Abstract
Estrogen receptor activity was quantitated in the cytosol and nucleus of normal rat liver and in regenerating rat liver at several time intervals after 75% hepatectomy. Cytosolic estradiol binding in regenerating liver decreases at 12, 24, and 48 h after hepatectomy and at 48 h is 30% of that in normal rat liver. Nuclear estrogen binding 48 h after surgery is elevated fivefold over normal values. No alterations in affiriity of the receptor for estrogen have been observed. Specificity studies indicate that the estrogen receptors from both normal and regenerating liver were similar and are highly specific for estrogens. These changes in cellular distribution of receptors parallel increases in nuclear deoxyribonucleic acid synthesis and mitotic indices in the liver.
The discovery of estrogen (1–7) receptors in the cytosol of hepatocytes has stimulated research attempting to define a possible role for steroid hormones, particularly estrogens, in the pathogenesis of hepatic disorders. Adenoma (8,9), focal nodular hyperplasia (10–13), hepatoma, and angiosarcoma (14–17) are disorders which have been associated with the long-term use of steroidal agents such as estrogen, androgens, and prednisone. The effect of estrogen in promoting diethylnitrosamine-induced liver tumors has been addressed recently by Wanless et al. (18). These studies have shown that estrogens promote the development of hepatic neoplasms associated with increasing hepatocyte mitogenic activity. In contrast, Mishkin et al. (19) has demonstrated that in animals with acetylaminofluorine-induced hepatic hyperplastic nodules, estradiol in combination with tamoxifen resulted in regression of the nodules. In a recent study, Porter et al. (20) found an increase of estrogen receptor activity in the liver of individuals with focal nodular hyperplasia occurring in association with oral contraceptive use, and a decrease in estrogen binding activity was noted in hepatic adenoma. In addition, Francavilla et al. (21) recently reported a very low level of cytosolic estrogen binding activity in Morris hepatoma 7777 as compared to normal rat liver.
Because a decrease in cytosolic estrogen binding activity (i.e., receptors) appears to be associated with an increased hepatocyte proliferative activity in such neoplastic conditions, we investigated whether partial hepatectomy, known to induce profound changes in the remaining liver, also results in alterations in cellular estrogen receptor distribution. We report herein that liver resection induces a decrease in cytosolic receptor and a concomitant increase in nuclear receptor; each of these changes parallel increases in nuclear deoxyribonucleic acid (DNA) synthesis and mitotic index.
Materials and Methods
Animals
Male Sprague–Dawley rats (250 g) used in these studies were maintained on standard rat laboratory diet and water ad libitum in a temperature- and light-controlled room. Partial hepatectomy was performed according to the methods of Higgins and Anderson (22). All operations were performed between 7:30 and 9:00 am using ketamine anesthesia (10 mg/100 g body wt). In sham-operated animals, the liver was manipulated in the same manner as the livers of resected animals, and returned into the abdomen. After partial hepatectomy, the animals were killed by decapitation 3, 12, 24, 48, 72, 96, 144, and 192 h later.
Materials
Estrone (E1), estradiol (E2), and estriol (E3) were purchased from Steraloids, Wilton, N.H. Diethylstilbesterol (DES), testosterone, 5-α-dihydrotestosterone (DHT), progesterone, cortisone, bovine serum albumin, sodium molybdate, and protamine sulfate were purchased from Sigma Chemical Company, St. Louis, Mo. Norit A and dextran C were obtained from Fisher Scientific Company, Pittsburgh, Pa. [2,4,6,7,16,17-3H]estradiol, [3H]E2, 151 Ci/mmol, and [methyl-3H]thymidine, 77 µCi/mmol, were obtained from New England Nuclear, Boston, Mass. The radiolabeled estrogens used in these studies were assayed periodically for purity by thin-layer chromatography on silica gel G in ethyl acetate/hexane/ethanol (85 : 10 : 5), and were used only if purity was >95%.
Preparation of Cytosol
Approximately 10 g of liver was used for each assay. All procedures were performed at 0°C. The liver was minced and homogenized with a Brinkmann Polytron (Brinkmann Instruments, Inc., Westbury, N.Y.) in 2 vol of TED buffer: 0.01 M Tris-HCl, 1.5 mM ethylenediaminete-traacetate (EDTA), 0.01 mM dithiothreitol, pH 7.4. The resultant homogenate was centrifuged for 60 min at 105,000 g. The supernate was removed carefully to prevent lipid contamination and treated with Norit A and dextran C according to the technique of Chamness (3,23) to remove endogenous free steroids. The final protein concentration in the cytosol samples so prepared was 15–25 mg/ml. Immediately before use, the cytosol was diluted to a final protein concentration of 5 mg/ml.
Protamine Sulfate Assay
The protamine sulfate precipitate method was used to assay cytosolic estrogen receptor; this method avoids interference of [3H]E2 binding to a high-capacity estrogen binder of male rat liver cytosol (7). Two-tenths milliliter of cytosol and 0.2 ml of protamine sulfate solution (1.5 mg/ml in TED buffer) were combined in multiple replicate tubes and mixed briefly. After standing for 5 min at 0°C, the reaction tubes were centrifuged at 800 g for 10 min and the supernate was removed by aspiration. A 250-µl aliquot of radioactive steroid solution in TED buffer, with or without unlabeled competing hormone, was then added to each tube. After incubation for 18 h at 0°C, the supernate was removed, and the precipitate in each tube was washed with three 2-ml aliquots of cold TED buffer. The bottom of the reaction tube containing the washed precipitate was placed in a scintillation vial with 2 ml of absolute ethanol and extracted for 1 h, after which 10 ml of Instagel was added (Packard Instrument Co., Inc., Downers Grove, Ill.).
Cytosol Binding Studies
Protamine sulfate precipitates of 200-µl aliquots of hepatic cytosol were prepared and incubated with varying concentrations of [3H]E2 over a range of 0.15–5 nM in the absence (total binding) and presence (nonspecific binding) of 100-fold excess of unlabeled E2 for 18 h at 0°C. These conditions yield maximum binding and represent equilibrium conditions (7). Specific binding was obtained by subtracting nonspecific binding from total binding. Scatchard analysis (24) was performed using the specific binding values. The slope and y-intercept of the apparent linear relationship were determined by use of the unweighted linear regression program of the TI 55 calculator (Texas Instruments, Houston, Tex.). Metabolism of [3H]E2 was assessed by extracting a standard 18-h incubation sample with 5 vol of ether; the extract was dried under N2, redissolved in ethanol, and subjected to thin-layer chromatography in benzene/ethanol (9 : 1, vol/vol) with appropriate standards. Metabolism to estrone occurred in all samples to some extent in 18 h; 23% and 56% of the available [3H]E2 was converted to estrone in normal and 48-h posthepatectomy liver, respectively. Sufficient estrogen is present for binding however, in that <10% of the total [3H]E2 added is bound to the receptor. Furthermore, most of the estrogen binding to receptor is complete at 2 h (7), but metabolism of [3H]E2 is undetectable at this time. In studies to assess specificity of binding, a single concentration (1.5 nM) of [3H]E2 was used in the presence and absence of 100-fold excess concentration of various unlabeled hormones, including E2, DES, E1, E3, progesterone, testosterone, DHT, and cortisone.
Preparation of Nuclei
Control or hepatectomized liver (5 g) was homogenized in three volumes of TEM buffer: 10 mM Tris, 1.5 mM EDTA, 20 mM sodium molybdate, pH 7.4, using a Brinkmann PT 10–35 Polytron. The homogenate was centrifuged at 48,200 g for 20 min to pellet a crude nuclei preparation. The crude nuclear pellet was then resuspended in SMH buffer: 0.25 M sucrose, 3 mM MgCl2, 10 mM HEPES, pH 7.4, to the original volume of homogenate. The resuspension was centrifuged at 800 g for 10 min. The resulting pellet was then resuspended in SMH containing 0.1% Triton X-100 and homogenized in a Kontes Dounce homogenizer (Kontes Co., Vineland, N.J.). The nuclear suspension was then washed two more times with SMH and centrifuged at 800 g. The washed pellet was then resuspended to original volume in SMH. Cytosolic contamination of nuclei was assayed by determining the activity of the alcohol dehydrogenase enzyme in cytosol (25) and the washed nuclei. The cytosol contamination was found to be no more than 2% in the nuclear preparation. The average recovery of DNA in the nuclear preparation was 72.8% of that in the homogenate.
Determination of Nuclear Binding Sites by [3H]Estradiol Exchange Assay
Nuclear suspensions (0.2 ml) were incubated in the presence of 10 nM [3H]E2 in TEM buffer with and without a 100-fold excess concentration of unlabeled DES in ethanol; each assay was done in triplicate. The exchange assay was accomplished by incubation at 30°C for 1 h, conditions determined to be optimal for the exchange assay. The reaction was stopped by chilling the tubes on ice for 5 min, and free steroid was removed by first washing with the addition of 2 ml SMH containing 0.1% Triton X-100 to incubation tubes and then following with centrifugation at 800 g for 10 min. The nuclear pellet was then washed three more times with 2 ml of SMH. The bound steroid was then extracted from the nuclear receptor complex with 2 ml of absolute ethanol at 30°C for 30 min. The ethanol extract was counted with 10 ml of ACS in a Packard Tri-Carb spectrometer. The remaining pellet was used for DNA quantification.
Mitotic Index
The mitotic index of the liver was determined at different times after hepatectomy. Hepatic tissues were fixed in 10% buffered formalin, obtained by dilution of 37% aqueous solution of formaldehyde. Tissue sections were stained with hematoxylin and eosin; the percentage of cells in metaphase was determined across 1000 cells under oil immersion (×950).
Deoxyribonucleic Acid Synthesis
Deoxyribonucleic acid synthesis was estimated on the 1st, 2nd, 3rd, 4th, 6th, and 8th day after partial hepatectomy. Two hours before death, 50 µCi of [3H]thymidine solution was administered by intraperitoneal injection of the rats. Extraction and purification of hepatic DNA were carried out according to the method described by Ove et al. (26,27), and the DNA content was measured by the diphenylamine method of Burton (28) using calf thymus DNA as standard. The incorporation of [3H]thymidine into DNA was determined by measurement of specific activity of pure DNA obtained (counts per minute/milligram of DNA).
Auxiliary Methods
Protein concentrations of cytosol were determined using the method of Lowry et al. (29) and BSA as the standard. The radioactivity content of samples was determined in a Packard Tri-Carb liquid scintillation spectrometer. Results are expressed as mean ± SEM. Statistical analysis was performed using the Student’s nonpaired t-test.
Results
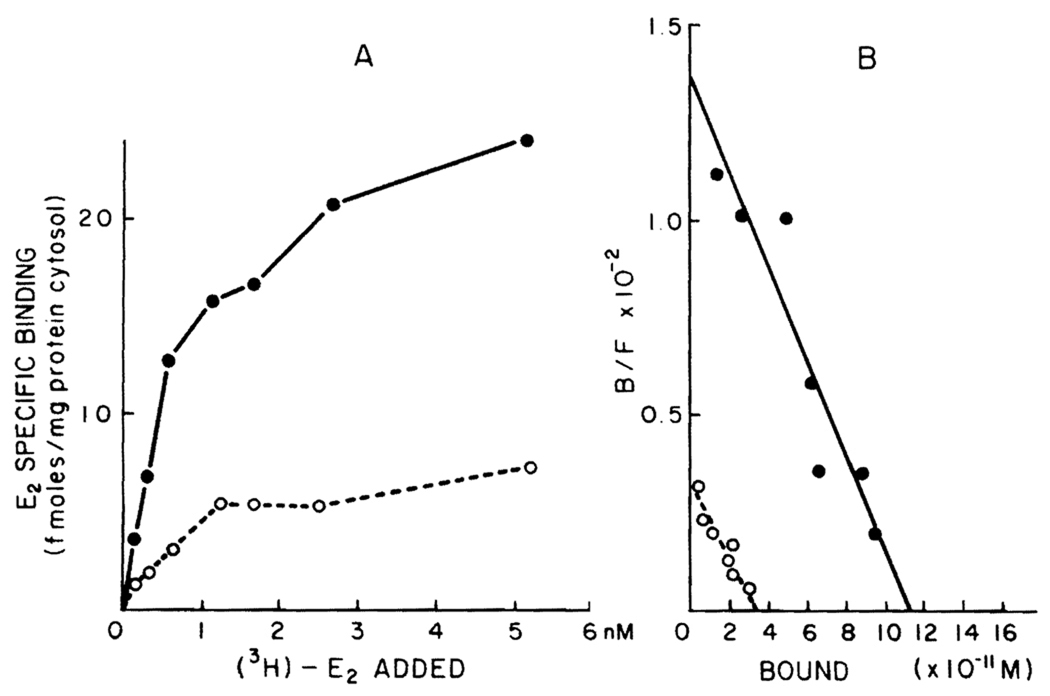
A typical binding curve obtained by incubating protamine sulfate precipitates of normal male rat liver with various concentrations of [3H]E2 is shown in Figure 1A. It is evident that the specific estradiol binding observed is of limited capacity and saturable. Figure 1B demonstrates the specific binding data plotted according to the method of Scatchard. A single class of binding sites for estradiol with a uniform affinity is found (r = 0.92). In this particular experiment, the binding capacity is 28.13 fmol of E2/mg protein and the Kd is 0.83 nM.
Figure 1.
A. Specific binding of [3H]E2 by cytosol of normal and regenerating male rat liver. Two-hundred microliter aliquots of cytosol (5 mg/ml) from normal or regenerating rat liver 48 h posthepatectomy precipitated with protamine sulfate were incubated with seven different concentrations of [3H]E2 (0.15–5 nM) for 18 h at 0°C in the absence (total binding) and presence (nonspecific binding) of 100-fold unlabeled E2. Specific binding was calculated by subtracting nonspecific binding from total binding. Each point is the average of triplicate determinations. B. Scatchard plot analysis of the specific [3H]E2 binding data obtained using normal and regenerating male rat liver. The specific binding data illustrated in A was replotted according to the method of Scatchard. Closed circles represent normal liver; open circles represent regenerating liver in both A and B.
Figure 1A also shows typical estrogen binding data obtained using cytosol from the regenerating liver of adult male rat 48 h after hepatectomy. As in normal rat liver, the binding of [3H]E2 in cytosol of regenerating liver is of limited capacity and high affinity. Figure 1B illustrates a Scatchard plot of the specific binding data. A single class of binding sites for estradiol with a uniform affinity was found (r = 0.93). The binding capacity was determined to be 8.6 fmol/mg and the Kd is 1.1 nM. In five separate experiments on normal and regenerating liver (48 h after hepatectomy), no alterations were noted in affinity of the receptor for E2 [normal liver: Kd = 0.66 ± 0.10 nM; regenerating liver: Kd = 0.74 ± 0.19 (p = NS)]. Sham-operated animals did not differ from control animals in their hepatic content of estrogen receptors.
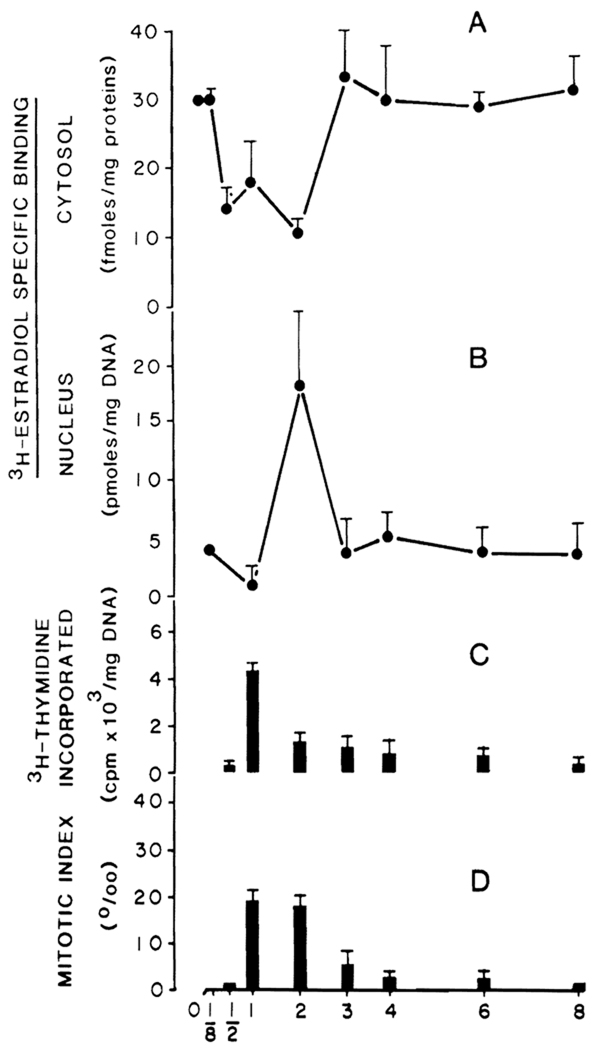
Figure 2 illustrates the changes in cellular distribution of estrogen receptors, DNA synthesis, and mitotic index as a function of time after partial hepatectomy. Cytosolic estrogen receptor content at 3 h after surgery (Figure 2A) does not differ from normal values (29.7 ± 6.1 fmol of E2/mg protein). However, cytosolic receptor content is significantly decreased at 12, 24, and 48 h. At 72 h and thereafter, receptor levels return to normal. A parallel increase in nuclear receptor content is evident in Figure 2B and most likely represents a translocation of cytosolic receptor to the nucleus as a sequela of resection.
Figure 2.
Cytosolic and nuclear specific [3H]E2 binding, DNA synthesis, and mitotic index in normal and regenerating livers at different days posthepatectomy. A. Two-hundred microliter aliquots of cytosol (5 mg/ml) precipitated with protamine sulfate were incubated with 1.5 nM E2 for 18 h at 0°C in the presence and absence of 100-fold unlabeled E2. Each point is the mean ± SEM of six experiments. B. Two-hundred microliter aliquots of nuclear suspension were incubated at 30°C for 60 min with 10 nM of [3H]E2 in the presence and absence of 100-fold excess concentration of unlabeled DES (exchange assay). Each point is the mean ± SEM of 5 animals; each assay is performed in triplicate. C. Deoxyribonucleic acid synthesis was determined as described in Materials and Methods for 1 mg of DNA at different times after partial hepatectomy. D. Mitotic index as an expression of the number of hepatocytes in metaphase for 1000 cells was determined as outlined in Materials and Methods.
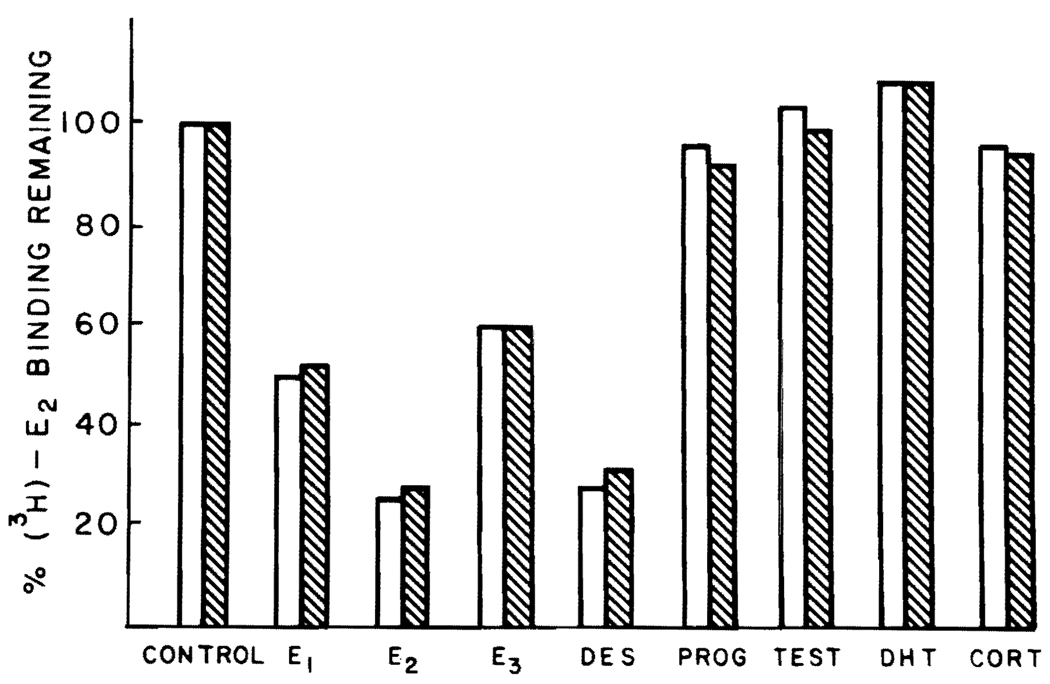
In order to establish that the binding of the labeled estrogen observed in these studies was specific, the ability of various unlabeled steroids to compete with [3H]E2 binding in cytosol of normal and regenerating liver was determined. The data shown in Figure 3 demonstrate that the binding is specific for only the estrogenic substances. Further, [3H]E2 binding is not inhibited by testosterone, DHT, cortisone, and progesterone in either kind of cytosol. The nuclear estrogen receptors are similarly highly specific for estrogenic substances (data not shown).
Figure 3.
Specificity of binding to cytosolic proteins in normal and regenerating male rat liver cytosol. Two-hundred microliter aliquots of male rat liver cytosol were precipitated with protamine sulfate and were incubated with 1.5 nM [3H]E2 in the presence and absence of 100-fold excess of competing ligand for 18 h at 0°C. Each bar represents the mean results of triplicate determinations for each competing ligand. The solid bars represent data obtained using normal liver; the hatched bars represent data obtained using regenerating liver. Progesterone (PROG), testosterone (TEST), cortisone (CORT).
Discussion
Recent research by our group and others has shown a low level of estrogen receptor in the cytosol of an hepatic neoplastic tissue, Morris hepatoma 7777 (21), and a reduction of receptor content in cytosol of hepatic adenoma obtained from patients (20) and the animal model (19) at the time of operation or death as compared with that of normal liver.
These observations suggest that a decrease of cytosolic estrogen receptors is associated with hepatocyte division. To define the behavior of estrogen receptors during hepatocyte division, the cytosolic and nuclear binding of these hormones was studied at different times during rat liver regeneration, a model in which the cellular division rate is comparable with that observed in some rapidly growing tumors. The results obtained demonstrate a decrease in estrogen binding activity in hepatic cytosol associated with an increase in nuclear estrogen binding. Both effects are maximum at 48 h after partial hepatectomy. Scatchard analysis of cytosolic estrogen binding data demonstrated a decrease in the number of receptors, rather than an alteration in the affinity of the receptors for the ligand used. At the same time, a clear increase of total nuclear receptors is demonstrated, which suggests an active translocation of the hormone receptor complex into the nucleus. These data may suggest that the decrease of cytosolic receptor found by us and others (20,21) in neoplastic tissues could be an expression of an active nuclear translocation.
The hormonal receptor redistribution starts at the same time as the stimulation of DNA synthesis and reaches its maximum at 48 h, coinciding with the high values of mitotic index. The relationship of this phenomenon with hepatocyte division is also demonstrated by the fact that a normal distribution of receptors is found in the cytosol and the nucleus at 72 h after hepatectomy when few mitoses are found in the liver. This active translocation of the estrogen receptor complex into the nucleus also correlates well with other markers of hepatic regeneration, including several biochemical activities, such as DNA polymerase, protein synthesis, and deoxythymidine kinase (30–32).
In relation to liver regeneration, the active nuclear translocation of estrogen receptors with its metabolic influences represents a further endocrine change in addition to those that are known to occur (33,34). Specifically, recent reports have documented an increase in glucagon and a decrease in T3 and insulin plasma levels after hepatic resection and subsequent regeneration (35,36). Some of these hormonal changes have been correlated with changes in binding of these same hormones to receptors on the hepatocyte plasma membrane during regeneration. A rapid, sustained decrease in glucagon binding and a transitory increase in insulin binding to hepatic plasma membranes has been demonstrated to occur during regeneration (36,37). In addition, the level of cytosolic glucocorticoid receptor in liver decreases rapidly after resection and returns to normal levels within 72 h (38).
From our data, we cannot claim, as is true for the other hormonal events reported above, a cause and effect relationship between the nuclear estrogen receptor levels and DNA synthesis. However, in light of these data, we may speculate on the role of the estrogen receptor in regeneration. The redistribution of estrogen receptor to the nucleus is an early event in regeneration and, therefore, must be important. possibly in stimulation of cell division or in induction of specific proteins necessary for this process, or in both. Further, estrogen is a known inducer of the hepatic synthesis of plasma transport proteins (39), a function likely to be important during the regenerative period. Clearly, more work needs to be done to establish the links between the estrogen and other hormone receptors, and the important and essential molecular events that occur during hepatic regeneration.
Acknowledgments
Supported by research grants from the Veterans Administration, by project grants AM-29961 and AM-30001 from the National Institutes of Health, by grant RR-000084 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health, and by grant 82/0031096 from Consiglio Nazionale Delle Ricerche.
The authors thank Joan E. Willett for invaluable technical expertise in the quantitation of nuclear receptors and John Prelich for excellent and skillful assistance with many of the experiments.
References
- 1.Eisenfeld AJ, Aten RF, Haselbacher GK, et al. Specific macromolecular binding of estradiol in the mammalian liver supernate. Biochem Pharmacol. 1977;26:919–926. doi: 10.1016/0006-2952(77)90466-x. [DOI] [PubMed] [Google Scholar]
- 2.Viladiu P, Delgado C, Pensky J, et al. Estrogen binding protein of rat liver. Endocr Res Commun. 1975;2:273–280. doi: 10.3109/07435807509053854. [DOI] [PubMed] [Google Scholar]
- 3.Chamness GC, Costlow ME, McGuire WL. Estrogen receptor in rat liver and its dependence on prolactin. Steroids. 1975;26:363–371. doi: 10.1016/0039-128x(75)90081-1. [DOI] [PubMed] [Google Scholar]
- 4.Powell-Jones W, Davies P, Griffiths K. Specific binding of (3H)-estradiol by cytoplasmic protein components of female liver. J Endocrinol. 1976;69:167–168. doi: 10.1677/joe.0.0690167. [DOI] [PubMed] [Google Scholar]
- 5.Beers PC, Rosner W. The binding of estrogens in the liver of the rat: demonstration and endocrine influences. J Steroid Biochem. 1977;8:251–258. doi: 10.1016/0022-4731(77)90017-6. [DOI] [PubMed] [Google Scholar]
- 6.Aten RF, Dickson RB, Eisenfeld AJ. Estrogen receptor in adult male rat liver. Endocrinology. 1978;131:1629–1635. doi: 10.1210/endo-103-5-1629. [DOI] [PubMed] [Google Scholar]
- 7.Eagon PK, Fisher SE, Imhoff AF, et al. Estrogen binding proteins of male rat liver: influences of hormonal changes. Arch Biochem Biophys. 1980;201:486–499. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 8.Baum JK, Holtz F, Bookstein JJ, et al. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;ii:926–929. doi: 10.1016/s0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- 9.Edmondson HA, Henderson B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 10.Fechner RE. Benign hepatic lesions and orally administered contraceptives. A report of seven cases and a critical analysis of the literature. Hum Pathol. 1977;8:255–268. doi: 10.1016/s0046-8177(77)80022-1. [DOI] [PubMed] [Google Scholar]
- 11.Ishak KG. Primary hepatic tumors in childhood. In: Popper H, Schaffner F, editors. Progress in liver diseases. New York: Grune and Stratton; 1976. pp. 636–667. [PubMed] [Google Scholar]
- 12.Ishak KG, Rabil L. Benign tumors of the liver. Med Clin North Am. 1975;59:995–1013. doi: 10.1016/s0025-7125(16)31998-8. [DOI] [PubMed] [Google Scholar]
- 13.Knowles DM, Wolff M. Focal nodular hyperplasia of the liver. A clinicopathologic study and review of the literature. Hum Pathol. 1976;7:533–545. doi: 10.1016/s0046-8177(76)80101-3. [DOI] [PubMed] [Google Scholar]
- 14.Christopherson WM, Mays ET, Barrows GH. Liver tumors in women on contraceptive steroids. Obstet Gynecol. 1975;48:221–223. [PubMed] [Google Scholar]
- 15.Christopherson WM, Mays ET, Barrows GH. A clinicopathologic study of steroid related tumors. Am J Surg Pathol. 1977;1:31–41. doi: 10.1097/00000478-197701010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Neuberger J, Nummerley HB, Davis M, et al. Oral contraceptive-associated liver tumors: occurrence of malignancy and difficulties in diagnosis. Lancet. 1980;i:273–276. doi: 10.1016/s0140-6736(80)90776-x. [DOI] [PubMed] [Google Scholar]
- 17.Mays ET, Christopherson WM, Mahr MM, et al. Hepatic changes in young women ingesting contraceptive steroids: hepatic hemorrhage and primary hepatic tumors. JAMA. 1976;235:71–72. [PubMed] [Google Scholar]
- 18.Wanless IR, Medline A. Role of estrogens as promoters of hepatic neoplasia. Lab Invest. 1982;46:313–320. [PubMed] [Google Scholar]
- 19.Mishkin SY, Farber E, Ho RK, et al. Evidence for the hormone dependency of hepatic hyperplastic nodules: inhibition of malignant transformation after exogenous 17-β3-estradiol and tamoxifen. Hepatology. 1983;3:308. doi: 10.1002/hep.1840030306. [DOI] [PubMed] [Google Scholar]
- 20.Porter LE, Elm MS, Van Thiel DH, Eagon PK. Estrogen receptor in human liver disease. Clin Res. 1983;31:287A. doi: 10.1016/0016-5085(87)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Francavilla A, Eagon PK, DiLeo A, et al. Estrogen binding protein activity in Morris hepatoma 7777 compared to normal rat liver. Gastroenterology. (in press). [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins EM, Anderson RM. Experimental pathology restoration of the liver of the white rat following surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 23.Chamness GC, Huff K, McGuire WL. Protamine precipitated estrogen receptor. A solid phase ligand exchange assay. Steroids. 1975;25:627–635. doi: 10.1016/0039-128x(75)90017-3. [DOI] [PubMed] [Google Scholar]
- 24.Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 25.Valle BL, Hoch FL. Zinc, a component of yeast alcohol dehydrogenase. Proc Natl Acad Sci USA. 1955;41:327. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ove P, Coetzee ML, Morris HP. DNA synthesis and the effect of sucrose in nuclei of host liver and Morris hepatomas. Cancer Res. 1971;31:1388–1395. [PubMed] [Google Scholar]
- 27.Ove P, Coetzee ML, Morris HP. Separable DNA polymerase activities in host liver and Morris hepatomas. Cancer Res. 1973;33:1272–1283. [PubMed] [Google Scholar]
- 28.Burton K. Determination of DNA concentration with diphenylamine. Methods Enzymol. 1968;12:163–166. [Google Scholar]
- 29.Lowry OH, Rosenbrough NJ, Farr Al, et al. Protein measurement with the Folin reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Smith HC, Berezney R. DNA polymerase α is tightly bound to the nuclear matrix of actively replicating liver. Biochem Biophys Res Commun. 1980;97:1541–1547. doi: 10.1016/s0006-291x(80)80041-6. [DOI] [PubMed] [Google Scholar]
- 31.Bresnick E, William JS, Mosse H. Rates of turnover of deoxythymidine kinase and of its template RNA in regenerating and control liver. Cancer Res. 1967;27:463–475. [PubMed] [Google Scholar]
- 32.Tomashefsky P, Tannenbaum M. Seromucoid and albumin syntheses after uninephrectomy and partial hepatectomy in the rat. Proc Soc Exp Biol Med. 1974;146:921–925. doi: 10.3181/00379727-146-38220. [DOI] [PubMed] [Google Scholar]
- 33.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 34.Francavilla A, Porter KA, Benichou J, Jones AF, Starzl TE. Liver regeneration in dogs: morphologic and chemical changes. J Surg Res. 1978;25:409–419. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morley CGD, Kuku S, Rubenstein HN, Boyer SL. Serum hormone levels following partial hepatectomy in rat. Biochem Biophys Res Commun. 1975;67:663–668. doi: 10.1016/0006-291x(75)90862-1. [DOI] [PubMed] [Google Scholar]
- 36.Leffert H, Alexander NM, Faloona G, Rubalcava B, Unger R. Specific endocrine and hormonal receptor changes associated with liver regeneration in adult rats. Proc Natl Acad Sci USA. 1975;72:4033–4036. doi: 10.1073/pnas.72.10.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pezzino V, Vigneri R, Cohen D, Goldifine ID. Regenerating rat liver: insulin and glucagon serum levels and receptor binding. Endocrinology. 1981;108:2163–2169. doi: 10.1210/endo-108-6-2163. [DOI] [PubMed] [Google Scholar]
- 38.Leob JN, Rosner W. Fall in hepatic cytosolic glucocorticoid receptor induced by stress and partial hepatectomy: evidence for separate mechanisms. Endocrinology. 1979;104:1003–1006. doi: 10.1210/endo-104-4-1003. [DOI] [PubMed] [Google Scholar]
- 39.Anderson KE, Kappas A. Hormones and hepatic function. In: Schiff E, editor. Diseases of the liver. 5th ed. Philadelphia: J.P. Lippincott; 1982. pp. 167–235. [Google Scholar]