Abstract
In spite of the improved outcome of orthotopic liver transplantation (OLTx), primary graft nonfunction remains one of the life-threatening problems following OLTx. The purpose of this study was to evaluate plasma lecithin: cholesterol acyltransferase (LCAT) activity in multiple organ donors as a predictor of liver allograft viability prior to OLTx. Thirty-nine donors were studied during a 5-month period between April and August 1988. Allograft hepatectomy was performed using a rapid technique or its minor modification with hilar dissections, and the allografts were stored cold (4 °C) in University of Wisconsin (UW) solution. Early post-transplant allograft function was classified as good, fair, or poor, according to the highest SGOT, SGPT, and prothrombin time within 5 days following OLTx. Procurement records were reviewed to identify donor data, which included conventional liver function tests, duration of hospital stay, history of cardiac arrest, and graft ischemic time. Blood samples from the donors were drawn immediately prior to aortic crossclamp, and from these plasma LCAT activity was determined. Plasma LCAT activity of all donors was significantly lower than that of healthy controls (12.4 ± 8.0 vs 39.2 ± 13.3 μg/ml per hour, P < 0.01). LCAT activity (16.4 ± 8.3 μg/ml per hour) in donors of grafts with good function was significantly higher than that in those with fair (8.6 ± 4.5 μg/ml perhour, P < 0.01) or poor (7.3 ± 2.4 μg/ml per hour, P < 0.01) function. Information regarding procurement, which was complete in the records of 31 of 39 donors, was used in a multiple logistic regression analysis that revealed plasma LCAT activity to be the only factor able to discriminate the quality of the hepatic graft from other variables in multiple organ donors. The present study suggests that the determination of plasma LCAT activity in multiple organ donors is extremely useful for the assessment of hepatic allograft viability prior to OLTx.
Keywords: Viability test, human livers; Liver transplantation, plasma lecithin assessment; Lecithin, viability, liver donors; Cholesterol acyltransferase, in liver donors
In spite of the recent introduction of University of Wisconsin (UW) solution, which now allows safe preservation of hepatic allografts for up to 24 h [6, 20], the shortage of donor livers continues to be one of the major limiting factors for clinical liver transplantation. Immediate function of the allograft is essential for successful orthotopic liver transplantation (OLTx). Primary graft nonfunction (PGNF) is associated with an urgent need for retransplantation and, at one time, was associated with an 80 % mortality rate [13]. In order to maximally utilize the limited number of liver allografts and to avoid PGNF, it is extremely important to assess the viability of the hepatic allograft prior to the actual transplant procedure. However, little is known about a useful and practical technique that can reliably predict allograft viability prior to OLTx. In acute and chronic liver diseases, the decreased plasma activity of lecithin: cholesterol acyltransferase (LCAT) has been well documented [3, 15]. We have recently reported that the plasma LCAT activity of the recipient immediately after OLTx is a reliable indicator of allograft function in clinical OLTx [14]. The purpose of this study was to evaluate, in multiple organ donors, the significance of plasma LCAT activity as an indicator of hepatic allograft viability prior to OLTx.
Materials and methods
During a 5-month period between 1 April and 30 August 1988, 180 hepatic allografts were transplanted at the Presbyterian University Hospital at the University of Pittsburgh. Of these, donor blood was available in 39 donors. The selection was made at random and based primarily on the cooperation of eight donor surgeons rather than on the condition of the donors. The age of the 39 donors ranged from 12 to 42 years with a mean (± SD) of 24.1 ± 8.4 years. Twenty-six of the donors were male (67 %). The causes of brain death among the donors studied were: closed head injury (n = 21; 54 %), gunshot wound (n = 9; 23 %), subarachnoid hemorrhage (n = 5; 13 %), and other (n = 4; 10 %). Allograft hepatectomy was performed using a rapid perfusion technique, or its minor modification with hilar dissection, prior to the aortic crossclamp [16, 21]. The hepatic allograft was flushed through portal and aortic cannulae with chilled, lactated Ringer’s or Euro-Collins’ solution (4 °C) immediately after interruption of circulation and then excised following cardiectomy. The allograft was then perfused with two liters of UW solution through the portal vein and hepatic artery at a 9:1 ratio and stored cold in UW solution (4°C).
Donor variables evaluated included age, sex, conventional liver function tests [serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SG PT), serum total bilirubin, and prothrombin time], arterial blood gases (lowest pO2, highest pC02, and worst pH), blood pressure (lowest systolic pressure), cause of death, graft ischemic time, duration of hospital stay, and history of cardiac arrest. Pertinent recipient variables evaluated included age, sex, intraoperative blood loss, and early post-transplant liver functions (SGOT, SGPT, serum total bilirubin, and prothrombin time). For the classification of early allograft function, we used criteria described in the past [9, 14, 21]. According to the highest SGOT, SGPT, and prothrombin time within 5 days following OLTx, early post-transplant allograft function was classified as either good, fair, or poor (Table 1). Grafts were classified into the lowest (poorest) category into which any of the assessment values fell.
Table 1.
Classification of early post-transplant allograft function. OLTx, Orthotopic liver transplantation; PT, prothrombin time
| Variablea | Early allograft function |
||
|---|---|---|---|
| Good | Fair | Poor | |
| SGOT (IU/l) | ≤1500 | ≤3500 | >3500 |
| SOPT (IU/l) | ≤1000 | ≤2500 | >2500 |
| PT (sec) | ≤20.0 | ≤20.0 | >20.0 |
Data were collected within 5 days after OLTx
Blood samples from the donor were drawn through a portal cannula in a heparinized sterile tube immediately prior to the aortic crossclamp and were stored cold (4 °C) overnight. From these cold-stored samples, the plasma was separated by centrifugation at 2000 rpm for 5 min and then stored at −60 °C. Blood samples from nine healthy male volunteers were obtained by phlebotomy and used as controls. Plasma LCAT activity was determined using a LCAT test kit-S (Nippon Shoji, Osaka, Japan) and a spectrophotometer with a modification of the method described by Nagasaki and Akamuma [10]. A unit of LCAT activity was defined as the ability to esterify free cholesterol at 37°C (μg/ml per hour) in the assay mixture.
Thirty-three allograft biopsies were obtained using a Trucut biopsy needle (Travenol Laboratories, Deerfield, Illinois, USA) immediately prior to implantation.
The grafts were followed for up to 1 month after OLTx. Two grafts in the poor function group were lost due to pancreatitis and rejection, respectively, and the other grafts were functional 1 month after OLTx.
Statistical analysis was by Wilcoxon’s rank-sum test. The results are expressed as mean ± SD. The multiple logistic regression analysis of early post-transplant allograft function was performed in 31 of the 39 patients (79 %) from whom complete donor information was obtained. Computations were carried out using the statistical software "SAS LOGIST" on an IBM system 4381 computer [12].
Results
LCAT activity in the multiple organ donors was significantly lower than that in the healthy controls (12.4 ± 8.0 vs 39.2 ± 13.3 μg/ml per hour, P < 0.01). Figure 1 demonstrates the correlation between plasma LCAT activity of multiple organ donors and early post-transplant allograft function. The LCAT activity of the donors of grafts with good function (16.4 ± 8.3 μg/ml per hour) was significantly higher than that of those with fair (8.6 ± 4.5 μg/ml per hour, P < 0.01) or poor (7.3 ± 2.4 μg/ml per hour, P < 0.01) function.
Fig. 1.
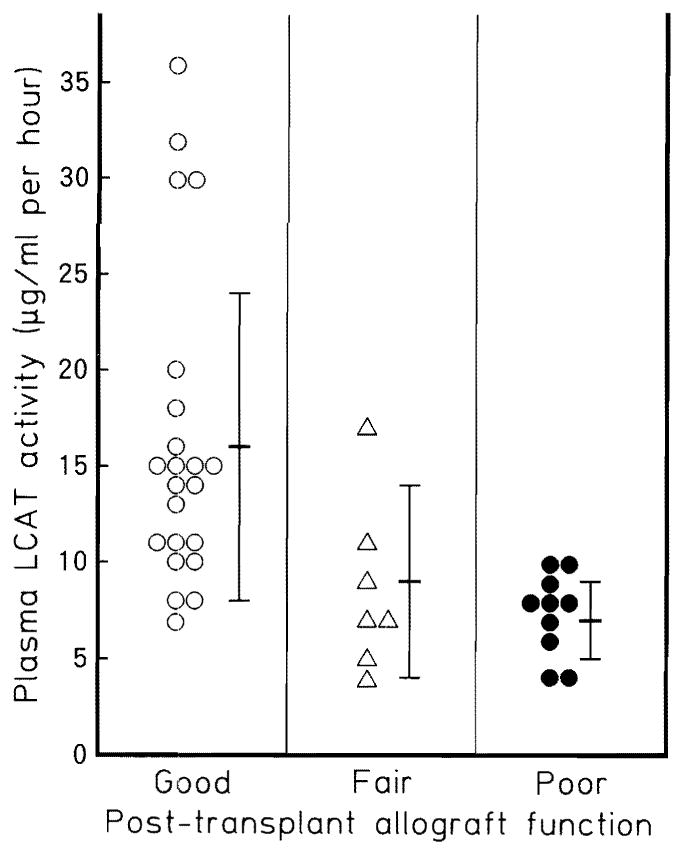
Correlation between the plasma LCAT activity in multi-organ donors and early post-transplant allograft function. The LCAT activity of the donors of grafts with good function (16.4 ± 8.3 μg/ml per hour) was significantly higher than that of those with fair (8.6 ± 4.5 μg/ml per hour, P < 0.01) or poor function (7.3 ± 2.4 μg/ml P < 0.01). LCAT Lecithin: cholesterol acyltransferase (normal range: 20–50 μg/ml per hour)
Table 2 shows the results of univariate analysis on the correlation between donor variables and early post-transplant allograft function following OLTx. The donor age of the grafts with fair function was higher than that of those with good function (29.7 ± 7.6 vs 22.4 ± 7.8 years, P < 0.05). The stay in the intensive care unit (ICU) of donors with fair grafts was also longer than those with good grafts (6.6 ± 3.9 vs 2.4 ± 1.8 days, P < 0.01). Other donor variables were comparable. Table 3 demonstrates the correlation between post-transplant allograft function and graft ischemic time or clinical recipient variables. The estimated operative blood loss of poor grafts was significantly lower than that of good grafts (6.8 ± 6.0 vs 17.8 ± 18.9, P < 0.05). Other variables did not show significant differences.
Table 2.
Univariate analysis on the correlation between donor variables and early allograft function following OLTx. OLTx, Orthotopic liver transplantation; ICU, intensive care unit; T.Bil, serum total bilirubin; PT, prothrombin time
| Variable | Early allograft function |
||
|---|---|---|---|
| Good (n = 22) | Fair (n = 7) | Poor (n = 10) | |
| Age (years) | 22.4 ± 7.8a,b | 29.7 ± 7.6b | 24.0 ± 9.4 |
| ICU stay (days) | 2.4 ± 1.8c | 6.6 ± 3.9c | 3.5 ± 2.5 |
| SGOT (IU/l) | 77.1 ± 54.1 | 48.6± 44.6 | 86.6 ± 59.6 |
| SGPT (IU/l) | 53.3 ± 54.0 | 47.0 ± 42.5 | 47.1 ± 36.2 |
| T.Bil (mg/dl) | 0.9 ± 0.6 | 1.0 ± 0.5 | 0.6 ± 0.3 |
| PT (s) | 13.2 ± 1.8 | 12.5 ± 1.0 | 13.5 ± 2.1 |
Mean ± SD
P < 0.05
P < 0.01
Table 3.
Correlation between early post-transplant allograft function and graft ischemic time or recipient variables. GIT, Graft ischemic time
| Variable | Early post-transplant allograft function |
||
|---|---|---|---|
| Good (n = 22) | Fair (n = 7) | Poor (n = 10) | |
| GIT (min) | 830 ± 327a | 685 ± 321 | 766 ± 291 |
| Age (year) | 39.8 ± 13.6 | 19.4± 11.6 | 43.3 ± 13.9 |
| Blood loss | 17.8± 18.9b | 9.9±5.3 | 6.8 ± 6.0b |
Mean ± SD
P < 0.02
Six of 33 biopsies (18.2 %) exhibited varying degrees of microscopic steatosis. The LCAT activity of the donors with steatitic grafts tended to be lower than that of those without steatosis (8.4 ± 4.3 vs 13.8 ± 8.8 μg/ml per hour).
As shown in Table 4, the multivariate analysis of the donor variables evaluated revealed that plasma LCAT activity was the only discriminating factor of allograft viability; the other variables – age, sex, cause of death, duration of ICU stay, SGOT, prothrombin time, serum total bilirubin, and biopsy findings – failed to achieve statistically significant differences.
Table 4.
Multivariate analysis on the correlation between donor variables and post-transplant allograft function. LCAT, Lecithin: cholesterol acyltransferase; T.Bil, serum total bilirubin; PT, prothrombin time; ICU, intensive care unit
| Variable | Coefficient | SE | P value |
|---|---|---|---|
| LCAT | −0.164 | 0.287 | 0.018 |
| T.Bil | −3.136 | 2.001 | 0.117 |
| Histology | 2.587 | 1.781 | 0.146 |
| SGOT | 0.009 | 0.011 | 0.398 |
| Age | 0.076 | 0.113 | 0.500 |
| PT | 0.234 | 0.350 | 0.504 |
| Death | 0.330 | 0.682 | 0.628 |
| Sex | 0.868 | 0.682 | 0.669 |
| ICU stay | −0.003 | 0.244 | 0.990 |
| Constant | 1.622 |
Discussion
Recently, hyperacute rejection of the hepatic graft has been suggested to be a cause of the primary nonfunctioning graft [1. 4]. However, for a reliable assessment of the quality of the donor liver, factors in the recipient, such as hyperacute rejection, should be excluded. We therefore excluded donors with primary nonfunctioning grafts from this study.
Different variables have been studied with regard to the post-transplant assessment of the hepatic allograft, including prothrombin time, free amino acid clearance [2, 5], recovery of adenosine triphosphate (ATP) [7] or ketone body ratio [19], and whole body oxygen consumption [17]. We have recently reported that plasma LCAT activity following OLTx is a reliable indicator of allograft viability [14].
The prediction of allograft quality prior to actual transplantation has, however, been less promising. In 1987, Makowka et al. [9] reported that none of the currently available donor variables could predict graft outcome following OLTx. To evaluate the viability of liver allografts prior to OLTx, Lanir et al. [8] demonstrated in 1988 that allografts with an ATP level over 2 nmol/mg protein and with an energy charge over 0.3 could predict the successful outcome of OLTx. Kamiike et al. [7], in 1988, also described three grafts with low levels of total adenine nucleotides (TAN) that failed to function and suggested that the allograft TAN level before recirculation might correlate with the viability of the hepatic allografts. Yet, neither of these techniques safely allows evaluation of the allografts before laparotomy of the donors, since the measurement of adenine nucleotide levels requires liver tissue. Moreover, these techniques require special equipment for the determination of adenine nucleotides. It seems unlikely that these techniques can be applied clinically at the present time.
Oellerich et al. [11] reported in 1989 that the formation of monoethyl glycinexylidine, after an intravenous injection of 1 mg/kg lidocaine, correlated well with graft outcome. This study, however, also required special equipment for the determination of lidocaine metabolites.
In contrast, LCAT activity can be determined with the use of a simple spectrophotometer and a commercially available test kit, which has already been used clinically after hepatic resections and other surgical procedures for esophageal varices [18].
In the correlation between LCAT activity in the donors and histology of the biopsies obtained prior to OLTx, allografts with steatosis tended to have lower plasma LCAT activities. These findings seem to reinforce the reliability of plasma LCAT activity in multiple organ donors as an indicator of liver allograft viability.
With regard to the significant difference in LCAT activity between the group with good function and the healthy volunteers, most donors had either cardiac arrest, hypotension, or poor nutritional support, and the initial management of patients with severe brain injury is focused on keeping patients dry to prevent brain edema rather than on maintenance of abdominal visceral perfusion. As a result, LCAT activities of the donors, even in the group with good function, was lower than that of healthy volunteers.
As for the higher estimated intra-operative blood loss of recipients of good grafts than those of poor grafts, this contradictory finding would appear to be explained by massive blood loss (39–63 μg/ml per hour) due to technical failure in four patients who received good grafts and who probably raised the mean value of blood loss.
In summary, plasma LCAT activity in multiple organ donors seems to be a discriminating and practical predictor of human liver allograft viability prior to actual OLTx.
References
- 1.Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Markus B, Mroczek E, Van Thiel DH, Sysyn G, Gordon R, Makowka L, Starzl TE. Antibody mediated rejection in human orthotopic liver allografts: a study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988;132:489–509. [PMC free article] [PubMed] [Google Scholar]
- 2.Fath J, Ascher NL, Konstantinideds FN, Bloomer J, Sharp H, Najarian JS, Cerra FB. Metabolism during hepatic transplantation: indicators of allograft function. Surgery. 1984;96:664–674. [PubMed] [Google Scholar]
- 3.Gjone E, Blomhoff JP, Wiencke I. Plasma lecithin: cholesterol acyltransferase activity in acute hepatitis. Scand J Gastroenterol. 1971;6:161–168. doi: 10.3109/00365527109180686. [DOI] [PubMed] [Google Scholar]
- 4.Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood groups barriers. Lancet. 1990;336:519–523. doi: 10.1016/0140-6736(90)92082-s. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins R, Bosari S, Khettry U, Clowes GHA, Jr, Pearl RH, Trey C. Survival from hepatic transplantation: relationship of protein synthesis to histological abnormalities in patient selection and postoperative management. Ann Surg. 1986;2004:364–374. doi: 10.1097/00000658-198610000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalayoglu M, Sollinger HW, Stratta RJ, D’Alessandro AM, Hoffmann RM, Pirsch JD, Belzer FO. Extended preservation of the liver for clinical transplantation. Lancet. 1988;I:617–619. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 7.Kamiike W, Burdelski M, Steinhoff G, Ringe B, Lauchart W, Pichlmayr R. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation. 1988;45:138–143. doi: 10.1097/00007890-198801000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Lanir A, Jenkins RL, Caldwell L, Lee RGL, Khettry U, Clouse ME. Hepatic transplantation survival: correlation with adenine nucleotide level in donor liver. Hepatology. 1988;8:471–475. doi: 10.1002/hep.1840080306. [DOI] [PubMed] [Google Scholar]
- 9.Makowka L, Gordon RD, Todo S, Ohkohchi N, Marsh JW, Tzakis AG, Yokoi H, Ligush J, Esquivel CO, Satake M, Iwatsuki S, Stanl TE. Analysis of donor criteria for the prediction of outcome in clinical liver transplantation. Transplant Proc. 1987;19:2378–2382. [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasaki T, Akanuma Y. A new colorimetric method for the determination of plasma lecithin: cholesterol acyltransferase activity. Clin Chim Acta. 1977;75:371–375. doi: 10.1016/0009-8981(77)90355-2. [DOI] [PubMed] [Google Scholar]
- 11.Oellerich M, Burdelski M, Ringe B, Lamesch P, Gubernatis G, Bunzendahl H, Pichlmayr R, Herrmann H. Lignocaine metabolite formation as a measure of pre-transplant liver function. Lancet. 1989;I:640–642. doi: 10.1016/s0140-6736(89)92144-2. [DOI] [PubMed] [Google Scholar]
- 12.SAS manual: SUGI Supplemental Library User’s Guide. SAS Institute; Cary, North Carolina, USA: 1986. [Google Scholar]
- 13.Shaw BW, Gordon RD, Iwatsuki S, Starzl TE. Hepatic re-transplantation. Transplant Proc. 1985;17:264–271. [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada M, Yanaga K, Makowka L, Kakizoe S, van Thiel DH, Starzl TE. Significance of lecithin: cholesterol acyltransferase (LCAT) activity as a prognostic indicator of early allograft function in clinical liver transplantation. Transplantation. 1989;48:600–603. [PMC free article] [PubMed] [Google Scholar]
- 15.Simon JB, Scheig R. Serum cholesterol esterification in liver disease: importance of lecithin: cholesterol acyltransferase. N Engl J Med. 1970;283:841–846. doi: 10.1056/NEJM197010152831604. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343–348. [PMC free article] [PubMed] [Google Scholar]
- 17.Svensson KL, Persson H, Henriksson BA, Karlberg I, Sonander H, Lundholm K, Stenquist O, Schersten T. Whole body gas exchange: amino acid and lactate clearance as indicators of initial and early allograft viability in liver transplantation. Surgery. 1989;105:472–480. [PubMed] [Google Scholar]
- 18.Takenaka K, Kanematu T, Sugimachi K, Inokuchi K. Serum lecithin: cholesterol acyltransferase (LCAT) activity is an accurate predictor of post-operative hepatic failure. Dis Markers. 1984;2:501–507. [Google Scholar]
- 19.Taki Y, Gubernatis G, Yamaoka Y, Oellerich M, Yamamoto Y, Ringe B, Okamoto R, Bunzendahl H, Beneking M, Burdelski M, Bornseheuer A, Ozawa K, Pichlmayr R. Significance of arterial ketone body ratio measurement in human liver transplantation. Transplantation. 1990;49:535–539. doi: 10.1097/00007890-199003000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation or human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 21.Yanaga K, Tzakis AG, Starzl TE. Personal experience with procurement of 132 hepatic allografts. Transplant Int. 1989;2:137–142. doi: 10.1007/bf02414600. [DOI] [PMC free article] [PubMed] [Google Scholar]


