Abstract
Carbonate-14C was used to label the hepatic intracellular arginine pool and direct measurement of albumin synthesis was made in six rabbits before and after an 18-36 hr fast. 18 perfusion studies were performed with livers derived from fed and fasted rabbits (18-24 hr). Microsomal amino acid-incorporating ability with leucine-3H and phenylalanine-14C was compared in 17 studies, using microsomes isolated from livers taken from fed and fasted rabbits and from isolated perfused livers whose donors were fed and fasted.
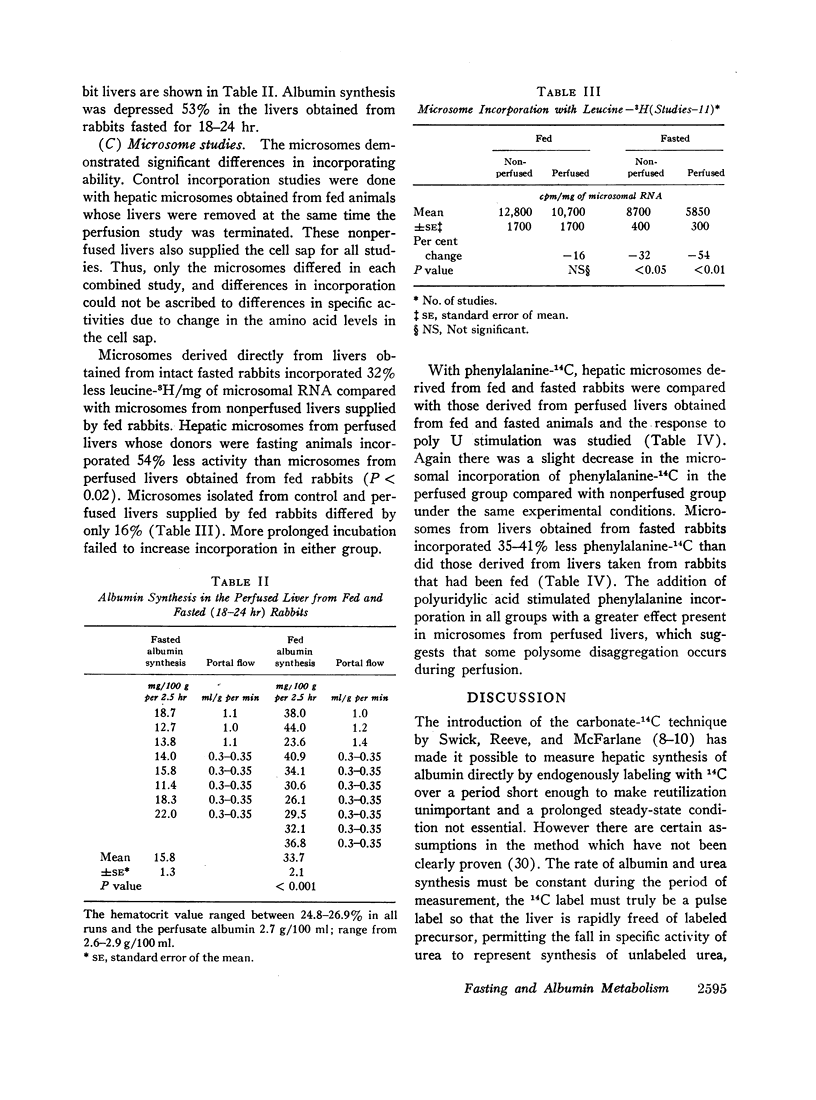
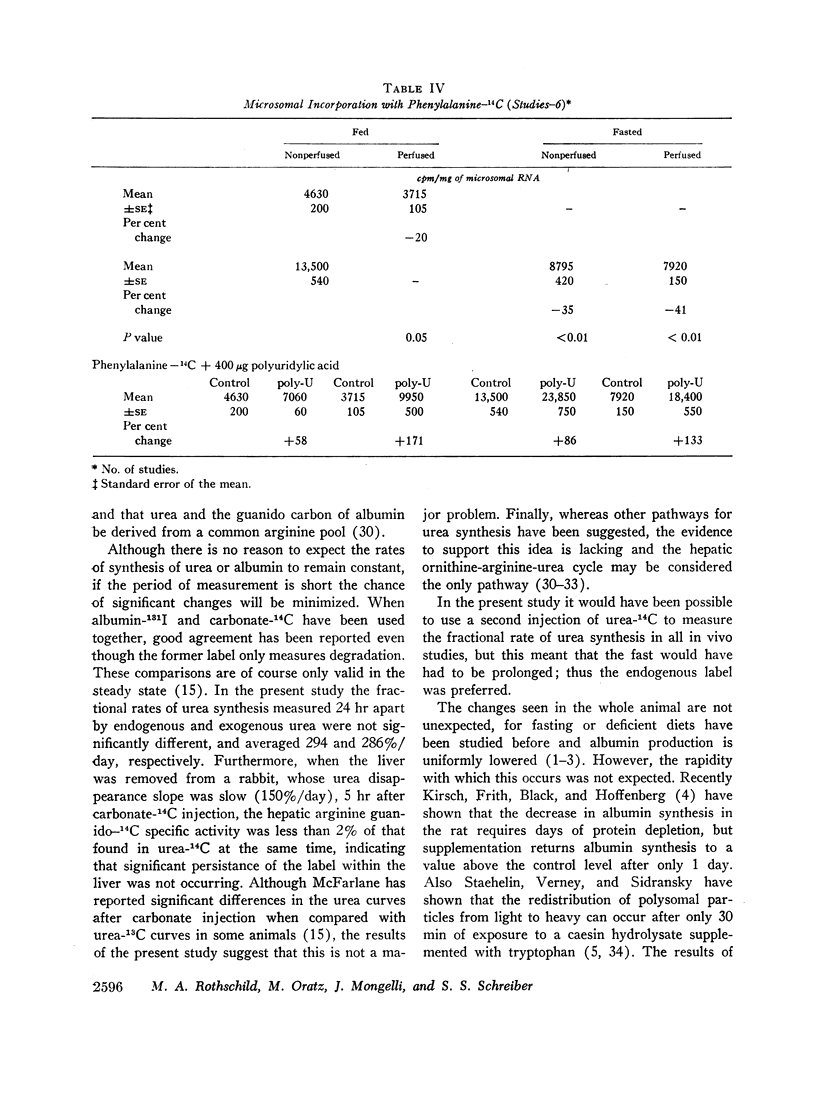
Albumin synthesis is rapidly inhibited by fasting. Albumin synthesis decreased 33% in vivo and 53% in the perfused liver. The microsomes from perfused livers taken from fed animals did not demonstrate a significantly reduced capacity to incorporate leucine-3H or phenylalanine-14C into protein. Microsomes derived from perfused and nonperfused livers whose donors were fasted incorporated 32-54% less tracer than microsomes obtained from fed donor rabbits. Microsomes separated from perfused livers removed from fed and fasted rabbits responded to polyuridylic acid stimulation and phenylalanine-14C incorporation rose from 58 to 171%.
An 18-36 hr fast inhibits albumin production in vivo and in the perfused liver. The microsomal system is less active in the fasted state and perfusion per se does not inhibit the microsomal response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENACERRAF B., BIOZZI G., HALPERN B. N., STIFFEL C., MOUTON D. Phagocytosis of heat-denatured human serum albumin labelled with 131I and its use as a means of investigating liver blood flow. Br J Exp Pathol. 1957 Feb;38(1):35–48. [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Byrne A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem J. 1933;27(2):419–429. [PMC free article] [PubMed] [Google Scholar]
- FISHER M. M., KERLY M. AMINO ACID METABOLISM IN THE PERFUSED RAT LIVER. J Physiol. 1964 Nov;174:273–294. doi: 10.1113/jphysiol.1964.sp007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN T., GORDON A. H. METABOLISM OF ALBUMIN AND GAMMA-GLOBULIN IN PROTEIN DEFICIENT RATS. Clin Sci. 1964 Feb;26:17–26. [PubMed] [Google Scholar]
- HYVARINEN A., NIKKILA E. A. Specific determination of blood glucose with o-toluidine. Clin Chim Acta. 1962 Jan;7:140–143. doi: 10.1016/0009-8981(62)90133-x. [DOI] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenberg R., Black E., Brock J. F. Albumin and gamma-globulin tracer studies in protein depletion states. J Clin Invest. 1966 Jan;45(1):143–152. doi: 10.1172/JCI105319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINGSBURY K. J., SHUTTLEWORTH K. E., MORGAN D. M. A study of plasma glyceride clearance. Clin Sci. 1962 Oct;23:251–263. [PubMed] [Google Scholar]
- Kirsch R., Frith L., Black E., Hoffenberg R. Regulation of albumin synthesis and catabolism by alteration of dietary protein. Nature. 1968 Feb 10;217(5128):578–579. doi: 10.1038/217578a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARSH J. B. Effects of fasting and alloxan diabetes on albumin synthesis by perfused rat liver. Am J Physiol. 1961 Jul;201:55–57. doi: 10.1152/ajplegacy.1961.201.1.55. [DOI] [PubMed] [Google Scholar]
- MCFARLANE A. S., IRONS L., KOJ A., REGOECZI E. THE MEASUREMENT OF SYNTHESIS RATES OF ALBUMIN AND FIBRINOGEN IN RABBITS. Biochem J. 1965 May;95:536–540. doi: 10.1042/bj0950536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFARLANE A. S. MEASUREMENT OF SYNTHESIS RATES OF LIVER-PRODUCED PLASMA PROTEINS. Biochem J. 1963 Nov;89:277–290. doi: 10.1042/bj0890277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthier B., Smuckler E. A., Hultin T. A comparison between preincubated and non-preincubated rat liver ribosomes in the poly (U)-directed phenylalanine incorporation and binding reactions. Biochim Biophys Acta. 1967 Sep 26;145(2):446–459. doi: 10.1016/0005-2787(67)90063-9. [DOI] [PubMed] [Google Scholar]
- REEVE E. B., PEARSON J. R., MARTZ D. C. Plasma protein synthesis in the liver: method for measurement of albumin formation in vivo. Science. 1963 Mar 8;139(3558):914–916. doi: 10.1126/science.139.3558.914. [DOI] [PubMed] [Google Scholar]
- ROTHSCHILD M. A., SCHREIBER S. S., ORATZ M., McGEE H. L. The effects of adrenocortical hormones on albumin metabolism studied with albumin-I 131. J Clin Invest. 1958 Sep;37(9):1229–1235. doi: 10.1172/JCI103711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SCHIMKE R. T. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962 Feb;237:459–468. [PubMed] [Google Scholar]
- SCHIMKE R. T. Differential effects of fasting and protein-free diets on levels of urea cycle enzymes in rat liver. J Biol Chem. 1962 Jun;237:1921–1924. [PubMed] [Google Scholar]
- SWICK R. W. Measurement of protein turnover in rat liver. J Biol Chem. 1958 Apr;231(2):751–764. [PubMed] [Google Scholar]
- Sidransky H., Sarma D. S., Bongiorno M., Verney E. Effect of dietary tryptophan on hepatic polyribosomes and protein synthesis in fasted mice. J Biol Chem. 1968 Mar 25;243(6):1123–1132. [PubMed] [Google Scholar]
- Staehelin T., Verney E., Sidransky H. The influence of nutritional change on polyribosomes of the liver. Biochim Biophys Acta. 1967 Aug 22;145(1):105–119. doi: 10.1016/0005-2787(67)90659-4. [DOI] [PubMed] [Google Scholar]
- Tavill A. S., Craigie A., Rosenoer W. M. The measurement of the synthetic rate of albumin in man. Clin Sci. 1968 Feb;34(1):1–28. [PubMed] [Google Scholar]
- Wannemacher R. W., Cooper W. K., Yatvin M. B. The regulation of protein synthesis in the liver of rats. Mechanisms of dietary amino acid control in the immature animal. Biochem J. 1968 May;107(5):615–623. doi: 10.1042/bj1070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler M. E., Gelboin H. V. Studies on the removal of endogenous messenger ribonucleic acid activity from rat liver microsomes. Effect of removal on polyuridylic acid-directed incorporation of phenylalanine. J Biol Chem. 1967 Feb 25;242(4):727–735. [PubMed] [Google Scholar]
- White S. W., Chalmers J. P., Hilder R., Korner P. I. Local thermodilution method for measuring blood flow in the portal and renal veins of the unanaesthetized rabbit. Aust J Exp Biol Med Sci. 1967 Oct;45(5):453–468. doi: 10.1038/icb.1967.45. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Hill H. Z., Hoagland M. B. Physiology of rat-liver polysomes. Protein synthesis by stable polysomes. Biochem J. 1967 May;103(2):567–572. doi: 10.1042/bj1030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. H., Hoagland M. B. Physiology of rat-liver polysomes. The stability of messenger ribonucleic acid and ribosomes. Biochem J. 1967 May;103(2):556–566. doi: 10.1042/bj1030556. [DOI] [PMC free article] [PubMed] [Google Scholar]



