Abstract
Progressive liver failure or hepatic complications of the primary disease led to orthotopic liver transplantation in eight children with glycogen storage disease over a 9-year period. One patient had glycogen storage disease (GSD) type I (von Gierke disease) and seven patients had type IV GSD (Andersen disease). As previously reported [19], a 16.5-year-old-girl with GSD type I was successfully treated in 1982 by orthotopic liver transplantation under cyclosporine and steroid immunosuppression. The metabolic consequences of the disease have been eliminated, the renal function and size have remained normal, and the patient has lived a normal young adult life. A late portal venous thrombosis was treated successfully with a distal splenorenal shunt. Orthotopic liver transplantation was performed in seven children with type N GSD who had progressive hepatic failure. Two patients died early from technical complications. The other five have no evidence of recurrent hepatic amylopectinosis after 1.1–5.8 postoperative years. They have had good physical and intellectual maturation. Amylopectin was found in many extrahepatic tissues prior to surgery, but cardiopathy and skeletal myopathy have not developed after transplantation. Postoperative heart biopsies from patients showed either minimal amylopectin deposits as long as 4.5 years following transplantation or a dramatic reduction in sequential biopsies from one patient who initially had dense myocardial deposits. Serious hepatic derangement is seen most commonly in types T and IV GSD. Liver transplantation cures the hepatic manifestations of both types. The extrahepatic deposition of abnormal glycogen appears not to be problematic in type I disease, and while potentially more threatening in type IV disease, may actually exhibit signs of regression after hepatic allografting.
Keywords: Liver transplantation, Types I and IV glycogen storage disease, Amylopectinosis
Introduction
By evaluation of products of hepatic synthesis such as haptoglobin [17, 22, 29] group specific component [17, 18], and others [1, 2, 24, 32, 36] it has been demonstrated that liver homografts retain their original metabolic specificity after liver transplantation. It has been well recognized that hepatic transplantation has been effective in the treatment of certain inborn errors of metabolism that result partly or completely from defects in hepatic function [18]. Some of these, notably the glycogenoses, also result in anatomic impairment of the liver and progressive liver failure.
Type I glycogen storage disease (GSD) results in glycogen overloading in liver, kidney, and intestinal cells which are deficient in glucose 6 phosphatase [8, 23]. Hypoglycemia, deficient gluconeogenesis, and accumulation of lactic acid underlie the clinical manifestations of seizures, systemic acidosis, hyperlipidemia, and growth retardation [10]. Such pre-operative clinical signs prevailed in the first patient that was transplanted for type I GSD nearly 9 years ago.
In Type IV GSD (Andersen disease, amylopectinosis) [3], branching enzyme alpha-1, 4-glucan: alpha-1,4-glucan 6-g1ycosyl transferase activity is notably absent in hepatic tissue as well as in cultured skin fibroblasts and other tissues [6, 15]. Fatal hepatic complications usually occur by the age of 2–4 years, but in exceptional cases involvement in other organ systems may be prominent mortality factors [4, 5, 12, 14, 27, 35]. Liver transplantation for this disease was first attempted in 1972, but the recipient died 110 days later after uncontrolled rejection of his first liver and attempted retransplantation [31]. The first successful liver replacement was in September 1984 in patient 1 of the present series, and since then, we have made six more such attempts. These seven cases are the basis of this report.
Methods
Type I GSD
This liver recipient was diagnosed clinically and histologically at age 2.5 years and transplanted at age 16.5. An older sibling had died in infancy with the same disease.
Type IV GSD
The seven liver transplantations were performed between 6 September 1984 and 26 May 1989 (Table 1). All patients were males with two sets of brothers. The mean age at diagnosis was 11 months (range prenatal to 24 months) and the mean age at the time of transplantation was 29 months (11–46). The mean time from diagnosis until transplantation was 16.7 months.
Table 1.
Clinical data of patients with type IV glycogen storage disease undergoing liver transplantation
For both types of glycogenosis, orthotopic liver transplantation was done in the usual fashion, and postoperative immunosuppression was with cyclosporine and prednisone.
Clinical features
Type I GSD
As a young child the patient had symptoms of recurrent hypoglycemia, epistaxis, and growth retardation. Chemically, she had persistent systemic acidosis, marked transaminase elevation, hyperbilirubinemia and hyperlipidemia. End-to-side portacaval shunting at age 8 ameliorated most of the symptoms except for the hypoglycemia, and her clinical course until liver transplantation was characterized by persistent feeding problems. Continuous night-time feedings and occasional hyperalimentation were employed. In addition to massive hepatosplenomegaly the patient developed multiple hepatic adenomas evident on liver scanning. These became apparent about 2 years before her transplant, and with the progressive adenomatosis the liver function deteriorated and her encephalopathy worsened.
Type IV GSD
Ascites and growth delays were always noted. In all of the children, hepatomegaly and splenomegaly were massive, and there were persistent moderate to marked elevations of the transaminases. Bilirubin ranged from 6 to 170 µmol/l, five of the seven children being jaundiced. The degree of liver disease was classified as severe in five cases and moderate in two. None of the patients required treatment preoperatively for hypoglycemia.
Psychomotor and cardiac assessment
Type IV GSD
The percentile height position of the patients on Harvard growth charts was < 5 in six of the seven children and ten in the other; weight percentiles were distorted by ascites and liver mass. Gross and fine motor development and language ability generally were retarded in all seven patients. No cardiac complaints or abnormal heart findings were present preoperatively in any of the seven patients. Cardiac biopsies were not obtained, mainly for safety reasons, until after transplantation.
Tissue studies
Type I GSD
Multiple hepatic adenomas were present in the native liver. Biochemical studies of samples quick frozen at −70°C and stored on dry ice were performed by Dr. Barbara Illingworth Brown, Washington University, St. Louis. This showed the glucose 6 phosphatase activity at 0.08 µm/min per gram liver with control values in the range of 3.7–14.4. The liver contained 8.7% glycogen (normal <5%).
Type IV GSD
Pre-transplant liver biopsies as well as the whole livers were examined with light microscopy and showed PAS inclusions after diastase digestion. Electron microscopic studies demonstrated the fibrillar aggregations that are typical of amylopection [16, 25, 26]. Cultured skin fibroblasts and homogenates of liver tissue were assayed at Washington University, St. Louis, for branching enzyme activity. The phosphorylase coupled assay measured the rate of formation of inorganic phosphate from glucose-1-phosphate as glucose was polymerized to glycogen by the homogenates [6, 7, 14]. The branching enzyme activity in skin fibroblasts averaged 0.08 µmoles/min per mg protein, which was less than 10% of the activity of normal controls (Table 1). The liver homogenate results were confirmatory (not shown).
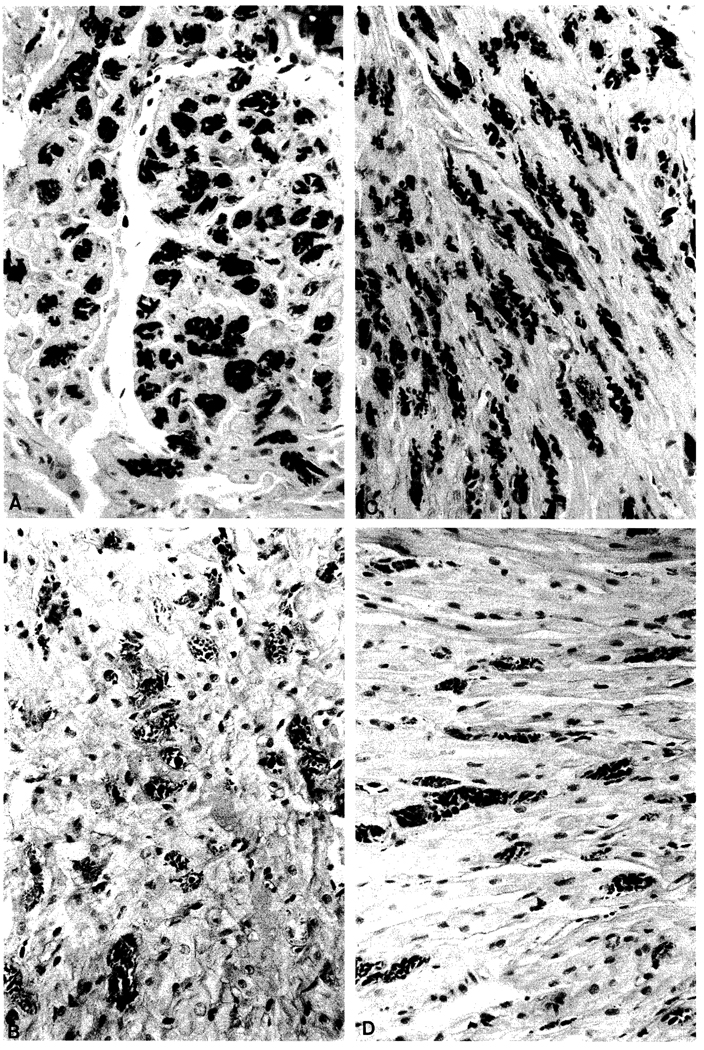
Special morphometric studies were performed on PAS diastase stained heart tissue which was obtained at autopsy in one patient and with endomyocardial biopsies in three others at variable times after transplantation (Table 2). The morphometry was done with the Bioquant System IV software on an Everex 286/12 computer with SummaSketch Plus eyepiece micrometer (Summa Graphics, 60 Silver Mine Road, Seymour, Connecticut 06483), and an Olympus BH-2 microscope. A 200 µm × 200 µm grid at 40 × magnification was randomly placed in multiple locations on each piece of PAS-diastase stained myocardium. Each inclusion was outlined in the field and the calculations made by the instrument. To eliminate artefact caused by vessels or compression, interstitial and vascular spaces in the field were eliminated from the final calculations. Sections were examined which had a predominantly longitudinal (Fig. 1B, D) or cross-sectional cut (Fig. 1A, C). To see the extent of sample variations, multiple specimens were examined from a large piece of right ventricle obtained at autopsy from patient 3. All had much the same amylopectin distribution with the single exception of a superficial subendocardial field. The technology was double checked with a point counting method which gave similar results.
Table 2.
PAS-diastase positive deposits in the myocardium of four patients with type IV glycogen storage disease after liver transplantation
| Patient | Time sample (months post-transplant) | No of inclusions per 3.6 × 105 µm2 | Mean % area occupied by inclusions | Mean size of inclusions (µm2) |
|---|---|---|---|---|
| 1 | 54.0 | 24 | 0.5% | 140 |
| 3 | 1.2 (Autopsy) | 46 | 2 % | 141 |
| 6 | 0.9 | 332 | 13 % | 146 |
| 14.0 | 129 | 5.9% | 164 | |
| 7 | 1.0 | Too few to quantitate | ||
| 11.0 | Too few to quantitate |
Fig.1.
A–D. Heart biopsies from patient 6. 3.5 weeks after liver transplantation (B, D) and 14 months later (A, C). Note the marked decrease in the number of inclusions although many of the remainder are large. The sections in the A, B panels have a more longitudinal arrangement, and those in the C, D panels are more cross-sectional. The myocardial fibers are slightly larger than before. PAS diastase stain. Original magnification × 380
Results
Survival
Type I GSD
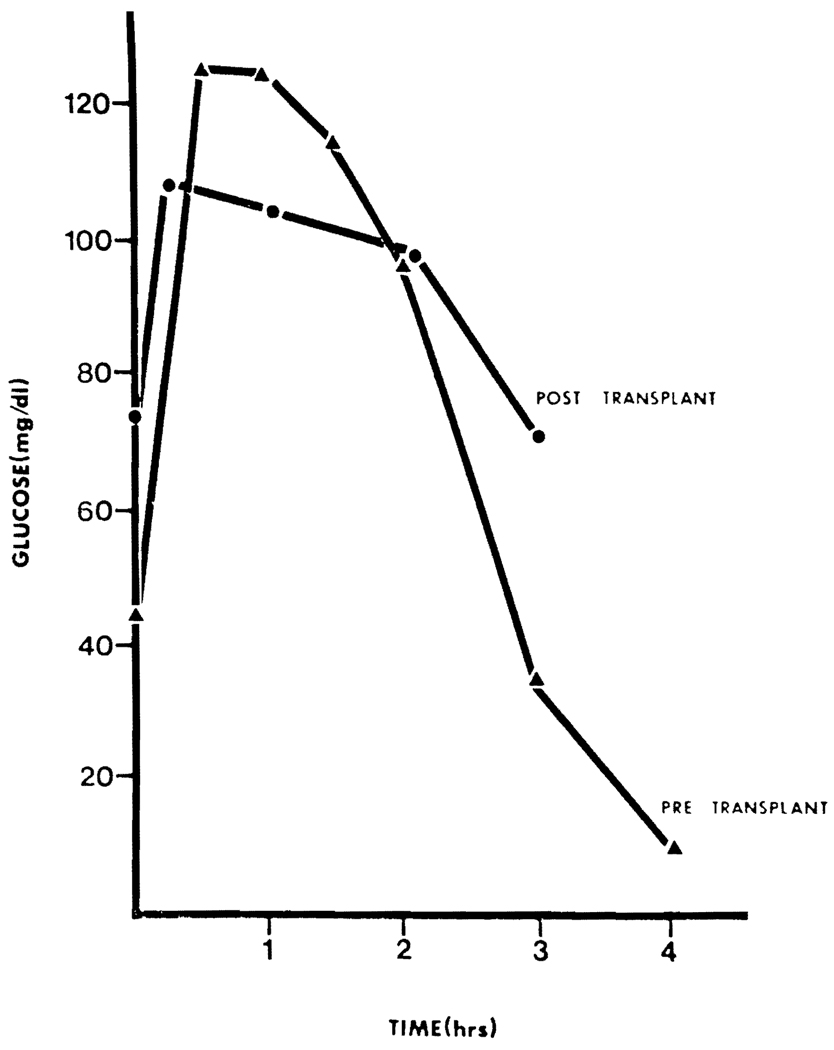
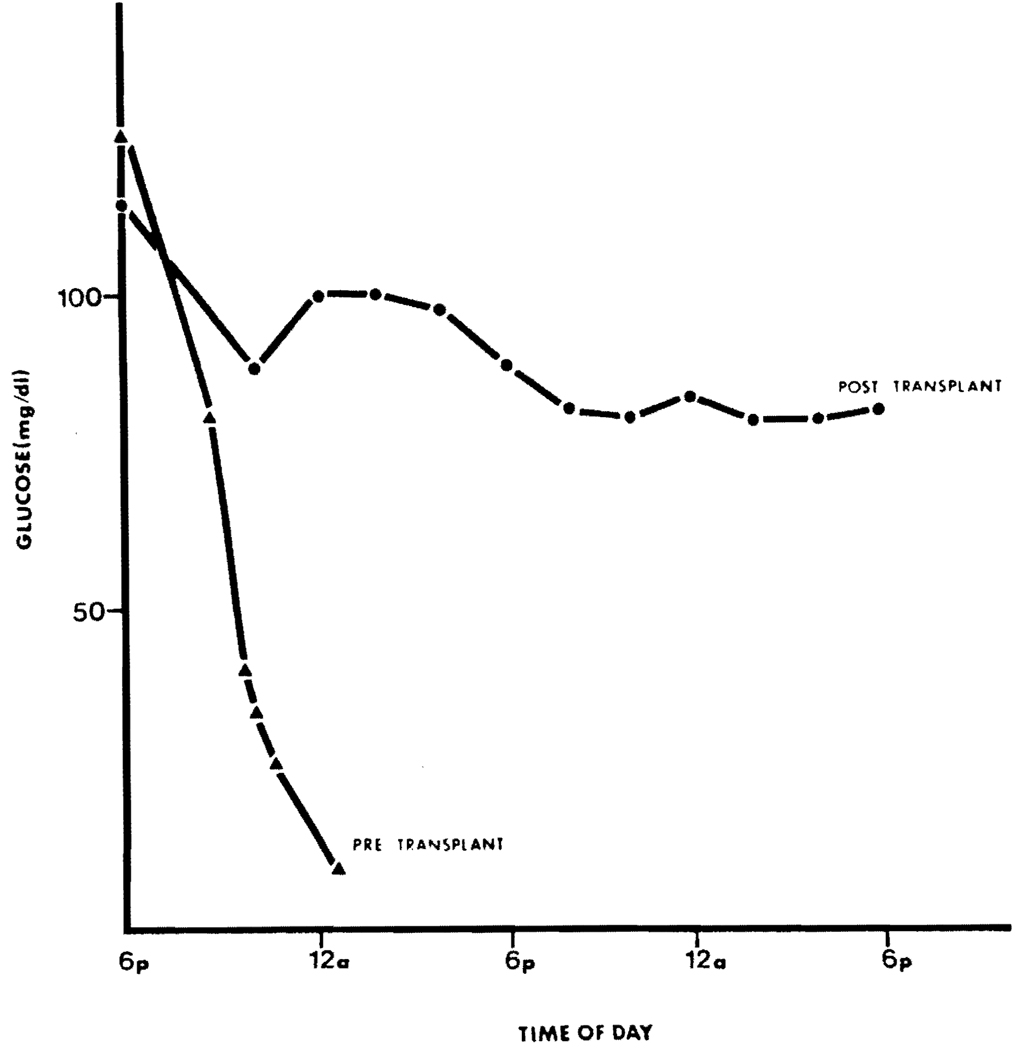
Nine years after transplantation this young adult continues to demonstrate normal carbohydrate metabolism in both fasting and challenged states (see Figs. 2, 3). She had a late portal venous thrombosis 7 months after transplantation which was treated with a distal splenorenal shunt and she now has normal bilirubin and transaminases. A renal biopsy has not been performed since both size and function of the kidneys has been normal.
Fig. 2.
Oral glucose tolerance test at age 8 years and 6 weeks after transplantation
Fig. 3.
Response of blood glucose concentrations during fasting at age 8 years, and 6 weeks after liver transplantation
Type IV GSD
Five children (71.4%) survived beyond the perioperative period. All five are still alive and healthy after a mean duration of 37.3 months (13.5–70). All have normal liver function, and with decompression of the portal hypertension via the new liver, the splenomegaly has resolved in all cases. The two deaths were caused 7 and 36 days postoperatively by septic complications from a bowel perforation in one child, and thrombosis of the hepatic artery in the other.
Studies of the transplanted livers
Type I GSD
Liver biopsy done 4 years after transplantation showed normal hepatic parenchyma and normal glycogen stores. This is based on a PAS stain showing a uniform distribution of fine granular material but varies in concentration between 1.5%–5.0% based on the nutritional state.
Type IV GSD
The liver graft lost from hepatic artery thrombosis showed extensive necrosis but contained no amylopectin. Autopsy was denied of the child who died after 7 days. The normally functioning liver grafts in the five surviving children were biopsied from 1–45 months postoperatively, as indicated for early management or arbitrarily for late follow-up. The livers were normal, and with special stains or electron microscopy, there was no evidence of amylopectin.
Cardiac studies
Type IV GSD
Chest X-ray films, clinical evaluation, and serial electrocardiographic and echocardiographic testing were done on all surviving children at frequent intervals postoperatively. In addition, one child had an MRI scan of the heart. No abnormalities were detected.
After transplantation, samples were available from four of the recipients’ hearts (Table 2). Amylopectin occupied about 2% of the area in the myocardium of the 36-month-old patient who died after 36 days (Table 2). Amylopectin was also found in his esophagus, bowel, bladder smooth muscle, skeletal muscle, central nervous system, and peripheral nerves.
Patient 1 whose liver replacement was at age 36 months, had a heart biopsy which was almost free of amylopectin 54 months later. His sibling (patient 6) was too ill preoperatively for an endomyocardial biopsy, but 3 weeks after transplantation, a biopsy in the younger boy showed involvement of 13% of the myocardial area (Table 2). Thirteen months after liver transplantation, a repeat heart biopsy showed remarkable improvement (Fig. 1) with a reduction of the amylopectin to 5.9% (Table 2). In patient 7, the heart biopsy 1 months after liver transplantation showed rare deposits in the myocytes; 10 months later, there was no change.
Psychomotor assessment
Type IV GSD
An intra-operative skeletal muscle biopsy in one of the surviving patients showed light deposits of amylopectin. The abnormal polysaccaride also was found in three of three intraoperative skin biopsies which included erector pili muscles, and in the smooth muscle of an excised segment of jejunum of one of these patients.
However, none of the five survivors has had any evidence of progressive muscular disease. All five had growth retardation before transplantation, but have developed at a normal rate afterwards, and the three with the longest postoperative follow ups have advanced into higher height or weight percentiles, or both. The physical prowess of all five children has improved.
Two of the children are performing well in grade school. Cognitive skills of the other three pre-school survivors are rated as good by the parents with no perceived abnormalities in intellectual growth. One child who was tested with the Gesell test 2 years postoperatively showed normal intellectual growth. None of the patients has myoclonus, seizures or other signs of abnormal neuromotor function.
Discussion
Since our initial report of this case in 1983 [19] there has only been one other reported case of liver transplantation for type I GSD [21] and the outcome was similar to ours with conversion to normal intermediary carbohydrate metabolism and elimination of the other metabolic derangements. Hepatic architecture remains normal in both patients and no extrahepatic organ dysfunction due to the glycogen deposition has been described in either case.
With respect to type IV GSD most of the clinical reports have emphasized variable but usually early hepatic manifestations [4, 5, 14]. However, all glycogen containing cells can be affected as was illustrated in the composite of tissue from our patients. The liver is usually the earliest casualty, but there can be an array of clinical presentations depending on the dominant organ system affected. Occasional cases of presenting cardiomyopathy [5, 12, 14, 27], neurological syndromes [9, 21] or musculoskeletal manifestations [11, 12, 35] have been reported. In these unusual patients, the clinical onset frequently is later than in the typical case of type IV disease, and death most often results from myocardial causes.
Because the metabolic phenotype of the hepatic graft permanently remains that of the donor [33], it was expected that the transplanted liver would not accumulate amylopectin. This prediction was verified. However, the question remained whether the disease would progress in other enzyme deficient organ systems. A Belgian child who underwent liver transplantation at the age of 14 months died of heart failure 11 months later. The etiology was suspected to have been the amylopectin which was demonstrated in the myocardium postoperatively and at autopsy. Amylopection was also widely distributed in other post mortem tissues and organs (personal communication Dr. Jean B. Otte, University of Louvain Medical School, Brussels, May 23, 1990).
Our observations are more optimistic. There have been no neuromuscular complications in our patients, and the generally retarded growth which was characteristic pre-transplantation has been restored toward normal in all survivors. Moreover, none of the surviving patients has had cardiac complications during 1.1 to nearly 6 postoperative years. Because a wide variety of tissues collected perioperatively in different cases contained cytoplasmic amylopectin, it must be assumed that all of the hearts had involvement. This was proved in the four hearts studied. However, the myocardial amylopectin was sparse in biopsies obtained a year or more after transplantation. Particularly striking was the diminution of amylopectin in a child whose endomyocardial serial biopsies showed a decrease in myocardial amylopectin over a period of 13 months following the liver transplantation. This child's older sibling whose only heart biopsy showed minimal heart involvement 54 months postoperatively is still well almost 6 years after liver transplantation. We speculate that he may have cleared amylopectin from his extrahepatic tissues by the time this first heart biopsy was obtained.
Finally, the possibility cannot be arbitrarily dismissed that chronic cyclosporine therapy was an ameliorating factor. This drug [20] as well as the new immunosuppressive agent FK 506 [34] have hepatotrophic qualities similar to those of insulin [30] which include enrichment of normal hepatic glycogen stores. Both of these immunosuppressive drugs attach to cytosolic binding sites which are rich in the ubiquitous enzyme peptidyl-prolyl isomerase [13, 28] and both cause wide ranging imunologic and non-immunologic effects. The action of cyclosporine and FK 506 could result from peptidyl-prolyl-isomerase inhibition and consequent triggering of second signals which may variably influence many physiologic processes including carbohydrate, cholesterol, and uric acid metabolism [34].
Acknowledgements
Aided by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland, USA
Abbreviation
- GSD
glycogen storage disease
References
- 1.Alper CA, Johnson AM, Birtch AG, Moore FD. Human C′3: Evidence for the liver as the primary site of synthesis. Science. 1969:163286–163288. doi: 10.1126/science.163.3864.286. [DOI] [PubMed] [Google Scholar]
- 2.Alper CA, Raum D, Awdeh Z, Petersen BH, Taylor PD, Starzl TE. Studies of hepatic synthesis in vivo of plasma proteins, including orosomucoid, transferrin, alpha-1-antitrypsis, C8, and Factor B. Clin Immunol Immunopath. 1980;16:84–89. doi: 10.1016/0090-1229(80)90169-5. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DH. Studies on glycogen disease with report of a case in which the glycogen was abnormal. In: Najjar A, editor. Carbohydrate metabolism. Baltimore: Johns Hopkins Press; 1952. pp. 28–42. [Google Scholar]
- 4.Bannayan GA, Dean WJ, Howell RR. Type IV glycogen storage disease. Am J Clin Path. 1976;66:702–709. doi: 10.1093/ajcp/66.4.702. [DOI] [PubMed] [Google Scholar]
- 5.Brown BI. Debranching and branching enzyme deficiencies. In: Engel AG, Banker BQ, editors. Myology. New York: McGraw-Hill; 1986. pp. 1653–1661. [Google Scholar]
- 6.Brown BI, Brown DH. Lack of an α-1,4-glucan: α-1,4-glucan 6-glycosyl transferase in a case of type IV glycogenosis. Proc Nat Acad Sci. USA. 1966;56:725–729. doi: 10.1073/pnas.56.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown BI, Brown DH. Branching enzyme activity of cultured amniocytes and chorionic villi: prenatal testing for type IV glycogen storage disease. Am J Hum Genet. 1989;44:378–381. [PMC free article] [PubMed] [Google Scholar]
- 8.Cori GT, Cori CF. Glucose-6-phosphatase in the liver in glycogen storage disease. J Biol Chem. 1952;199:661–667. [PubMed] [Google Scholar]
- 9.Ferguson IT, Mahon M, Cumming WJ. An adult case of Andersen’s disease-Type IV glycogenosis. A clinical, histochemical, ultrastructural and biochemical study. J Neurol Sci. 1983;60:337–351. doi: 10.1016/0022-510x(83)90144-2. [DOI] [PubMed] [Google Scholar]
- 10.Greene HL, Slonim AE, Burr IM. Type I glycogen storage disease: a metabolic basis for advances in treatment. Adv Pediatr. 1979;26:63–92. [PubMed] [Google Scholar]
- 11.Guerra AS, Van Diggelen OP, Carneiro F, Tsou RM, Simoes S, Santos NU. A juvenile variant of glycogenosis IV (Andersen disease) Eur J. 1986;145:179–181. doi: 10.1007/BF00446059. [DOI] [PubMed] [Google Scholar]
- 12.Greene GM, Weldon DC, Ferrans VJ, Cheatham JP, McComb RD, Brown BI, Gumbiner CH, Vanderhoff JA, Itkin PG, McManus BM. Juvenile polysaccharidosis with cardioskeletal myopathy. Arch Pathol Lab Med. 1987;111:977–982. [PubMed] [Google Scholar]
- 13.Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 14.Hers HG, Van Hoof F, De Barsy T. Glycogen storage diseases. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. 6th edn. New York: McGraw Hill; 1989. pp. 425–453. [Google Scholar]
- 15.Illingworth B, Corti GT. Structures of glycogen and amylopectins: III. Normal and abnormal human glycogen. J Biol Chem. 1952;199:653–660. [PubMed] [Google Scholar]
- 16.Ishihara T, Yokota T, Yamashita Y, Takahashi M, Kawano H, Uchino F, Kamei T, Matsumoto N, Kusunose Y, Yamada M. Comparative study of the intracytoplasmic inclusions in Lafora disease and Type IV glycogenosis by electron microscopy. Acta Pathol Jpn. 1987;37:1591–1601. doi: 10.1111/j.1440-1827.1987.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi N, Groth CG, Starzl TE. Changes in serum haptoglohin and group specific component after orthotopic liver homotransplantation in humans. Proc Soc Exp Biol Med. 1968;128:247–250. doi: 10.3181/00379727-128-32988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashiwagi N. Specila immunochemical studies. In: Starzl TE, editor. Experience in hepatic transplantation. Vol. 163. Philadelphia: WB Saunders Co; 1969. pp. 286–288. [Google Scholar]
- 19.Malatack JJ, Iwatsuki S, Gartner JL, Zitelli BJ, Roe T, Starzl TE. Liver transplantation for type I glycogen storage disease. Lancet I. 1983:1073–1075. doi: 10.1016/s0140-6736(83)91910-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazzaferro V, Porter K, Scotti-Foglieni CL, Ventakataramanan R, Makowka L, Rossaro L, Froncavilla A, Todo S, Van Thiel D, Starzl TE. The hepatotrophic effect of cyclosporine. Surgery. 1990;107:533–539. [PMC free article] [PubMed] [Google Scholar]
- 21.McMaster KR, Powers JM, Hennigar GR, Jr, Wohlbmann HJ, Farr OR., Jr Nervous system involvement in Type IV Glycogenosis. Arch Pathol Lab Med. 1979;103:105–111. [PubMed] [Google Scholar]
- 22.Merrill DA, Kirkpatrick CH, Wilson WEC, Riley CM. Change in serum haptoglobin type following human liver transplantation. Proc Soc Exp Biol Med. 1964;116:748–751. doi: 10.3181/00379727-116-29363. [DOI] [PubMed] [Google Scholar]
- 23.Nordlie RC. Glucose-6-phosphatase, hydrolytic and synthetic activities. In: Boyer PD, editor. The enzymes. New York: Academic Press; 1971. pp. 543–610. [Google Scholar]
- 24.Raum D, Marcus D, Alper CA, Levey R, Taylor PD, Starzl TE. Synthesis of human plasminogen by the liver. Science. 1980;208:1036–1037. doi: 10.1126/science.6990488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed GB, Dixon JFP, Neustein HB. Type IV glycogen. Lab Invest. 1969;19:546–557. [Google Scholar]
- 26.Schochet SS, McCormick WF, Zellweger H. Type IV glycogenosis. 1970 [PubMed] [Google Scholar]
- 27.Servidei S, Riepe R, Langston C, Tani LY, Bricher JT, CrispLindgren JT, Travers H, Armstrong D, DiMauro S. Severe cardiopathy in branching enzyme deficiency. J Pediatr. 1987;111:51–56. doi: 10.1016/s0022-3476(87)80341-4. [DOI] [PubMed] [Google Scholar]
- 28.Siekierka JJ, Hung SHY, Poe M, Lin CS, Sigal NH. A cytosolic binding protein for the immunosuppressant FK 506 has peptidyl-prolyl isomerase activity but is distinct from cyelophilin. Nature. 1989;341:755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- 29.Starzl TE, Marchioro TL, Rowlands DT, Jr, Kirkpatrick CH, Wilson WEC, Rifkind D, Waddell WR. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg. 1964;160:411–439. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet I. 1976:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 31.Starzl TE, Koep LJ, Halgrimson GC, Hood J, Schroter GPJ, Porter KA, Weil R. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Ieatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW, Jr, Hakala TR, Rosenthal JT, Porter KA. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starl TE, Demetris AJ, Van Thiel DH. Medical progress: Liver transplantation. N Engl J Med (Part I) 1989;321:1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Starzl TE, Porter KA, Mazzaferro V, Todo S, Fung J, Francavilla A. Hepatotrophic effects of FK 506 in dogs. Transplantation. doi: 10.1097/00007890-199101000-00010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zellweger H, Mueller S, Ionasescu V, Schochet SS, McCormick W. Glycogenosis IV: a new cause of infantile hypotonia. J Pediatr. 1972;80:842–844. doi: 10.1016/s0022-3476(72)80144-6. [DOI] [PubMed] [Google Scholar]
- 36.Zitelli BJ, Malatack JJ, Gartner JC, Shaw BW, Jr, Iwatsuki S, Starzl TE. Orthotopic liver transplantation in children with hepatic-based metabolic disease. Transplant Proc. 1983 (in press) [PMC free article] [PubMed] [Google Scholar]