted amounts of matrix metalloproteinase (MMP)-2 and MMP-9, two different pure EVT pools were treated with 5 and 50 IU/ml CG for 48 h. After concentration (see below) supernatants were snap frozen and stored at −80 C for further analyses.
Cultivation of SGHPL-5 cells
Cytotrophoblastic SGHPL-5 cells exhibiting features of invasive trophoblasts cells, such as human leukocyte antigen-G and cytokeratin 7 expression, were cultivated in a 1:1 mixture of DMEM/Ham’s F-12 supplemented with 10% FCS (Gibco BRL Life Technologies, Paisley, UK) as described (36, 37).
Invasion and migration assays
To analyze effects of CG on trophoblast migration, serum-free explant cultures were seeded for 4 h on collagen I, allowing for anchorage, and then stimulated with 5 IU or 50 IU/ml CG. Eight anchoring villi were analyzed per condition and experiments were repeated with three different placentae. After 48 h explant cultures were digitally photographed. For quantification the area of outgrowth was measured using the imaging software CellP̂ (Olympus, Hamburg, Germany). To analyze migration and invasion of SGHPL-5, cells were seeded on either uncoated transwells or growth factor-reduced Matrigel invasion chambers (BD Biosciences, Bedford, MA; 50,000 cells/chamber). After attachment cells were starved for 6 h and subsequently incubated with either 5 IU or 50 IU/ml CG. After 24 h noninvaded cells on the upper side of the inserts were removed by a cotton swap. Cells on the lower surface were fixed in ice-cold methanol and stained with Papanicolaou’s solution 1a Harris’ hematoxylin solution (Merck, Darmstadt, Germany). Filters were excised and mounted in Aquatex (Merck). For evaluation cells were counted using Olympus cell imaging software. In blocking studies organ cultures or SGHPL-5 cells were preincubated for 1 h with 10 μm UO126 [MAPK kinase (MEK) inhibitor] or 10 μm LY294002 [phosphatidylinositide 3-kinase (PI3K) inhibitor] before supplementation of CG.
Proliferation assays
For detection of proliferation in first-trimester villous explant cultures, 5-bromo-2′-deoxyuridine (BrdU) labeling and detection kit I (Roche, Mannheim, Germany) was used as previously mentioned (34, 35). Briefly, serum-free floating villous explants were treated with 10 μm BrdU and incubated for 24 h in the absence or presence of CG. Subsequently tissues were snap frozen in liquid nitrogen, and eight serial sections per explant were cut and fixed in ethanol-Fixans (15 mm glycine per 70% ethanol, 20 min, −20 C). BrdU was detected with primary monoclonal anti-BrdU and secondary antimouse Ig fluorescein antibodies. Sections were counterstained with 4′,6′-diamino-2-phenylindole (DAPI) and cytokeratin 7 and digitally photographed. The ratio (percentage) of BrdU/4′,6′-diamino-2-phenylindole labeling was counted in cytotrophoblasts of cell columns using Olympus CellP̂ imaging software. Seventeen untreated explants (36207 nuclei), 18 explants stimulated with 5 IU/ml CG (39803 nuclei), and 17 explants incubated with 50 IU/ml CG (36446 nuclei) of three different placentae were analyzed. For evaluation of SGHPL-5 cell proliferation, serum-free cultures were seeded on plastics (1000 cells/cm2) and stimulated with 5 IU or 50 IU/ml CG for 24, 48, and 72 h. Cumulative cell numbers were counted using a multichannel electronic cell counter (CASY-I; Schärfe Systems, Reutlingen, Germany).
RNA extraction and semiquantitative RT-PCR
Tissues of total placenta and explant cultures were frozen in liquid nitrogen and homogenized with a microdismembrator (Braun; Biotech International, Melsungen, Germany). Total RNA of primary cultures, cell lines, and tissues was isolated using Tri-reagent (Molecular Research Center Inc., Cincinnati, OH) as previously mentioned (35). Quality and quantity of the extracted RNA was analyzed with the Bioanalyzer 2100 (Agilent, Palo Alto, CA). Reverse transcription and PCR were done as previously mentioned (38). Cycle numbers were optimized within the linear range of individual PCRs. Oligonucleotide primers, annealing temperatures, product sizes, and cycle numbers were as follows: LH/CG-R receptor-s, 5′-ACCTTGAAGTTGTCCACAT TGC-3′, LH/CG receptor-a, 5′-ACCGAATTG AACAGTGCA TCTC-3′ (57 C, 508 bp, 40 cycles), β-actin-s, 5′-GACAGCAGTCGG TTGGAG C-3′, β-actin-a, 5′-CAGGTAAGCCCT GGCTGC-3′ (55 C, 398 bp, 22 cycles). In all experiments, a possible DNA contamination was checked by negative control RT-PCR in which reverse transcriptase was omitted in the reverse transcription step. The PCR products were analyzed on 1.5% agarose gels containing ethidium bromide and photographed under UV radiation. PCR fragments were sequence verified on a 16-capillary sequencer by using the nonradioactive ABI PRISM terminator cycle sequencing ready reaction kit as specified by the supplier (Applied Biosystems, Foster City, CA).
Western blot analyses
Western blot analyses were performed using standard protocols as recently done (35, 38). For MMP analyses, 1 ml of supernatant of either SGHPL-5 cells or pure EVT pools, which had been stimulated for 48 h with CG, was concentrated 25-fold (to ~200 μg/ml) using Ultrafree-MC filter tubes (Millipore, Billerica, MA). Equal amounts of protein lysate (10 μg) were separated on 10% sodium dodecyl sulfate/polyacrylamide gels and transferred onto polyvinyl difluoride membranes (Hybond-P; GE Healthcare, Buckinghamshire, UK). After blocking filters were incubated overnight (4 C) with polyclonal rabbit antibodies against human LH/CG receptor [1:5000 (39)], p44/42 MAPK (1:1000, Cell Signaling, Beverly, MA), phospho(Thr202/ Tyr204)-p44/42 MAPK (1:1000; Cell Signaling), AKT (1:1000; Cell Signaling), phospho-AKT (Ser473, 1:1000; Cell Signaling, Danvers, MA), MMP-2 (1:1000; Cell Signaling), or MMP-9 (1:1000; Cell Signaling). After 1 h of treatment (room temperature) with secondary antibodies (antimouse or antirabbit Ig horseradish peroxidase linked; Amersham; 1:50,000) signals were developed by using ECL Plus Western blotting detection system (GE Healthcare). To analyze protein loading, filters were stripped as previously mentioned (35) and incubated with actin antibodies (mouse monoclonal, 1:5000; Sigma). PageRuler prestained protein ladder (Fermentas, St. Leon-Rot, Germany) was used as a molecular size marker.
Gelatin zymography
Gelatinase (MMP-2, MMP-9) secretion was evaluated in 50-fold concentrated supernatants of SGHPL-5 cells or pooled villous explant cultures (eight explants per stimulation, three different placentae) using substrate gel zymography as recently mentioned (34). Briefly, conditioned medium containing 500 ng of protein was mixed with the same volume of 2× sample buffer [0.125 m Tris-HCl (pH 6.8), 20% glycerol, 4% sodium dodecyl sulfate, 0.005% bromophenol blue], incubated for 10 min at room temperature, and loaded onto nonreducing 10% polyacrylamide gels containing 0.1% gelatin (Sigma). Proteins were separated by electrophoresis at a constant voltage of 125 V/gel for 90 min as described by the manufacturer. Gels were washed with Novex renaturing buffer (Invitrogen, Carlsbad, CA) with gentle agitation for 30 min and incubated overnight at 37 C with Novex developing buffer. Gels were stained with 0.5% Coomassie brilliant blue R-250 and destained overnight in 10% acetic acid per 30% methanol.
Statistical analyses
Statistical analyses were performed with Student’s t test or ANOVA using SPSS 14 (SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
Expression analyses of LH/CG receptor in different trophoblast model systems
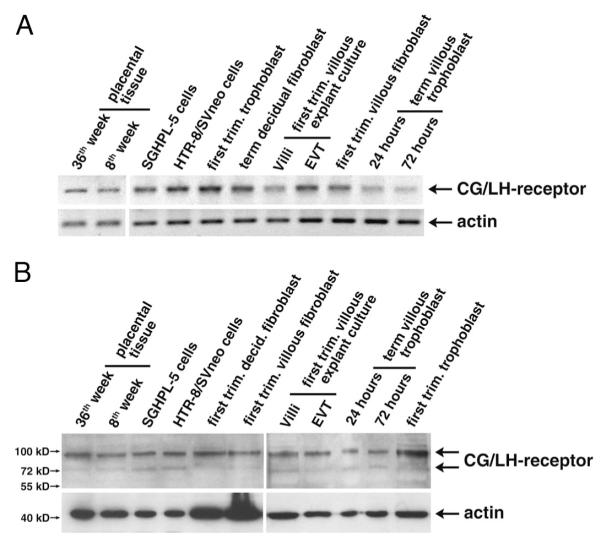
To test the suitability of cell systems for analyses of CG-dependent effects, LH/CG receptor expression was investigated in different trophoblast cultures (Fig. 1). Semiquantitative RT-PCR analyses suggested receptor expression in placental tissues, primary villous and extravillous trophoblasts, and villous and decidual fibroblasts as well as trophoblast cell lines (Fig. 1A). Accordingly, in Western blot analyses, specific signals at 90 and 75 kDa, corresponding to the mature and precursor forms of the LG/CG receptor (40), were detected in the different cultures systems (Fig. 1B).
Fig. 1.
Expression analyses of LH/CG receptor in placental tissues and different trophoblast model systems. RNA and protein lysates were prepared from placental tissues, first-trimester villous explant cultures, primary trophoblasts and fibroblasts, and trophoblastic cell lines. RT-PCR and Western blot analyses were done as described in Materials and Methods. Representative examples are shown. A, Semiquantitative RT-PCR analyses detecting a 508-bp LH/CG receptor fragment. β-Actin (398 bp) was used as a loading control. B, Western blot analyses using specific antibodies against LH/CG receptor. Actin was used as loading control. Specific signals are indicated by arrows; marker bands are depicted.
CG promotes trophoblast invasion and migration
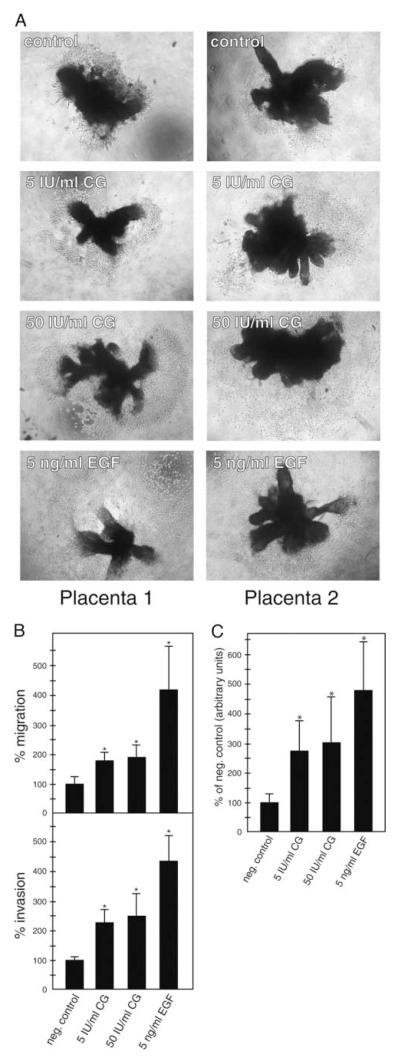
Subsequently the influence of CG on trophoblast cell migration and invasion was tested in a dose-dependent manner (Fig. 2). Compared with controls (100%), 50 IU/ml of the hormone increased migration and invasion of trophoblastic SGHPL-5 cells to 192 and 247%, respectively (Fig. 2A). EGF (5 ng/ml), a well-known stimulator of trophoblast motility (27, 41, 42), increased migration and invasion to 415 and 444% each. Similarly, 5 and 50 IU/ml of CG stimulated trophoblast outgrowth and migration in first-trimester villous explant cultures seeded on collagen I (Fig. 2B). Quantification revealed that the area of outgrowth increased to 271, 301, and 480% in the presence of 5 IU/ml CG, 50 IU/ml CG, and 5 ng/ml EGF, respectively (Fig. 2C).
Fig. 2.
CG increases migration and invasion in different trophoblast model systems. A, Migration and invasion of SGHPL-5 cells through uncoated and Matrigel-coated transwells, respectively. Assays and stimulation with CG was performed as described in Materials and Methods. EGF was used as a positive control. Bars represent mean values of three (migration) and four (invasion) different experiments performed in duplicates; error bars indicate SD. Mean value of untreated cultures (negative control) was arbitrarily set to 100%. *, P < 0.05. B, CG-induced migration in first-trimester villous explant cultures cultivated on collagen I. Preparation of organ cultures and CG treatment was done as mentioned above. Representative examples of explants isolated from two different placentae were chosen and digitally photographed (10-fold magnification). C, Quantification of the area of outgrowth in CG-treated explant cultures. Mean values ± SD of 24 explants per condition derived from three different placentae are depicted. *, P < 0.05.
CG does not affect trophoblast proliferation
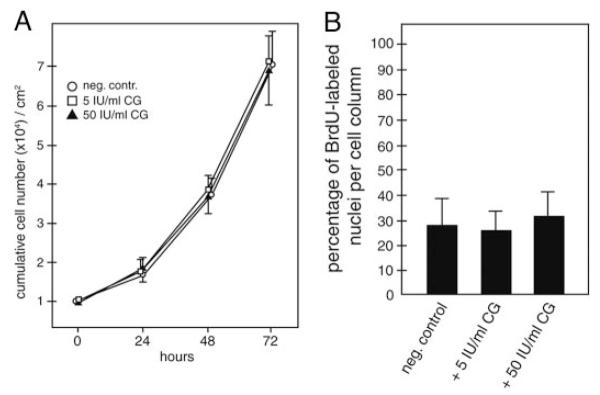
To analyze whether CG may influence SGHPL-5 cell proliferation, cumulative cell numbers were determined in the absence or presence of CG (Fig. 3A). The hormone did not significantly change proliferation at 24, 48, or 72 h of incubation. Similarly, CG-dependent proliferation was investigated in villous explant cultures using BrdU labeling (Fig. 3B). No significant changes in numbers of BrdU-labeled nuclei of cell columns could be observed at 24 h of stimulation using 5 or 50 IU/ml CG.
Fig. 3.
Proliferation of SGHPL-5 cells and villous explant cultures in the absence or presence of CG. A, Determination of cumulative cell numbers. SGHPL-5 cells (n = 3) were cultivated up to 72 h with different doses of CG and counted as mentioned in Materials and Methods. Mean values of three different experiments performed in duplicates are shown; error bars depict SD. *, Significant change (P < 0.05) at 72 h. B, Proliferation in villous explant cultures. BrdU labeling, CG stimulation, and determination of number of BrdU-labeled nuclei in cell columns were done as described above. Mean values (percentage of BrdU labeled nuclei) ± SD of three different placentae are depicted. *, P < 0.05.
CG stimulates activation of ERK and AKT in SGHPL-5 cells
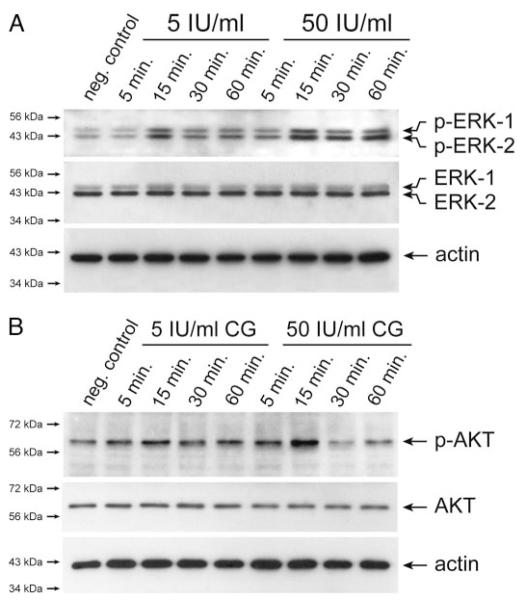
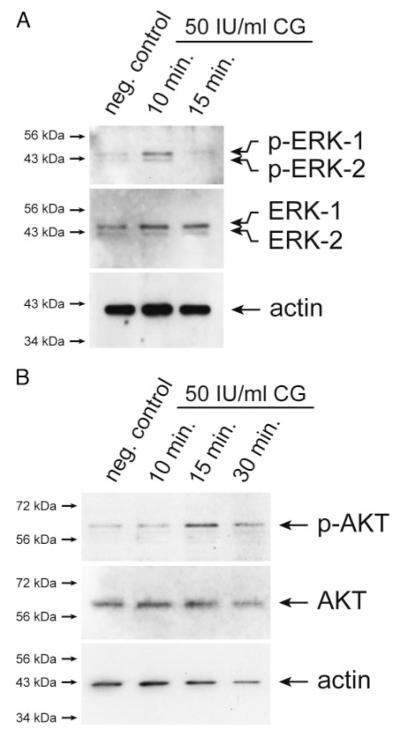
To gain insights into the molecular mechanisms of CG-induced trophoblast migration and invasion, activation of critical signal transduction pathways was investigated in SGHPL-5 cells (Fig. 4). Western blot analyses revealed that CG dose-dependently induced phosphorylation of ERK with peak levels at 15 min of treatment (Fig. 4A). Densitometrical scanning of Western blots (n = 3) revealed that phosphorylation of both ERK-1 and -2 increased to 260% ± 49 sd at 15 min of incubation with 50 IU/ml CG. (controls set at 100%). Similarly, strongest CG-induced phosphorylation of AKT could be observed at 15 min of stimulation with either 5 or 50 IU/ml of hormone (Fig. 4B). At the latter condition, signals of phospho-AKT increased to 228% ± 13 sd.
Fig. 4.
Western blot analyses showing CG-dependent phosphorylation of ERK and AKT in SGHPL-5 cells. Serum-free cultures were treated with either 5 or 50 IU/ml CG for indicated time periods. Preparation of protein lysates and Western blot analyses were performed as described in Materials and Methods. Marker bands (kilodaltons) are depicted on the left side. Actin (42 kDa) was used as a loading control. Representative examples of n = 3 are shown. A, CG-stimulated activation of ERK. Specific signals of phosphorylated ERK-1 (44 kDa) and ERK-2 (42 kDa) and total ERK-1/2 are indicated by arrows. B, Phosphorylation of AKT after CG treatment. Arrows indicate specific signals of phosphorylated and total AKT protein (60 kDa), respectively.
CG induces phosphorylation of ERK and AKT in pure, EVTs
To study whether CG had similar effects on invasive primary trophoblasts, activation of ERK and AKT was studied in pure EVTs isolated from villous explant cultures (Fig. 5). Western blot analyses suggested that stimulation with 50 IU/ml CG increased phosphorylation of both ERK (Fig. 5A) and AKT (Fig. 5B).
Fig. 5.
Western blot analyses demonstrating CG-stimulated phosphorylation of ERK and AKT in pure, EVTs. Preparation of EVT, CG stimulation, and detection of ERK and AKT phosphorylation were performed as described in Materials and Methods. Marker bands (kilodaltons) are depicted on the left side. Actin (42 kDa) was used to evaluate protein loading. Representative examples are shown. A, Phosphorylation of ERK. Specific bands of p-ERK-1/2 and total ERK-1/2 are indicated. B, Activation of AKT. Arrows specify signals of phosphorylated and total AKT protein.
Inhibition of ERK and AKT reduces phosphorylation and CG-induced SGHPL-5 cell invasion
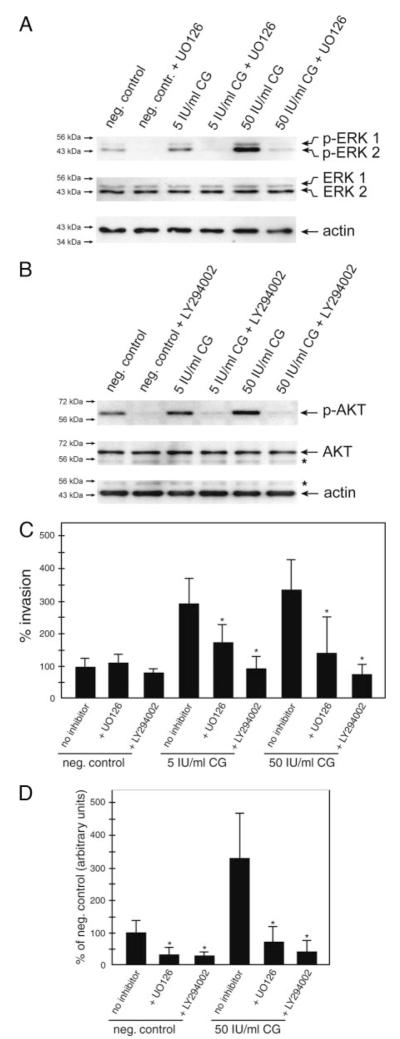
Western blot analyses were performed to study the effects of either ERK or AKT inhibition in SGHPL-5 cells. Treatment of cultures with the specific MEK inhibitor UO126 reduced basal as well as CG-stimulated ERK phosphorylation (Fig. 6A). Similarly, basal and CG-induced AKT phosphorylation was diminished in the presence of the PI3K inhibitor LY294002 (Fig 6B). To evaluate whether CG-induced activation of ERK and AKT might play a role in CG-mediated trophoblast invasion, Matrigel transwell assays in the presence of the inhibitors were performed (Fig. 6C). Compared with controls, basal migration was not significantly affected upon preincubation with LY294002 or UO126. However, compared with CG-stimulated motility at either 5 or 50 IU/ml, invasion was significantly reduced in the presence of either UO126 or LY294002.
Fig. 6.
Inhibition of ERK and AKT diminishes phosphorylation and CG-dependent cell motility. Cultures pretreated with either UO126 or LY294002 were stimulated for 15 min (Western blotting), 24 h (SGHPL-5 cell invasion assay), or 48 (outgrowth from explant cultures) with 5 or 50 IU/ml CG. Western blot analyses (n = 3) and invasion assays were done as described above. A, Western blot analyses showing UO126-mediated inhibition of ERK. Specific signals are indicated by arrows. B, Western blot analyses demonstrate LY294002-mediated inhibition of AKT. Specific signals are indicated by arrows, asterisk marks unspecific bands. C, Invasion assays. Bars represent mean values of three different experiments performed in duplicates; error bars indicate SD. D, Mean value of untreated cultures (negative control) was arbitrarily set to 100%. D, Quantification of the area of outgrowth in CG/inhibitor-treated explant cultures. Mean values ± SD of at least 21 explants per condition derived from three different placentae are depicted. *, P < 0.05.
Inhibition of ERK and AKT reduces CG-dependent outgrowth of villous explant cultures
Similar to their effects on SGHPL-5 cells, pretreatment of villous organ cultures with UO126 or LY294002 significantly diminished the area of CG-stimulated outgrowth (Fig. 6D).
CG stimulates MMP-2 activity
Gelatin zymography revealed that CG stimulated accumulation of the pro-form (72 kDa) of MMP-2 in SGHPL-5 cell supernatants (Fig. 7A). Pro-MMP-9 was hardly detectable in the concentrated supernatants, and its expression was only slightly induced in the presence of the hormone. Similarly, CG induced secretion of 72 and 62 kDa (active) MMP-2 in first-trimester villous explant culture (Fig. 7B), whereas pro-MMP-9 was less stimulated.
Fig. 7.
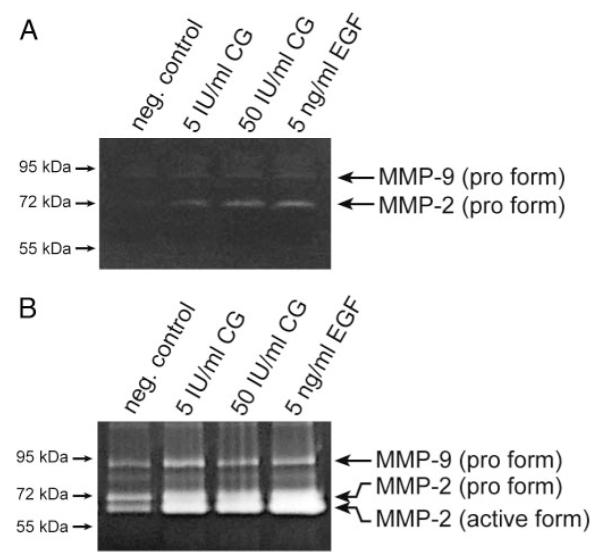
Gelatin zymography showing CG-dependent MMP-2 and MMP-9 activity in culture supernatants. Activity of gelatinases in concentrated supernatants after 48 h of CG stimulation was determined as described in Materials and Methods. Marker bands (kilodaltons) are depicted on the left side. Representative examples are shown. A, SGHPL-5 cells. B, First-trimester villous explant cultures.
CG-dependent MMP-2 expression involves ERK and AKT signaling
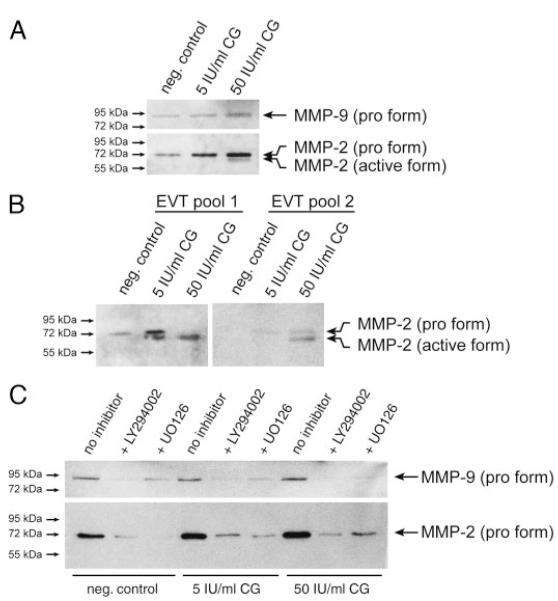
CG-stimulated secretion of the 72-kDa pro-MMP-2 was also noticed in Western blot analyses of SGHPL-5 supernatants (Fig. 8A). Production of active MMP-2 could be observed in the presence of 50 IU/ml CG. However, this was not consistently observed in every experiment and required long exposure of films. Densitometrical scanning (n = 3) revealed that compared with controls (100%), CG increased pro-MMP-2 to 166% ± 36 sd and 266% ± 45 sd at 5 IU and 50 IU/ml CG, respectively. Low levels of pro-MMP-9 were only significantly stimulated at 50 IU/ml (164 ± 8%). Accordingly, CG treatment of two different pools of pure EVT promoted expression of soluble, pro- and active MMP-2 (Fig. 8B). However, MMP-9 was undetectable in these highly concentrated supernatants (not shown). Incubation of SGHPL-5 cells with either UO126 or LY294002 reduced basal as well as CG-stimulated MMP-2 and MMP-9 expression (Fig. 8C). Compared with controls (100%), MMP-2 stimulated by 50 IU/ml CG (255% ± 50 sd) decreased to 51% ± 25 sd and 35 ± 17 sd in the presence of UO126 and LY294002, respectively. Similarly, MMP-9 expression at 50 IU/ml CG (170% ± 28 sd) decreased to 29% ± 14 sd and 18% ± 11 sd upon incubation with UO126 and LY294002, respectively.
Fig. 8.
Western blot analyses of MMP-2 and MMP-9 protein expression after CG stimulation. Concentration of cell supernatants and Western blot analyses using specific MMP-2 and MMP-9 antibodies were done as described in Materials and Methods. Marker bands (kilodaltons) are depicted on the left side. Representative examples of three (A and C) and two (B) different experiments are shown. A, Secretion of gelatinases from SGHPL-5 cells. B, Immunodetection of soluble MMP-2 in different EVT pools. C, MMP secretion of SGHPL-5 cells in the presence of MEK/ERK or PI3K/AKT inhibitors. After 1 h of preincubation, cells were incubated for 48 h with CG.
Discussion
Expression of the LH/CG receptor in diverse trophoblast and fibroblast subtypes of the fetal-maternal interface supports the assumption that CG has a pivotal role in implantation, decidualization, and placental differentiation. Here we confirm previous results demonstrating LH/CG receptor expression in villous and invasive trophoblasts as well as in fibroblasts of the placental bed (20, 40, 43). Whereas the multiple functions of CG on diverse target cells of reproductive tissues are still being elucidated, we attempted to shed light on its role in trophoblast invasion. Besides using SGHPL-5 trophoblast cells, retaining features of EVTs (36, 37), first-trimester villous explant cultures and pure EVT were used to analyze CG-dependent effects on proliferation, motility, signaling, and MMP expression.
CG-dependent Matrigel invasion assays of SGHPL-5 cells as well as evaluation of trophoblast migration in villous explants cultures suggest that the hormone indeed promotes trophoblast motility. Therefore, data obtained in primary trophoblasts and nonimmortalized SGHPL-5 cells are in accordance with previous results demonstrating that CG increased invasion/migration of immortalized and tumorigenic trophoblasts (23, 24). With respect to proliferation, CG may negatively affect growth (44) but was also shown to provoke a mitogenic response in various reproductive cell types (45–47). However, in SGHPL-5 cells, CG did not affect proliferation up to 72 h of stimulation, suggesting that the increase in invasion cannot be explained by an elevated mitogenic response. Similarly, CG did not significantly alter BrdU labeling in cell column trophoblasts of first-trimester explant cultures, indicating that CG-dependent outgrowth could be mainly due to elevated cell motility. Hence, abundant levels of the hormone during early pregnancy may predominantly stimulate trophoblast invasion but not affect placental growth.
Different forms of CG are present in urinary preparations such as intact, cleaved, nicked, and glycosylated proteins (48). Whether these various hormones may have distinct effects on proliferation or invasion still has to be determined. Along those lines, it was recently suggested that supernatants of primary EVT may promote invasion through its high proportion of hyperglycosylated CG (49). Interestingly, CG of villous trophoblast supernatants was inefficient in promoting motility, compared with the hormone of EVT supernatants (49). This may suggest that the motility-promoting effects of the urinary hormone used in our study could be mainly due to its portion of hyperglycosylated CG.
Signaling pathways involved in CG-induced trophoblast invasion remain largely elusive. In HTR-8/SVneo cells, CG-stimulated trophoblast migration may involve the IGF-II system. The hormone was suggested to increase the externalization rate of IGF-II/mannose 6-phosphate receptor and hence bioavailability of IGF-II (24). Here we demonstrate that activation of ERK and AKT signaling are crucially involved in CG-dependent trophoblast motility. Indeed, Raf/MEK/ERK and PI3K/AKT signaling are thought to play key roles in the invasive differentiation process because abundant growth factors of the fetal-maternal interface, such as EGF or IGF-II, stimulate trophoblast migration through activation of both pathways (28). CG activated ERK and AKT in not only SGHPL-5 cells but also pure, nonproliferating EVT, suggesting a direct role of the signaling pathways in the regulation of invasive trophoblasts. Although ERK and/or AKT signaling are known to mediate a mitogenic response upon activation of G protein-coupled receptors (50), we failed to detect significant CG-dependent changes in proliferation. However, inhibition of the kinases reduced CG-stimulated SGHPL-5 cell invasion as well as trophoblast motility in explant cultures. Interestingly, inhibition also affected basal migration in villous explant cultures but not SGHPL-5 cells. This could suggest that the organ cultures/EVT produce higher levels of ERK/AKT-activating growth factors acting in a paracrine or autocrine manner.
CG-induced signaling through these pathways may have multiple effects on migratory trophoblasts such as changes in the cytoskeleton, expression of invasion-promoting growth factors, or secretion of matrix-digesting proteinases. Among the latter, expression of the gelatinases, MMP-2 and MMP-9, has been closely linked to trophoblast invasiveness because various promigratory growth factors were shown to activate the particular enzymes (51). MMP-2 is thought to be the predominant enzyme in early placentae, but its expression decreases after 11 wk gestation. In contrast, MMP-9 is undetectable at 6 wk of pregnancy but gradually increases after wk 7. Accordingly, blocking of MMP-9 did not affect trophoblast invasion before the ninth week of pregnancy (52). Compared with MMP-2, gelatin zymography and Western blot analyses detected only low levels of the proform of MMP-9 in concentrated supernatants of SGHPL-5 cells or first-trimester villous explant cultures. This could be due to the fact that the majority of first-trimester placentae were usually obtained between 7 and 9 wk gestation. Interestingly, MMP-9 was absent from two different pure EVT pools of 8 and 10 wk of pregnancy despite its weak expression in explant cultures. This might be explained by the fact that during early pregnancy MMP-2 is predominantly expressed in EVT, whereas MMP-9 is mainly secreted from villous trophoblasts (53), the latter being present in the whole organ cultures.
Cell models used in this study mainly mimic interstitial trophoblast invasion of early pregnancy. In these cultures low levels of pro-MMP-9 were only marginally stimulated by CG, compared with MMP-2, suggesting that MMP-9 may play only an ancillary role in early, hormone-dependent trophoblast invasion. However, it is possible that the effects of CG on MMP-9 secretion become more important when expression of the protease increases at later stages of pregnancy. Interestingly, CG induced not only total MMP-2 expression but also production of its active 62-kDa form in the different trophoblast model systems. Hence, it is possible that the hormone also activates MMP-2-processing enzymes such as MT1- or MT2-MMP, which had been detected in first-trimester placentae (54, 55).
Both AKT and ERK signaling are involved in CG-dependent regulation of MMP expression because inhibition of either kinase reduced hormone-stimulated MMP-2 or MMP-9 production. Thus, CG acts similar to other promigratory growth factors such as EGF increasing MMP expression through activation of both ERK and AKT signaling (56). Besides direct effects of the hormone on protease expression, CG may also indirectly affect trophoblast invasiveness by stimulating other inducers of gelatinases such as vascular EGF (vEGF) (57). However, CG could also play a more central role in trophoblast invasion. Growth factors such as leptin may promote MMP expression partly through stimulation of CG secretion (58).
In conclusion, the data suggest that CG increases trophoblast invasion and migration through activation of ERK and AKT signaling. CG-stimulated production of active MMP-2, particularly in nonproliferating EVT, supports the assumption that the gelatinase might be crucially involved in hormone-dependent trophoblast invasion. Further studies are needed to gain more insights into the diverse effects of the pregnancy hormone on invasive trophoblasts.
Acknowledgments
We are grateful to G. Whitley and A. Fazleabas for providing SGHPL-5 cells and LH/CG receptor antibody, respectively. We thank G. Puller for preparation of graphics.
This work was supported by Grant 10719 of the “Jubilaümsfond” of the Austrian National Bank. S.S. is supported by Grant P-17894-B14 from the Fonds zur Förderung der wissenschaftlichen Forschung, Austria.
Abbreviations
- BrdU
5-Bromo-2′-deoxyuridine
- CG
chorionic gonadotropin
- EGF
epidermal growth factor
- EVT
extravillous trophoblast
- FCS
fetal calf serum
- MEK
MAPK kinase
- MMP
matrix metalloproteinase
- PI3K
phosphatidylinositide 3-kinase
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 2.Tulchinsky D, Hobel CJ. Plasma human chorionic gonadotropin, estrone, estradiol, estriol, progesterone, and 17α-hydroxyprogesterone in human pregnancy. 3. Early normal pregnancy. Am J Obstet Gynecol. 1973;117:884–893. doi: 10.1016/0002-9378(73)90057-4. [DOI] [PubMed] [Google Scholar]
- 3.Rao CV, Lei ZM. The past, present and future of nongonadal LH/hCG actions in reproductive biology and medicine. Mol Cell Endocrinol. 2007;269:2–8. doi: 10.1016/j.mce.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132:1387–1395. doi: 10.1210/endo.132.3.7679981. [DOI] [PubMed] [Google Scholar]
- 5.Han SW, Lei ZM, Rao CV. Treatment of human endometrial stromal cells with chorionic gonadotropin promotes their morphological and functional differentiation into decidua. Mol Cell Endocrinol. 1999;147:7–16. doi: 10.1016/s0303-7207(98)00240-8. [DOI] [PubMed] [Google Scholar]
- 6.Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. In vivo infusion of interleukin-1β and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology. 2005;146:4097–4104. doi: 10.1210/en.2005-0380. [DOI] [PubMed] [Google Scholar]
- 7.Ticconi C, Zicari A, Belmonte A, Realacci M, Rao CV, Piccione E. Pregnancy-promoting actions of HCG in human myometrium and fetal membranes. Placenta. 2007;28(Suppl A):S137–S143. doi: 10.1016/j.placenta.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
- 9.Toth P, Lukacs H, Gimes G, Sebestyen A, Pasztor N, Paulin F, Rao CV. Clinical importance of vascular LH/hCG receptors—a review. Reprod Biol. 2001;1:5–11. [PubMed] [Google Scholar]
- 10.Than NG, Itakura A, Rao Ch V, Nohira T, Toth P, Mansell JP, Isaka K, Nishi H, Takayama M, Than GN. Clinical applications of pregnancy-related proteins—a workshop report. Placenta. 2003;24(Suppl A):S60–S64. doi: 10.1053/plac.2002.0947. [DOI] [PubMed] [Google Scholar]
- 11.Huber AV, Huber JC, Kolbus A, Imhof M, Nagele F, Loizou D, Kaufmann U, Singer CF. Systemic HCG treatment in patients with endometriosis: a new perspective for a painful disease. Wien Klin Wochenschr. 2004;116:839–843. doi: 10.1007/s00508-004-0296-5. [DOI] [PubMed] [Google Scholar]
- 12.Huber AV, Saleh L, Prast J, Haslinger P, Knöfler M. Human chorionic gonadotropin attenuates NF-κB activation and cytokine expression of endometriotic stromal cells. Mol Hum Reprod. 2007;13:595–604. doi: 10.1093/molehr/gam032. [DOI] [PubMed] [Google Scholar]
- 13.Rao CV, Li X, Manna SK, Lei ZM, Aggarwal BB. Human chorionic gonadotropin decreases proliferation and invasion of breast cancer MCF-7 cells by inhibiting NF-κB and AP-1 activation. J Biol Chem. 2004;279:25503–25510. doi: 10.1074/jbc.M400683200. [DOI] [PubMed] [Google Scholar]
- 14.Ringler GE, Strauss JF., 3rd In vitro systems for the study of human placental endocrine function. Endocr Rev. 1990;11:105–123. doi: 10.1210/edrv-11-1-105. [DOI] [PubMed] [Google Scholar]
- 15.Knöfler M, Saleh L, Bauer S, Galos B, Rotheneder H, Husslein P, Helmer H. Transcriptional regulation of the human chorionic gonadotropin β gene during villous trophoblast differentiation. Endocrinology. 2004;145:1685–1694. doi: 10.1210/en.2003-0954. [DOI] [PubMed] [Google Scholar]
- 16.Knöfler M, Saleh L, Bauer S, Vasicek R, Griesinger G, Strohmer H, Helmer H, Husslein P. Promoter elements and transcription factors involved in differentiation-dependent human chorionic gonadotropin-α messenger ribonucleic acid expression of term villous trophoblasts. Endocrinology. 2000;141:3737–3748. doi: 10.1210/endo.141.10.7713. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Lei ZM, Rao CV. The central role of human chorionic gonadotropin in the formation of human placental syncytium. Endocrinology. 2003;144:1108–1120. doi: 10.1210/en.2002-220922. [DOI] [PubMed] [Google Scholar]
- 18.Braunstein GD, Rasor J, Danzer H, Adler D, Wade ME. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am J Obstet Gynecol. 1976;126:678–681. doi: 10.1016/0002-9378(76)90518-4. [DOI] [PubMed] [Google Scholar]
- 19.Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Human chorionic gonadotropin expression in human trophoblasts from early placenta: comparative study between villous and extravillous trophoblastic cells. Placenta. 2007;28:175–184. doi: 10.1016/j.placenta.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Tao YX, Lei ZM, Hofmann GE, Rao CV. Human intermediate trophoblasts express chorionic gonadotropin/luteinizing hormone receptor gene. Biol Reprod. 1995;53:899–904. doi: 10.1095/biolreprod53.4.899. [DOI] [PubMed] [Google Scholar]
- 21.Islami D, Mock P, Bischof P. Effects of human chorionic gonadotropin on trophoblast invasion. Semin Reprod Med. 2001;19:49–53. doi: 10.1055/s-2001-13910. [DOI] [PubMed] [Google Scholar]
- 22.Yagel S, Geva TE, Solomon H, Shimonovitz S, Reich R, Finci-Yeheskel Z, Mayer M, Milwidsky A. High levels of human chorionic gonadotropin retard first trimester trophoblast invasion in vitro by decreasing urokinase plasminogen activator and collagenase activities. J Clin Endocrinol Metab. 1993;77:1506–1511. doi: 10.1210/jcem.77.6.8263134. [DOI] [PubMed] [Google Scholar]
- 23.Lei ZM, Taylor DD, Gercel-Taylor C, Rao CV. Human chorionic gonadotropin promotes tumorigenesis of choriocarcinoma JAR cells. Trophoblast Research. 1999;13:147–159. [Google Scholar]
- 24.Zygmunt M, McKinnon T, Herr F, Lala PK, Han VK. HCG increases trophoblast migration in vitro via the insulin-like growth factor-II/mannose-6 phosphate receptor. Mol Hum Reprod. 2005;11:261–267. doi: 10.1093/molehr/gah160. [DOI] [PubMed] [Google Scholar]
- 25.Klein M, Graf A, Kiss H, Czerwenka K, Beck A, Egarter C, Husslein P. The relation between depth of trophoblastic invasion and β-HCG levels in tubal pregnancies. Arch Gynecol Obstet. 1995;256:85–88. doi: 10.1007/BF00634713. [DOI] [PubMed] [Google Scholar]
- 26.Oktay K, Brzyski RG, Miller EB, Krugman D. Association of serum β-hCG levels with myosalpingeal invasion and viable trophoblast mass in tubal pregnancy. Obstet Gynecol. 1994;84:803–806. [PubMed] [Google Scholar]
- 27.Saleh L, Prast J, Haslinger P, Husslein P, Helmer H, Knöfler M. Effects of different human chorionic gonadotropin preparations on trophoblast differentiation. Placenta. 2007;28:199–203. doi: 10.1016/j.placenta.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Pollheimer J, Knöfler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005;26(Suppl A):S21–S30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Fisher SJ, Cui TY, Zhang L, Hartman L, Grahl K, Zhang GY, Tarpey J, Damsky CH. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989;109:891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 31.Selam B, Kayisli UA, Garcia-Velasco JA, Arici A. Extracellular matrix-dependent regulation of Fas ligand expression in human endometrial stromal cells. Biol Reprod. 2002;66:1–5. doi: 10.1095/biolreprod66.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Genbacev O, Schubach SA, Miller RK. Villous culture of first trimester human placenta—model to study extravillous trophoblast (EVT) differentiation. Placenta. 1992;13:439–461. doi: 10.1016/0143-4004(92)90051-t. [DOI] [PubMed] [Google Scholar]
- 33.Vicovac L, Jones CJ, Aplin JD. Trophoblast differentiation during formation of anchoring villi in a model of the early human placenta in vitro. Placenta. 1995;16:41–56. doi: 10.1016/0143-4004(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 34.Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knöfler M. Tumor necrosis factor-α inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- 35.Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P, Knöfler M. Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168:1134–1147. doi: 10.2353/ajpath.2006.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choy MY, Manyonda IT. The phagocytic activity of human first trimester extravillous trophoblast. Hum Reprod. 1998;13:2941–2949. doi: 10.1093/humrep/13.10.2941. [DOI] [PubMed] [Google Scholar]
- 37.Choy MY, St. Whitley G, Manyonda IT. Efficient, rapid and reliable establishment of human trophoblast cell lines using poly-l-ornithine. Early Pregnancy. 2000;4:124–143. [PubMed] [Google Scholar]
- 38.Leisser C, Saleh L, Haider S, Husslein H, Sonderegger S, Knöfler M. Tumour necrosis factor-α impairs chorionic gonadotropin β-subunit expression and cell fusion of human villous cytotrophoblast. Mol Hum Reprod. 2006;12:601–609. doi: 10.1093/molehr/gal066. [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee S, Gurevich VV, Preninger A, Hamm HE, Bader MF, Fazleabas AT, Birnbaumer L, Hunzicker-Dunn M. Aspartic acid 564 in the third cytoplasmic loop of the luteinizing hormone/choriogonadotropin receptor is crucial for phosphorylation-independent interaction with arrestin2. J Biol Chem. 2002;277:17916–17927. doi: 10.1074/jbc.M110479200. [DOI] [PubMed] [Google Scholar]
- 40.Pidoux G, Gerbaud P, Tsatsaris V, Marpeau O, Ferreira F, Meduri G, Guibourdenche J, Badet J, Evain-Brion D, Frendo JL. Biochemical characterization and modulation of LH/CG-receptor during human trophoblast differentiation. J Cell Physiol. 2007;212:26–35. doi: 10.1002/jcp.20995. [DOI] [PubMed] [Google Scholar]
- 41.Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, Fisher SJ. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev Biol. 1994;164:550–561. doi: 10.1006/dbio.1994.1223. [DOI] [PubMed] [Google Scholar]
- 42.Qiu Q, Yang M, Tsang BK, Gruslin A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod. 2004;10:677–684. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- 43.Licht P, von Wolff M, Berkholz A, Wildt L. Evidence for cycle-dependent expression of full-length human chorionic gonadotropin/luteinizing hormone receptor mRNA in human endometrium and decidua. Fertil Steril. 2003;79(Suppl 1):718–723. doi: 10.1016/s0015-0282(02)04822-7. [DOI] [PubMed] [Google Scholar]
- 44.Ku SY, Choi YM, Suh CS, Kim SH, Kim JG, Moon SY, Lee JY. Effect of gonadotropins on human endometrial stromal cell proliferation in vitro. Arch Gynecol Obstet. 2002;266:223–228. doi: 10.1007/s00404-002-0292-9. [DOI] [PubMed] [Google Scholar]
- 45.Gubbay O, Guo W, Rae MT, Niven D, Howie AF, McNeilly AS, Xu L, Hillier SG. Anti-inflammatory and proliferative responses in human and ovine ovarian surface epithelial cells. Reproduction. 2004;128:607–614. doi: 10.1530/rep.1.00272. [DOI] [PubMed] [Google Scholar]
- 46.Herr F, Baal N, Reisinger K, Lorenz A, McKinnon T, Preissner KT, Zygmunt M. HCG in the regulation of placental angiogenesis. Results of an in vitro study. Placenta. 2007;28(Suppl A):S85–S93. doi: 10.1016/j.placenta.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Horiuchi A, Nikaido T, Yoshizawa T, Itoh K, Kobayashi Y, Toki T, Konishi I, Fujii S. HCG promotes proliferation of uterine leiomyomal cells more strongly than that of myometrial smooth muscle cells in vitro. Mol Hum Reprod. 2000;6:523–528. doi: 10.1093/molehr/6.6.523. [DOI] [PubMed] [Google Scholar]
- 48.Cole LA, Shahabi S, Butler SA, Mitchell H, Newlands ES, Behrman HR, Verrill HL. Utility of commonly used commercial human chorionic gonadotropin immunoassays in the diagnosis and management of trophoblastic diseases. Clin Chem. 2001;47:308–315. [PubMed] [Google Scholar]
- 49.Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. hCG produced by the invasive trophoblast but not by the villous trophoblast promotes cell invasion and is down regulated by PPARγ. Endocrinology. 2007;148:5011–5019. doi: 10.1210/en.2007-0286. [DOI] [PubMed] [Google Scholar]
- 50.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 51.Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24:53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 54.Xu P, Wang YL, Zhu SJ, Luo SY, Piao YS, Zhuang LZ. Expression of matrix metalloproteinase-2, -9, and -14, tissue inhibitors of metalloproteinase-1, and matrix proteins in human placenta during the first trimester. Biol Reprod. 2000;62:988–994. doi: 10.1095/biolreprod62.4.988. [DOI] [PubMed] [Google Scholar]
- 55.Bjorn SF, Hastrup N, Larsen JF, Lund LR, Pyke C. Messenger RNA for membrane-type 2 matrix metalloproteinase, MT2-MMP, is expressed in human placenta of first trimester. Placenta. 2000;21:170–176. doi: 10.1053/plac.1999.0447. [DOI] [PubMed] [Google Scholar]
- 56.Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- 57.Islami D, Bischof P, Chardonnens D. Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol Hum Reprod. 2003;9:395–398. doi: 10.1093/molehr/gag053. [DOI] [PubMed] [Google Scholar]
- 58.Chardonnens D, Cameo P, Aubert ML, Pralong FP, Islami D, Campana A, Gaillard RC, Bischof P. Modulation of human cytotrophoblastic leptin secretion by interleukin-1α and 17β-oestradiol and its effect on HCG secretion. Mol Hum Reprod. 1999;5:1077–1082. doi: 10.1093/molehr/5.11.1077. [DOI] [PubMed] [Google Scholar]