Abstract
Objective
To investigate the clinical efficacy of individual endovascular management for the treatment of different traumatic pseudoaneurysms presenting as intractable epistaxis.
Materials and Methods
For 14 consecutive patients with traumatic pseudoaneurysm presenting as refractory epistaxes, 15 endovascular procedures were performed. Digital subtraction angiography revealed that the pseudoaneurysms originated from the internal maxillary artery in eight patients; and all were treated with occlusion of the feeding artery. In six cases, they originated from the internal carotid artery (ICA); out of which, two were managed with detachable balloons, two with covered stents, one by means of cavity embolization, and the remaining one with parent artery occlusion. All of these cases were followed up clinically from six to 18 months, with a mean follow up time of ten months; moreover, three cases were also followed with angiography.
Results
Complete cessation of bleeding was achieved in all the 15 instances (100%) immediately after the endovascular therapies. Of the six patients who suffered from ICA pseudoaneurysms, one presented with a permanent stroke and one had an episode of rebleeding requiring intervention.
Conclusion
In patients presenting with a history of craniocerebral trauma, traumatic pseudoaneurysm must be considered as a differential diagnosis. Individual endovascular treatment is a relatively safe, plausible, and reliable means of managing traumatic pseudoaneurysms.
Keywords: Traumatic, Pseudoaneurysm, Epistaxis, Endovascular therapy
Epistaxis is a common medical condition. Most cases are due to bleeding from the anterior nasal septum and are easily managed with local measures, such as applying pressure to the nostrils, chemical or electrocautization, topical hemostatic or vasoconstricting agents, cryotherapy, hot water irrigation, or anterior nasal packing together with the management of underlying risk factors such as hypertension and oral anticoagulation (1). However, in about 5% of the cases, the origin of the epistaxis lies in a more posterior part of the nasal cavity; this epistaxis is more severe with a distinct source of bleeding, which is often difficult to localize (2). Historically, the definitive treatment for intractable posterior epistaxis consisted of transantral surgical ligation of the branches of the internal maxillary artery (IMA). The first endovascular treatment of epistaxis was presented as an alternative to surgery by Sokoloff et al. (3) in 1974. Nowadays, endovascular management of refractory epistaxis is in the clinical mainstream and has yielded impressive results.
However, quite rarely, life-threatening hemorrhages associated with craniocerebral trauma are encountered by neurosurgeons; angiographic procedures reveal traumatic pseudoaneurysms arising from the external carotid artery (ECA) branches or internal maxillary artery (ICA) along its cervical, petrous, or cavernous segments. As can be predicted, the pseudoaneurysm often complicates the therapy of epistaxis. Here, we retrospectively reviewed 14 consecutive patients who suffered from traumatic pseudoaneurysms presenting as massive epistaxes, which were managed with endovascular techniques in our hospital from June 2005 to June 2009.
MATERIALS AND METHODS
From June 2005 to June 2009, 14 consecutive patients underwent endovascular intervention as a means of managing traumatic pseudoaneurysms presenting as intractable epistaxes in our hospital. We retrospectively reviewed their medical records with the approval of the Institutional Review Board. The group consisted of twelve male and two female patients ranging from 17 to 56 years of age (mean age: 28.1 years). Various clinical manifestations, such as delayed massive epistaxes, othemorrhagia, cerebrospinal fluid leakage, acrosia, pulsatile exophthalmos, etc., as well as multiple maxillofacial and/or basalis bone fractures were seen in the CT scans. All of the data indicted a definite history of maxillofacial or cranial trauma in all patients (Tables 1, 2).
Table 1.
Demographics and Outcome of Eight Traumatic Pseudoaneurysms of External Carotid Artery Managed with Endovascular Procedures
Note.-IOA = infraorbital artery, No. = number, PVA = polyvinyl alcohol, SPA = sphenopalatine artery
Table 2.
Demographics and Outcome of Six Traumatic Pseudoaneurysms of Internal Carotid Artery Managed with Endovascular Procedures
Note.-*Cavernous segment was involved in all cases. CCF = carotid cavernous fistula, GDC = Guglielmi detachable coils, ICA = internal carotid artery, No. = number
All cases had previously been managed with conventional management procedures, for instance, posterior nasal packing, which unfortunately failed to bring a complete cessation of the massive epistaxis. Five of the patients had to be treated with blood transfusions; however, three were hemodynamically unstable during admission and had to go through resuscitation maneuvers. Cerebral angiographies, which included bilateral ECA, ICA, and vertebral arteries, were performed in all cases, and traumatic pseudoaneurysms arising from the ECA branches were identified in eight cases (Table 1, Patient numbers 1, 2, 3, 5, 6, 9, 11 and 13), with collateral ECA being harbored in Patient numbers 3 and 9. Six cases (Table 2, Patient numbers 4, 7, 8, 10, 12 and 14) had origins of the traumatic pseudoaneurysms in the ipsilateral ICA along its petrous (Patient number 8) or cavernous segments (Patient numbers 4, 7, 10, 12 and 14).
Our routine protocol for managing IMA traumatic pseudoaneurysm has been occlusion of the parent arteries. The occlusion procedures were performed under local anesthesia. A 3-Fr microcatheter (COOK, Bloomington, IN) was navigated into the parent arteries as close to the pseudoaneurysm as possible (Fig. 1), followed by the frequent use of spiral coils - and similar materials - with or without the preliminary addition embolization of polyvinyl alcohol particles ranging in size from 500 to 700 µm. Each and every individual case was unique in itself. For those who had pseudoaneurysms originating in the ICA, the decision of whether to occlude the ICA or to embolize the aneurysm was made on the basis of balloon occlusion test (BOT) result. The BOT protocol was as follows: administration of 5000 U of intravenous heparin to all the patients before the procedure. The subsequent hypotension BOT was managed by administration of intravenous Perdipine under the guide of an anesthesia monitor.
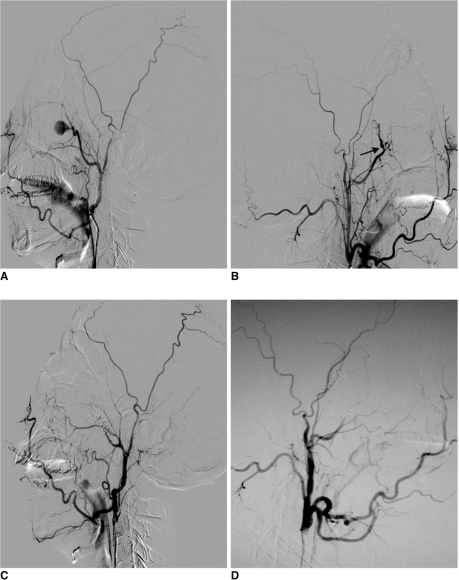
Fig. 1.
Patient number 3, massive recurrent epistaxis resulting from bilateral internal maxillary artery traumatic pseudoaneurysm which was managed with spiral coils.
A. Lateral view of right external carotid artery, demonstrating traumatic pseudoaneurysm arising from internal maxillary artery.
B. Same patient, lateral view of left external carotid artery, demonstrating micro pseudoaneurysm located in left internal maxillary artery (arrow).
C. Post parent artery embolization, lateral view of right external carotid artery, pseudoaneurysm is not visualized through internal maxillary artery and external carotid artery branches.
D. Post parent artery embolization, lateral view of left external carotid artery, pseudoaneurysm is not visualized through internal maxillary artery and external carotid artery branches.
Patient numbers 8 and 12 tolerated the BOT as well as hypotension BOT uneventfully. In Patient number 12, the traumatic pseudoaneurysm was associated with ipsilateral carotid cavernous fistula, which was managed by occluding the ICA with detachable balloons, preceded by confirmation of absence of any collateral blood flow. Patient numbers 4 and 7 could not tolerate the BOT, thus giving us no other option than pseudoaneurysm cavity occlusion.
Patient numbers 10 and 14 (Fig. 2) suffered from ICA pseudoaneurysms in the intracavernous segments. During those days, endovascular covered stents were a newly introduced measure in this hospital. Two covered stents (Jostent Coronary Stent Graft, Jomed GmbH, Rangendingen, Germany) with dimensions of 3.5×12 mm and 4×19 mm, respectively, were deployed across the corresponding segments of the pseudoaneurysms under general anesthesia. After the procedure, subcutaneous Low Molecular Weight Heparin (40 mg Q 12 h) was delivered for 72 hours followed by a four week regimen of oral Clopidrogel (75 mg/day) and a long term oral aspirin treatment (150 mg/day) were given.
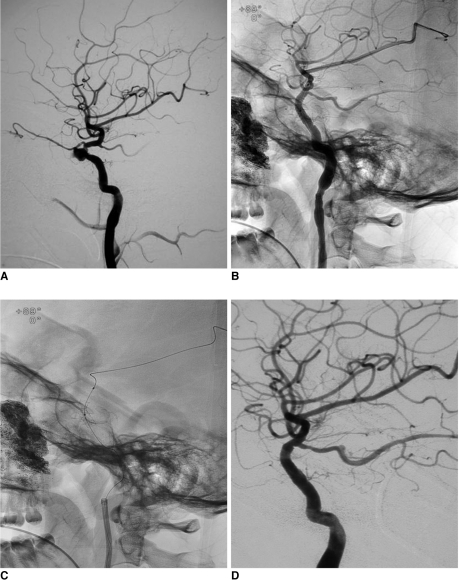
Fig. 2.
Patient number 14, application of covered stent in management of traumatic pseudoaneurysm presenting as massive epistaxis.
A. Lateral view demonstrating traumatic pseudoaneurysm which is located in cavernous sinus of right internal carotid artery.
B. After covered stent deployment, lateral view, clearly demonstrating Jostent covered stent in cavernous segment of internal carotid artery along guide wire.
C. Un-subtracted carotid angiography, lateral view, showing Jostent covered stent in disease segment of right internal carotid artery and gauze of right accessory nasal cavity. Note vascular spasm of internal carotid artery.
D. Follow-up angiogram at 13 months shows no recanalization of pseudoaneurysm and patency of stent.
RESULTS
The results and follow-up findings are listed in Tables 1 and 2. Complete cessation of bleeding was achieved in all 15 instances (100%) immediately after the endovascular therapies.
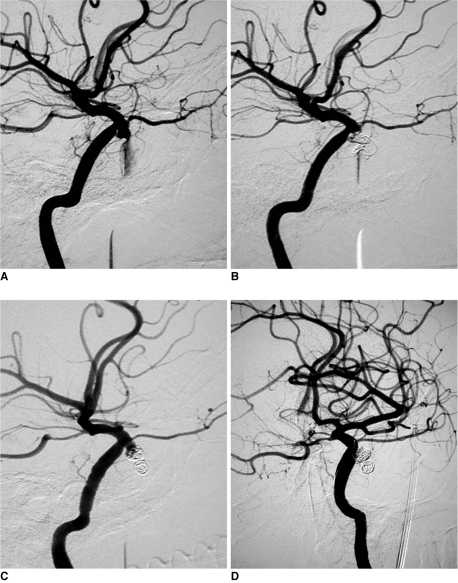
However, an ICA pseudoaneurysm is significantly different from an IMA pseudoaneurysm with its high fatality and invalidity rates. In Patient number 4 (Fig. 3), the epistaxis was halted after embolization of the pseudoaneurysm cavity using four Guglielmi detachable coils (GDCs; 2 of the 4 mm×8 cm GDC-10 soft, 2 of the 3 mm×6 cm GDC-10 soft) while keeping the ICA lumen intact (Fig. 2B); unfortunately, the patient suffered from an episode of epistaxis two months after the procedure, as revealed from the repeat digital subtraction angiography examination. This was due to a partial recanalization of the pseudoaneurysm that was successfully managed by a supplementary embolization using two GDCs of dimensions 3 mm×6 cm and 2 mm×2 cm, respectively (Fig. 2D). His further hospital stay for the next seven days was uneventful and his follow up for next 18 months as well showed no recurrence.
Fig. 3.
Patient number 4, massive recurrent epistaxis resulting from cavernous traumatic pseudoaneurysm which was managed with two cavity embolization procedures.
A. Lateral view demonstrating traumatic pseudoaneurysm of right internal carotid artery which is shown as actively bleeding into sphenoidal sinus.
B. After first embolization with four Guglielmi detachable coils, pseudoaneurysm cannot be visualized in carotid angiography while internal carotid artery was kept intact.
C. Two months later, lateral carotid angiography demonstrating partial recanalization of pseudoaneurysm; image of coils changed in contrast with B, and location of coils is displaced.
D. After supplemental embolization with two Guglielmi detachable coils, relapsing and recanalized pseudoaneurysm cavity is tightly embolized.
Patient number 7 had an aneurysm rupture during the cavity filling procedure, after which, due to massive and possibly fatal epistaxis, he was hemodynamically unstable. We did perform a partial cavity embolization and successive emergency occlusion of the parent ICA using spiral coils; nevertheless, as predictable, the patient suffered from permanent hemiparalysis of the right side.
The complicated exophthalmos of Patient number 12 was healing well.
In addition to clinically following all the cases for about six to eight months (with a mean of ten months), Patient numbers 4, 10 and 14 were also followed up with repeat angiographies; and as mentioned above, only Patient number 4 had a recurrent episode. Cerebral angiography of the remaining two patients showed complete occlusion of the pseudoaneurysm as well as no signs of intra-stent stenosis.
DISCUSSION
With an incidence of less than 1% of the intracranial aneurysms, traumatic pseudoaneurysm is a rare complication (4) and even rarer when it comes to being one of the reasons behind intractable epistaxes. Nevertheless, rare though it may be, when it does occur it is indeed life threatening. Reportedly, ICA traumatic pseudoaneurysms have a mortality rate of around 30-50% (5). The etiology is mainly caused by bone fracture due to trauma such as gunshot wounds, motor-vehicle accidents, auto-pedestrian, and falls. Recently, iatrogenic injury is another notable etiology, like, for example, radiation therapy and transsphenoidal surgery (6, 7). Contrary to the past, current endovascular management of intractable epistaxes has gained increasing acceptance.
In diagnosing the pseudoaneurysm of the ICA, even though MRI and three dimensional CT angiography are performed first, angiographic imaging is still the gold standard technique for definite diagnosis (8, 9). Further, as long as the suspicion index for traumatic pseudoaneurysms presenting as intractable epistaxes is sufficiently high, angiography becomes mandatory.
Lasjaunias et al. (10) had stressed the importance of obtaining a diagnostic pre-embolization angiogram of the ECA and ICA. It may reveal specific abnormalities indicating the cause and location of the intractable epistaxis. Among the cases described above, even though bilateral common carotid artery angiograms of Patient number 6 were normal, multiple micro traumatic pseudoaneurysms were revealed in the ECA angiograms; this may be associated with many limiting parameters, like the pressure limit, the flow rate, or the injection volume. But sometimes, provided that the posterior nasal cavity is tightly and effectively packed, the ECA or ICA angiography may demonstrate abnormal findings; this has been reported by Cockroft et al. (11). If no abnormal signs are seen in both the ECA and ICA angiograms, then it is prudent to assume that the most conceivable source of bleeding should be any one or more branches of the ECA. Nishioka et al. (12) pointed out that the empirical embolization of both the distal internal maxillary arteries resulted in a complete remission of the epistaxis without further complications.
Patients with epistaxes, due to arterial damage and consequent pseudoaneurysm formation, usually present after a latent period ranging from several days to many years during which there is possible weakening of the arterial wall and/or adjacent bone erosion, with most cases usually presenting at about the third week (88%) (8, 13).
Bleeding is usually characterized by numerous episodes, which become more and more severe over time; though the first hemorrhage may also be fatal in rare instances (8). The initial bleeding episodes that are not particularly drastic, like those that sometimes cease on their own and/or those which could be brought to a temporary halt by applying conservative measures, e.g. nasal packing, usually become a cause of delayed diagnosis and treatment. Thus, we cannot underestimate the importance of carotid angiography as long as we have a sufficiently high suspicion index for traumatic pseudoaneurysm.
Pseudoaneurysms can arise from the branches of the ECA as well as from the ICA system. The majority of traumatic pseudoaneurysms of the ICA responsible for massive epistaxes are based in the cavernous segment. The close proximity of the sphenoid sinus with the ICA and the carotid artery bulging into the sphenoid sinus in about 71% of cases, along with formation of a thin bony layer (<1 mm in thickness) in 66% of the cases, has been well described by Renn and Rhoton (14) in their cadaver studies. So, as described in the few cases above, it is quite predictable that the pseudoaneurysms have a higher predilection for rupturing into the sphenoid sinus.
When the traumatic pseudoaneurysm is located in the ECA branches, parent artery occlusion may be considered as an effective and a simple procedure. Endovascular trapping is also a time tested technique that is preferred for the treatment of pseudoaneurysms. However, because of the fear of rupturing the fragile pseudoaneurysm due to extraneous procedures, rather than to opt for pseudoaneurysm cavity filling or pseudoaneurysm trapping, we preferred to occlude only the feeding artery. Although recurrent epistaxes is the main limiting factor of this approach (15), in the cases described above, there were no retrograde filling of the lesion via the indirect collateral, and there were no recurrent episodes during the entire clinical follow up period. Regardless of these results, we still opine that we will have to work this out on a larger number of patients to come to a definite conclusion regarding the pros and cons of both of the approaches.
Owing to the high morbidity and mortality rates of the epistaxes resulting from an ICA pseudoaneurysm, as we can see, out of six patients, one succumbed to permanent hemiparalysis and another had to be readmitted. Though the management technicalities have improved drastically over the past few decades, theoretically, we still have only two options: to preserve or to occlude the carotid artery. Parent artery occlusion is a very important issue laden with grave complications and should be avoided at all costs. Anatomic constraints limiting operative exposure and distal control add up to the fact that direct clipping of the fragile lesion is by itself difficult to achieve, making a direct surgical approach to the cavernous and petrous ICA a very challenging issue (16). Surgical ligation or carotid artery occlusion carries with themselves greater preponderances of cerebrovascular events. Reportedly, even among those who well tolerated the BOT and the hypotension BOT prior to the intervention, 5-22% developed ischemic complications, including cerebral infarction (17). There also remains a risk of developing collateral blood flow into the pseudoaneurysm leading to recurrent epistaxes (8, 18). Presently, endovascular management of traumatic pseudoaneurysms presenting as refractory epistaxis has gained considerable clinical recognition. The decision on how to approach an individual endovascular should be based on the location of the pseudoaneurysm, presence or absence of the collaterals, the management team's own clinical judgment and experience, and, most importantly the results of the BOT.
Temporary balloon occlusion for 30 minutes of testing requires marked anticoagulation with an activated clotting time in the range of 300 seconds. It seems to carry a risk of hemorrhage in patients with pseudoaneurysms. However, the risk of severe cerebrovascular events arising from blind ICA occlusion is far outweighed by the risk of hemorrhage. Moreover, the patients were under some initial treatment, such as posterior nasal packing (Fig. 3B, C).
Patient numbers 4 and 7 could not tolerate the BOT. For them, except for bypass extracranial to intracranial surgery, aneurysm cavity embolization was the only remaining option. Embolization with detachable platinum coils or stent-assisted coils are available to preserve the patency of the ICA and to eradicate the cerebral pseudoaneurysm (19, 20). Lempert et al. (21) describes a successful direct coiling of eleven consecutive post traumatic pseudoaneurysms with preservation of the parent vessels. The difficulty is, a pseudoaneurysm consists of only fibrous tissue and does not contain any of the normal vessel wall elements, thus giving us no walls to confine the coils. Furthermore, owing to pseudoaneurysm's fragility, there is always a fear of rupture. In our opinion, direct tight coiling of the pseudoaneurysm is much more difficult than of a true aneurysm. Two of our cases suffered from some iatrogenic mishaps while undergoing intervention with the coils, i.e. rupture of the pseudoaneurysm in Patient number 7 and recurrence after two months in Patient number 4. A similar event was described by Lempert et al. (21). This kind of recanalization may be related to the recompression or displacement of the coils under the impact of the artery pulse. Struffert et al. (22) reported two similar cases, in which the coils migrated after endovascular coil occlusion of the ICA pseudoaneurysms. In our case, a supplementary embolization with two coils proved to be a permanent curative management, which may be explained by the fact that subacute pseudoaneurysms have walls that are more mature, containing fibroblasts and other elements to strengthen and encapsulate the walls, and can be treated as a true aneurysm.
Recently, endovascular covered stents have been applied in similar lesions (6, 23). Managing traumatic pseudoaneurysm with covered stents is considered to be more logical, safer, and easier in comparison to the deployment of an embolizing material in the aneurismal sac (24).
In Patient numbers 10 and 14, right after employing the covered stents, the traumatic pseudoaneurysms were easily and successfully excluded with immediate effect, thus bringing about a complete halt to the epistaxes episodes. As one of the most upsetting drawbacks of covered stents is occlusion of adjoining small perforating arteries in the region of deployment, two of our cases inevitably suffered from permanent occlusion of the ipsilateral ophthalmic arteries. Fortunately, no blindness occurred in Patient number 10, thanks to the abundant compensatory circulation of the ophthalmic artery. The right side blindness of Patient number 14 was associated with primary trauma. After placement of the covered stents, reconstructions of the ophthalmic artery from the external carotid artery collaterals were seen in both of the patients, as anticipated. While the cavernous segment does have branches, the clinical significance of occluding these branches has not yet been elaborated. However, as seen in our cases, the fact that the patients tolerated covered stenting of the cavernous segments is well documented in previous literature. Apart from occlusion of the adjoining arteries, limited flexibility of the Jostent covered stent is another limitation. So, in some cases, significant vessel tortuosity was an obstacle that hindered us from attempting this approach. Possible complications that may result from this rigidity are dissection and endomembrane injury of the cerebral arteries. This may also explain its association perioperative vasospasm observed in Patient number 10, which was relieved merely after the withdrawal of the balloon and the guide wire. While several varieties of covered stents have been utilized by the neurovascular community, only the Willis covered stent reported by Li et al. (25) is specially designed for intracranial vasculature use. In the future, the development of special intracranial covered stents will provide physicians with the greater ability to reconstruct vessels and exclude anomalous vascular conditions. A recent report of a massive epistaxis resulting from an ICA pseudoaneurysm brought about to a complete halt while preserving the ICA, by means of overlapping self expanding stents, was very promising (26). It was supposedly a great new measure for handling this intricate entity; however, in contrast to the first angiographic follow-up, after three follow-ups it was revealed that some residual leaking into the pseudoaneurysm was occurring, thus implying that the risk of recurrent epistaxis was still eminent.
In conclusion, in our experience, refractory epistaxes were challenging lesions with no unified endovascular therapy mode. The importance of not omitting traumatic pseudoaneurysm as a differential diagnosis in all cases presenting with craniocerebral trauma cannot be neglected. Intractable epistaxis due to ICA pseudoaneurysm is severe and life threatening, which can be well managed with an individual endovascular approach depending on the pseudoaneurysm location, collateral circulation, and management experiences. Endovascular covered stenting is a safe, feasible and life-saving procedure and a prudent mode of therapy in the management of ICA pseudoaneurysms, although long-term follow-up and larger samples are required.
References
- 1.Tan LK, Calhoun KH. Epistaxis. Med Clin North Am. 1999;83:43–56. doi: 10.1016/s0025-7125(05)70086-9. [DOI] [PubMed] [Google Scholar]
- 2.Viducich RA, Blanda MP, Gerson LW. Posterior epistaxis: clinical features and acute complications. Ann Emerg Med. 1995;25:592–596. doi: 10.1016/s0196-0644(95)70169-9. [DOI] [PubMed] [Google Scholar]
- 3.Sokoloff J, Wickbom I, McDonald D, Brahme F, Goergen TC, Goldberer LE. Therapeutic percutaneous embolization in intractable epistaxis. Radiology. 1974;111:285–287. doi: 10.1148/111.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Quintana F, Diez C, Gutierrez A, Diez ML, Austin O, Vazquez A. Traumatic aneurysm of the basilar artery. AJNR Am J Neuroradiol. 1996;17:283–285. [PMC free article] [PubMed] [Google Scholar]
- 5.Karamoskos P, Dohrmann PJ. Traumatic internal carotid artery aneurysm and massive epistaxis. Aust N Z J Surg. 1989;59:745–747. doi: 10.1111/j.1445-2197.1989.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 6.Auyeung KM, Lui WM, Chow LC, Chan FL. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: emergency treatment with covered stent in two cases. AJNR Am J Neuroradiol. 2003;24:1449–1452. [PMC free article] [PubMed] [Google Scholar]
- 7.Nishioka H, Ohno S, Ikeda Y, Ohashi T, Haraoka J. Delayed massive epistaxis following endonasal transsphenoidal surgery. Acta Neurochir (Wien) 2007;149:523–526. doi: 10.1007/s00701-007-1134-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Concus AP, Halbach VV, Cheung SW. Epistaxis originating from traumatic pseudoaneurysm of the internal carotid artery: diagnosis and endovascular therapy. Laryngoscope. 1998;108:326–331. doi: 10.1097/00005537-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Feiz-Erfan I, Horn EM, Theodore N, Zabramski JM, Klopfenstein JD, Lekovic GP, et al. Incidence and pattern of direct blunt neurovascular injury associated with trauma to the skull base. J Neurosurg. 2007;107:364–369. doi: 10.3171/JNS-07/08/0364. [DOI] [PubMed] [Google Scholar]
- 10.Lasjaunias P, Marsot-Dupuch K, Doyon D. The radio-anatomical basis of arterial embolisation for epistaxis. J Neuroradiol. 1979;6:45–53. [PubMed] [Google Scholar]
- 11.Cockroft KM, Carew JF, Trost D, Fraser RA. Delayed epistaxis resulting from external carotid artery injury requiring embolization: a rare complication of transsphenoidal surgery: case report. Neurosurgery. 2000;47:236–239. doi: 10.1097/00006123-200007000-00052. [DOI] [PubMed] [Google Scholar]
- 12.Nishioka H, Haraoka J, Ikeda Y. Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery. Acta Neurochir (Wien) 2005;147:1163–1166. doi: 10.1007/s00701-005-0586-3. [DOI] [PubMed] [Google Scholar]
- 13.Fontela PS, Tampieri D, Atkinson JD, Daniel SJ, Teitelbaum J, Shemie SD. Posttraumatic pseudoaneurysm of the intracavernous internal carotid artery presenting with massive epistaxis. Pediatr Crit Care Med. 2006;7:260–262. doi: 10.1097/01.PCC.0000216418.01278.5E. [DOI] [PubMed] [Google Scholar]
- 14.Renn WH, Rhoton AL., Jr Microsurgical anatomy of the sellar region. J Neurosurg. 1975;43:288–298. doi: 10.3171/jns.1975.43.3.0288. [DOI] [PubMed] [Google Scholar]
- 15.Song HH, Won YD, Kim YJ, Kim BS. The endovascular management of saccular posterior inferior cerebellar artery aneurysms. Korean J Radiol. 2008;9:396–400. doi: 10.3348/kjr.2008.9.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bavinzski G, Killer M, Knosp E, Ferraz-Leite H, Gruber A, Richling B. False aneurysms of the intracavernous carotid artery--report of 7 cases. Acta Neurochir (Wien) 1997;139:37–43. doi: 10.1007/BF01850866. [DOI] [PubMed] [Google Scholar]
- 17.Eckert B, Thie A, Carvajal M, Groden C, Zeumer H. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1998;19:577–582. [PMC free article] [PubMed] [Google Scholar]
- 18.Goleas J, Mikhael MA, Paige ML, Wolff AP. Intracavernous carotid artery aneurysm presenting as recurrent epistaxis. Ann Otol Rhinol Laryngol. 1991;100:577–579. doi: 10.1177/000348949110000711. [DOI] [PubMed] [Google Scholar]
- 19.Quintana F, Diez C, Gutierrez A, Diez ML, Austin O, Vazquez A. Traumatic aneurysm of the basilar artery. AJNR Am J Neuroradiol. 1996;17:283–285. [PMC free article] [PubMed] [Google Scholar]
- 20.Karamoskos P, Dohrmann PJ. Traumatic internal carotid artery aneurysm and massive epistaxis. Aust N Z J Surg. 1989;59:745–747. doi: 10.1111/j.1445-2197.1989.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 21.Lempert TE, Halbach VV, Higashida RT, Dowd CF, Urwin RW, Balousek PA, et al. Endovascular treatment of pseudoaneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol. 1998;19:907–911. [PMC free article] [PubMed] [Google Scholar]
- 22.Struffert T, Buhk JH, Buchfelder M, Rohde V, Doerfler A, Knauth M. Coil migration after endovascular coil occlusion of internal carotid artery pseudoaneurysms within the sphenoid sinus. Minim Invasive Neurosurg. 2009;52:89–92. doi: 10.1055/s-0029-1215579. [DOI] [PubMed] [Google Scholar]
- 23.Celil G, Engin D, Orhan G, Barbaros C, Hakan K, Adil E. Intractable epistaxis related to cavernous carotid artery pseudoaneurysm: treatment of a case with covered stent. Auris Nasus Larynx. 2004;31:275–278. doi: 10.1016/j.anl.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Choi SY, Won JY, Lee do Y, Choi D, Shim WH, Lee KH. Percutaneous transabdominal approach for the treatment of endoleaks after endovascular repair of infrarenal abdominal aortic aneurysm. Korean J Radiol. 2010;11:107–114. doi: 10.3348/kjr.2010.11.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MH, Li YD, Gao BL, Fang C, Luo QY, Cheng YS, et al. A new covered stent designed for intracranial vasculature: application in the management of pseudoaneurysms of the cranial internal carotid artery. AJNR Am J Neuroradiol. 2007;28:1579–1585. doi: 10.3174/ajnr.A0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Juretschke F, Castro E, Mateo Sierra O, Iza B, Manuel Garbizu J, Fortea F. Massive epistaxis resulting from an intracavernous internal carotid artery traumatic pseudoaneurysm: complete resolution with overlapping uncovered stents. Acta Neurochir (Wien) 2009;151:1681–1684. doi: 10.1007/s00701-009-0294-5. [DOI] [PubMed] [Google Scholar]