Abstract
Matrix metalloproteinases (MMPs) play an important role in reperfusion-induced brain injury following ischemia. To define the effects of peroxynitrite decomposition catalyst on MMP activation and neurovascular reperfusion injury, FeTMPyP (5,10,15,20-tetrakis (2,4,6-trimethyl-3,5-disulfonatophenyl)-porphyrin iron (III)) was administered intravenously 30 min prior to reperfusion following a middle cerebral artery occlusion (MCAO). Activation of MMP was assessed by in situ and gel zymography. Neurovascular injury was assessed using endothelial barrier antigen (EBA), collagen IV immunohistochemistry and Cresyl violet staining. Results were compared to sham and ischemia alone groups. We found that administration of FeTMPyP just before reperfusion after ischemia inhibited MMP-9 activation and total MMP-2 increases in the cortex and decreased active MMP-9 along with the total amounts of active MMP-9 and active MMP-2 in the striatum. Reperfusion-induced injury to the basal lamina of collagen IV-immunopositive microvasculature and neural cells in cortex and striatum was ameliorated by FeTMPyP. Losses of blood vessel endothelium produced by ischemia or reperfusion were also decreased in the cortex. These results suggest that administration of FeTMPy prior to reperfusion decreases MMP activation and neurovascular injury after prolonged cerebral ischemia. This strategy may be useful for future therapies targeted at preventing breakdown of the blood-brain barrier and hemorrhagic transformation.
Keywords: Cerebral ischemia, Peroxynitrite, Matrix metalloproteinase, Brain damage, Neuroprotection
Matrix metalloproteinases (MMPs) play an important role in reperfusion-induced brain injury following ischemia (Cunningham et al. 2005; Rosell and Lo 2008). It has been reported that matrix metalloproteinase 2 (MMP-2, EC 3.4.24.24) and matrix metalloproteinase 9 (MMP-9, EC 3.4.24.35) are upregulated after transient cerebral ischemia in mouse, rat and baboon (Mun-Bryce and Rosenberg 1998; Gasche et al. 1999; Heo et al. 1999). Reperfusion following transient cerebral ischemia further accelerates and potentiates activations of MMP-2, MMP-9 and injury to microvasculature and neural cells (Lu et al. 2008)
Several metalloporphyrins react directly and catalytically to decompose peroxynitrite and attenuate the toxic effects of peroxynitrite in vitro and in vivo. These agents, including 5,10,15,20-tetrakis (2,4,6-trimethyl-3,5-disulfonatophenyl)-porphyrin iron (III) (FeTMPyP), FeTPPS and FP-15, isomerize peroxynitrite to the harmless nitrate anion (Salvemini et al. 1998; Cuzzocrea et al. 2001). Researchers have used these peroxynitrite decomposition catalysts to implicate peroxynitrite in lung, intestinal, and splanchnic ischemia–reperfusion injury (Cuzzocrea et al. 2000; Naidu et al. 2003; Stefanutti et al. 2007). In a rat MCAO model, FeTMPyP and FeTPPS treatment decreased infarct volume, brain edema and neurological deficits (Thiyagarajan et al. 2004).
Reperfusion after ischemia increases levels of reactive oxygen species, including superoxide radical (Dirnagl et al. 1995) and nitric oxide (NO) (Kumura et al. 1996; Yang et al. 2008). Oxidative radicals trigger activation of MMP-9 and MMP-2 within the ischemic mouse brain (Gasche et al. 2001). Nitric oxide directly activates MMP-9 after cerebral ischemia (Gua et al. 2002). Superoxide reacts with NO to form peroxynitrite (ONOO-). Oxygen-glucose deprivation or reperfusion after cerebral ischemia increases peroxynitrite in cerebral endothelial cells and neurons (Eliasson et al. 1999; Xu et al. 2000; Gursoy-Ozdemir et al. 2000; Kidd et al. 2005). Peroxynitrite is more permeable through lipid membrane than superoxide (Marla et al. 1997) and is more toxic (Gobbel et al. 1997). In vitro, peroxynitrite strongly activates MMP-1, −2, −8 and −9, (Rajagopalan et al. 1996; Okamoto et al. 1997; Okamoto et al. 2001) and inactivates tissue inhibitors of metalloproteinase-1 (TIMP-1) (Frears et al. 1996). It is possible that peroxynitrite formation after cerebral ischemia and reperfusion could activate MMPs, which contribute to neurovascular injury.
In the present study, we test whether administration of a peroxynitrite decomposition catalyst prior to reperfusion, after prolonged cerebral ischemia, decreases MMP activation and neurovascular injury. A sutured-rat middle cerebral artery occlusion model (MCAO) was used to produce focal ischemia and to test effects of reperfusion. We administered FeTMPyP to a subset of rats 30 min before reperfusion to assess its effects on reperfusion related MMP activation and neurovascular injury after MCAO.
Methods and materials
Animals
Male Sprague–Dawley rats weighing approximately 290–320 g were employed in the experiment. Rats were allowed to access to food and water ad libitum and were housed in a 12-h light–dark cycle. All experimental procedures were approved by the University of Cincinnati Animal Care Committee and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Middle cerebral artery occlusion model
To induce focal ischemia, a standard intraluminal middle cerebral artery occlusion method was used as previously described (Lu et al. 2008). Briefly, each rat was anesthetized with 3% isoflurane and maintained with 1.5% isoflurane in 28.5% oxygen and 70% nitrous oxide via a face mask. During surgery, the rectal temperature of all animals was maintained at 37 ± 0.5 °C with a feedback-controlled heating blanket. Following a midline incision, the left common carotid artery, external carotid artery (ECA) and internal carotid artery (ICA) were freed from surrounding tissues, and their branches were electrocoagulated. A 3/0 monofilament nylon suture was inserted into the ECA and advanced into the ICA until the tip occluded the junction of the MCA and anterior cerebral artery. The wound was closed and the suture was kept in place for 3.5 h. Rats were re-anesthetized, the neck skin was reopened and the nylon suture was removed to achieve reperfusion. EMLA cream was used at wound site for post-operation analgesia as needed. Intracranial reperfusion was confirmed by a laser Doppler flow-meter (5 mm lateral and 2 mm posterior to bregma) (Lu et al. 2008). Blood pressure was monitored through tail artery canalization. To prevent hypothermia after surgery, rats were transferred to a temperature-controlled incubator at 37 °C for 30 min until animals completely woke up, and then they were transferred to cages with a Delta Phase Isothermal Pad (Braintree Scientific, Inc., Braintree, MA, USA).
Experimental groups
Animals were divided into four groups (N = 8 for each group, total 32 rats): the sham operation group, the permanent ischemia group, the ischemia-reperfusion group and the ischemia-reperfusion with FeTMPyP group. In each group, half animals were used for gel zymogram and other half for in situ zymography, immunohistochemistry and Cresyl violet staining. The surgical procedure of sham operation group was same as the other three groups, but there was no suture insertion and occlusion of MCA. In the permanent ischemia group, the suture was not removed. In the ischemia-reperfusion group, the suture was removed at 3.5 h after MCAO. The FeTMPyP group was administered FeTMPyP (3 mg/kg in 0.9% saline, EMD Biosciences, Inc., San Diego, CA) intravenously 3 h after MCAO using an infusion pump over a period of 30 min, with reperfusion 3.5 h after MCAO. The dose of the FeTMPyP was chosen from a previous report which showed that it had better protection against brain infarction than lower dose (Thiyagarajan et al. 2004). The other three groups were given 0.9 % saline intravenously 3 h after sham operation or ischemia over a period of 30 min. All rats were sacrificed at 8 h after the sham operation or the onset of ischemia.
In situ zymography
Rats were sacrificed and brains were frozen in 2-methylbutane 8 h after MCAO. Frozen coronal sections (20 µm) were prepared using a microtome and subjected to gelatin in situ zymography. When the intramolecularly quenched FITC-labeled DQ gelatin was cleaved by MMPs, fluorescent light was released (Gasche et al. 2001; Jourquin et al. 2003). Sections were incubated with reaction buffer containing 100 µg/ml FITC-labeled DQ gelatin for 3 h at 37 °C, rinsed in phosphate butter solution (PBS), and mounted in ProLong® Gold antifade reagent (Invitrogen, Carlsbad, CA USA). Images were taken using fluorescence microscopy under constant exposure. To measure the fluorescent intensity of gelatinolytic activity, sections at 0.2 and −0.3 mm to bregma were analyzed where there are a good morphology of both striatum and cortex and reliable neurovascular injury. The somatosensory cortex was divided into two areas, lower and upper, based on a previous study (Aoki et al. 2002). Four images from each area were obtained at 20× resolution. Similarly, the striatum was divided into four regions (Fig. 1), and one 20× image was taken in each region.
Fig. 1.
Quantification was performed in the somatosensory cortex and striatum. The cortex was divided into lower and upper areas. Four 20× images from each area were obtained. Similarly, the striatum was divided into four regions, and one 20× image was taken in each region.
MMP gel zymogram
Metalloproteinase extraction from brains was performed according to a previously described method (Gasche et al. 1999; Lu et al. 2008). Briefly, brains were quickly removed and sliced into six 2-mm coronal slices at 4 °C. The cortex and striatum in the 2nd, 3rd, and 4th slices were dissected and homogenized immediately in a lysis buffer (50 mmol, L Tris-HCl pH 7.6, 150 mmol, L NaCl, 5 mmol/L CaCl2, 0.05% BRIJ-35, 0.02% NaN3, 1% Triton X-100). The homogenates were centrifuged at 12,000×g for 20 min. The supernatants were recovered and total protein concentrations were measured (BCA kit, Pierce, Rockford, IL, U.S.A.). The supernatant were incubated with gelatin-Sepharose 4B. After centrifugation, the pellets were eluted with elution buffer containing 10% dimethyl sulfoxide. The proteins in the eluent were separated by electrophoresis through 10% polyacrylamide zymogram gels containing gelatin (Invitrogen, Carlsbad, California, USA). After washing in 2.5% Triton X-100 to remove SDS, the gels were incubated overnight in a developing buffer (50 mM Tris-HCl, pH 7.5, 10 mM CaCl2, 1mM ZnCl2, and 0.05% BRIJ-35) at 37 °C. The gels were then stained with 0.1% Coomassie blue and destained. Activity of MMP, represented by clear digested regions, was photographed and analyzed by an automated MCID imaging system.
Immunohistochemistry and Cresyl violet staining
Immunohistochemistry analysis and cresyl violet staining were performed as previously described (Lu et al. 2008). Briefly, frozen coronal sections (20 µm) were used. After fixing in cold acetone or 2% paraformaldehyde for 10 min, sections were blocked for 1.5 h in blocking buffer (2% serum, 0.2% Triton X-100, 0.1% BSA in 0.1M PBS). Following incubation with primary antibodies, section were washed in PBS and incubated with biotinylated secondary antibodies for 1.5 h (Vector Labs). Next, ABC reagent (Vector Labs) was applied to sections after three 5-min PBS washes. Finally, diaminobenzidine was used to visualize the HRP. The primary antibodies used in the experiment were mouse anti-rat endothelial barrier antigen (EBA) antibody (SMI71, 1:1000; Sternberger Monoclonals, Lutherville, MD, USA) and mouse anti-rat collagen IV monoclonal antibody (M3F7; 1:200; Developmental Studies Hybridoma Bank at the University of Iowa, USA). The EBA serves as a marker of injury to endothelial cells. Anti-EBA recognizes an endothelial protein expressed in areas of the blood-brain barrier (BBB) or the blood-spinal cord barrier. Injury to the cerebral microvascular basal lamina after cerebral ischemia and reperfusion was assessed using collagen IV immunohistochemistry. To assess neuronal and glial cell injury, some sections were stained in 1% filtered cresyl violet solution.
For tissue measurements and counting, the cortex and striatum were photographed (10× images) in the same areas described above for in situ zymography. Based on previously published data (Lin and Ginsberg 2000; Zhang et al. 2006), the percentage of the EBA- or collagen IV-positive microvascular area in the measured image area (% of stained area) and the average microvascular number in each square millimeter of the image (numbers/mm2) were measured and calculated using the MCID imaging system. The average microvascular size, which is the microvascular area in the image divided by the total microvascular number in the image, was also calculated. Similarly, we calculated the areas, numbers and size of changes in neuronal cells in Cresyl violet stained sections.
Statistical analysis
Quantitative data are expressed as mean ± SD. Statistical comparisons were conducted using ANOVA followed by Tukey tests for inter-group comparisons. Differences with p < 0.05 were considered statistically significant.
Results
The Effects of FeTMPyP on cerebral blood flow and mean arterial blood pressure after ischemia and reperfusion
Regional cerebral blood flow (rCBF) is measured in ischemia-reperfusion cortex. After ischemia, perfusion dropped to approximately 18 % of baseline value (Table 1). The degree of ischemia was not statistically different between the groups. Reperfusion produced more than 80% recovery of average rCBF. FeTMPyP had no effect on rCBF (Table 1). Mean arterial pressure (MABP) was also monitored. Administration of FeTMPyP 30 min prior to reperfusion had no significant effect on MABP (monitored at 10 min after reperfusion) compared with the ischemia-reperfusion group (87.2 ± 3.8 mmHg vs 91.4 ± 8.5 mmHg, p > 0.05).
Table 1.
The change of regional cerebral blood flow after ischemia-reperfusion
| Groups | Before MCAO |
10 min after MCAO |
3 h 40 min after MCAO (10 min after reperfusion) |
8 h after MCAO |
|---|---|---|---|---|
| PMCAO 8 h | 100 | 17.8 ± 4.8** | 18.4 ± 8.1** | 17.3 ± 9.6** |
| I 3.5 h/R 4.5 h | 100 | 18.4 ± 4.3** | 82.1 ± 21.5 | 86.9 ± 28.1 |
| I 3.5 h/R 4.5 h + FeTMPyP |
100 | 18.5 ± 5.3** | 93.4 ± 29.4 | 82.7 ± 23.2 |
PMCAO: permanent middle cerebral artery occlusion. I: ischemia. R: reperfusion (n =4). FeTPyP: peroxynitrite decomposition catalyst.
p < 0.01 compared with before MCAO.
FeTMPyP reduces gelatinolytic activity of MMPs in ischemia-reperfusion rats
Using FITC-labeled DQ gelatin as substrate, in situ zymograms showed significant activations of gelatinolytic enzymes 8 h after permanent ischemia in cortex (Fig. 2a, c; p < 0.01) and striatum (Fig. 2b, d; p < 0.01) as compared to sham control. In comparison to permanent ischemia, 3.5 h of focal ischemia followed by 4.5 h of reperfusion (ischemia 3.5 h, R 4.5 h; 8 h total) markedly increased gelatinolytic enzyme activity in cortex (Fig. 2c; p < 0.01) and striatum (Fig. 2d; p < 0.01). Administered 30 min prior to reperfusion, FeTMPyP significantly reduced the reperfusion-induced activation of gelatinolytic enzymes both in the cortex (Fig. 2a, c; p < 0.01) and striatum (Fig. 2b, d; p < 0.05).
Fig. 2. In Situ gelatinolysis assays and quantification of fluorescent intensities in adult rats after focal MCA ischemia.
a, b Representative images show the effects of reperfusion and FeTPyP (peroxynitrite decomposition catalyst) on gelatinolytic activity in cortex (a) and striatum (b) after 3.5 h cerebral ischemia, scale bar = 100 µm; c. Quantification of fluorescent intensities in image a (c); d. Quantification of fluorescent intensities in image b (d). I = ischemia, R = reperfusion (n = 4). ## p < 0.01 compared with sham control. ** p < 0.01 compared with ischemia for 8 h. &p < 0.05 and && p < 0.01 compared with ischemia-reperfusion group.
Activation of MMPs and the effects of FeTMPyp after reperfusion were further evaluated and quantified using gelatin gel zymograms. Basal levels of MMP-2 and −9 were detected in the sham-operated control group (Fig. 3a, b). In the cortex, 8 h of permanent focal ischemia significantly increased pro- and active MMP-9 (Fig. 3a, c; p < 0.01) and total MMP-2 (Fig. 3a, d; p < 0.05) relative to the sham group. Reperfusion after 3.5 h of ischemia further elevated both pro- and active MMP-9 (Fig. 3a, c; p < 0.05) and total MMP-2 (Fig. 3a, d; p < 0.05) compared to the permanent ischemia group. Administration of FeTMPyP just before reperfusion inhibited MMP-9 activation and total MMP-2 increase, compared with the ischemia -reperfusion group (Fig. 3a, c, d; p < 0.05). In the striatum, pro- and active MMP-9 were increased in both the permanent ischemia group and the ischemia-reperfusion group, compared with sham controls (Fig. 3b, e; p < 0.05 for pro-MMP-9; p < 0.01 for active MMP-9). Active MMP-9 was markedly decreased by FeTMPyP relative to the 8-h permanent ischemia rats (Fig. 3e; p < 0.05). Ischemia for 3.5 h followed by 4.5 h of reperfusion also significantly increased pro-MMP-2 (Fig. 3b, f; p < 0.01), active MMP-2 (not shown; p < 0.05) and the total activity of active MMP-9 plus active MMP-2 (Fig. 3f; p < 0.01) compared with the sham control group. Administration of FeTMPyP just before reperfusion markedly reduced the total amount of active MMPs (active MMP-9 plus active MMP-2) in the striatum relative to the sham, permanent ischemia and ischemia-reperfusion groups (Fig. 3f; p < 0.05).
Fig. 3. Gel zymogram and quantification of band density in cerebral cortex (a, c and d) and in striatum (b, e and f) of adult rats after focal MCA ischemia.
I = ischemia. R = reperfusion (n = 4). #p < 0.05, ##p < 0.01 compared with sham group. * p < 0.05 compared with ischemia 8 h group. &p < 0.05 compared with I 3.5 h R 4.5 h group. MCA ischemia for 3.5 h followed by 4.5 h of reperfusion increased MMP-9 activation, total MMP-2 level in the cortex (a, c and d) and total activities of active MMP-9 plus active MMP-2 in the striatum (b and f). Administering FeTMPyP just before reperfusion (30 min before) decreased MMP activations (c and f)
FeTMPyP protects the basal lamina from reperfusion injury
The loss of collagen type IV was significant both in the cerebral cortex and striatum following 8 h of permanent ischemia, as determined by the percentage of collagen type IV-stained area compared to sham controls (Fig. 4a–d; p < 0.01). The collagen IV-positive microvessels were degraded to short segments with decreased average microvascular size (Table 2; p < 0.05 in the cortex; p < 0.01 in the striatum). Moreover, reperfusion significantly accelerated the degradation of collagen type IV as compared to ischemia alone. The percentages of collagen type IV-stained areas were further decreased in the cortex (Fig. 4c and d; p < 0.01 in the cortex; p < 0.05 in the striatum). The integrity of the microvasculature was also further jeopardized by a decreased average microvascular size in the cortex and striatum (Table 2; p < 0.05). In contrast, FeTMPyP ameliorated the reperfusion-induced deterioration from basal laminar injury in both the cortex and striatum. Loss of collagen type IV was significant decreased (Fig. 4c and d; p < 0.01). The integrity of microvasculature (average microvascular size) was also improved (Table 2; p < 0.01 in the cortex; p < 0.05 in the striatum).
Fig. 4. Collagen IV immunohistochemistry and quantification of the collagen IV staining in adult rats after focal MCA ischemia.
a and b. Representative figures (10×) in cortex (a) and striatum (b), scale bar = 100 µm; c,d. Quantification of collagen IV immunopositive microvasculature (% of collagen IV-stained area) in cortex (c) and striatum (d); I = ischemia, R = reperfusion. # p < 0.05 and ## p < 0.01 compared with sham control,*p <0.05 and ** p < 0.01 compared with permanent ischemia for 8 h. && p < 0.01 compared with ischemia-reperfusion group. Reperfusion after 3.5 h MCAO accelerated injury to basal lamina of collagen IV immunopositive microvasculature. Administration of FeTPyP just before reperfusion protected against the injury.
Table 2.
The effects of FeTPyP on average microvascular size and numbers of collagen IV-immunoreactive microvessels in cortex and striatum.
| Groups | Cortex | Striatum | ||
|---|---|---|---|---|
| Average microvascular size (µm2/profile) |
Microvascular numbers/mm2 tissue |
Average microvascular size (µm2/profile) |
Microvascular numbers/mm2 tissue |
|
| Sham | 311.9 ± 24.7 | 256.3 ± 23.7 | 315.0 ± 22.2 | 222.8 ± 24.4 |
| PMCAO 8 h | 225.4 ± 42.2# | 262.6 ± 46.8 | 176.4 ± 6.3## | 253.0 ± 24.7 |
| Isch 3.5 h/R 4.5 h | 137.8 ± 10.0*## | 294.2 ± 38.2 | 125.1 ± 9.8*## | 274.4 ± 25.4 |
| Isch 3.5 h/R 4.5 h + FeTPyP |
211.0 ± 27.0&&## | 303.6 ± 40.9 | 208.0 ± 38.6&## | 258.5 ± 28.9 |
PMCAO: permanent middle cerebral artery occlusion. Isch: ischemia. R: reperfusion (n = 4). FeTPyP: peroxynitrite decomposition catalyst.
p < 0.05 and
p < 0.01 compared with sham control,
p < 0.05 compared with permanent ischemia 8 h,
p < 0.05 and
p < 0.01 compared with ischemia-reperfusion group.
FeTMPyP protects endothelial cells from reperfusion injury
Endothelial barrier antigen (EBA) immunohistochemistry was performed to assess injury to microvascular endothelial cells after reperfusion and with FeTMPyP protection.
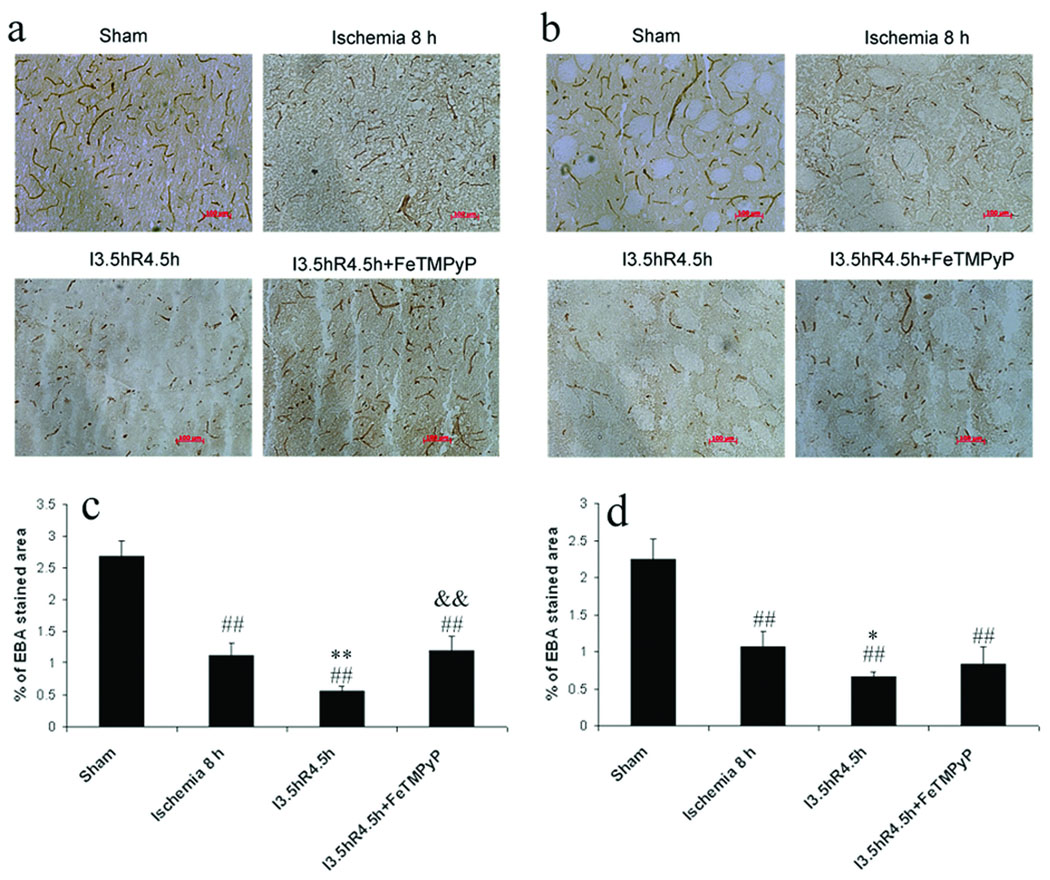
EBA-immunoreactive microvessels were significantly reduced following 8 h of permanent ischemia, as compared to sham controls. The percentage of EBA-stained areas and the average microvascular size were smaller in the cortex (Fig. 5a, c; Table 3; p < 0.01) and striatum (Fig. 5 b, d; p < 0.01, and Table 3; p < 0.05). Reperfusion after 3.5 h of ischemia markedly accelerated the injury to vascular endothelial cells. Many EBA-immunoreactive microvessels disappeared or degraded to short segments (Fig. 5a, b). The percentages of EBA-stained endothelial areas and microvacular numbers were significantly decreased in the cortex (Fig. 5c; p < 0.01, and Table 3; p <0.05) and in the striatum (Fig. 5 b, d, p < 0.05, and Table 3; p < 0.01). When FeTMPyP was administered 30 min before reperfusion, reperfusion-induced injury of blood vessel endothelia was ameliorated in the cortex where the microvascular area and microvascular numbers had been significantly increased, relative to the ischemia-reperfusion group (Fig. 5 c and Table 3; p < 0.01).
Fig. 5. EBA immunohistochemistry and quantification of the EBA staining in adult rats after focal MCA ischemia.
a and b. Representative figures (10×) in cortex (a) and striatum (b), scale bar = 100 µm; c,d. Quantification of EBA immunopositive microvasculature (% of EBA-stained area) in cortex (c) and striatum (d); I = ischemia, R = reperfusion. ## p < 0.01 compared with sham control, * p < 0.05 and ** p < 0.01 compared with permanent ischemia 8 h, && p < 0.01 compared with ischemia-reperfusion group. Reperfusion after 3.5 h MCAO accelerated injury to the microvasculature endothelium. Administration of FeTPyP just before reperfusion protected against the injury.
Table 3.
The effects of FeTPyP on average microvascular sizes and numbers of EBA-immunoreactive microvessels in cortex and striatum.
| Groups | Cortex | Striatum | ||
|---|---|---|---|---|
| Average microvascular size(µm2/profile) |
Microvascular numbers/mm2 tissue |
Average microvascular size (µm2/profile) |
Microvascular numbers/mm2 tissue |
|
| Sham | 139.8 ± 13.4 | 193.0 ± 20.5 | 109.3 ± 20.3 | 212.0 ± 38.8 |
| PMCAO 8 h | 61.2 ± 14.8## | 193.3 ± 46.9 | 65.2 ± 6.5# | 162.0 ± 20.9 |
| Isch 3.5 h/R 4.5 h | 60.1 ± 8.8## | 104.8 ± 11.7*## | 63.9 ± 10.7# | 106.5 ± 23.5**## |
| Isch 3.5 h/R 4.5 h + FeTPyP |
67.8 ± 12.9## | 179.1 ± 22.1&& | 56.9 ± 10.5## | 150.5 ± 47.3 |
PMCAO: permanent middle cerebral artery occlusion. Isch: ischemia. R: reperfusion(n = 4). FeTPyP: peroxynitrite decomposition catalyst.
p < 0.05 and
p < 0.01 compared with sham control,
p < 0.05 and
p < 0.01 compared with permanent ischemia 8 h,
p < 0.01 compared with ischemia-reperfusion group.
FeTMPyP protects neural cells from reperfusion injury
Cresyl violet staining was performed to assess injury to neuronal cells after reperfusion and with FeTMPyP protection. Relative to sham animals, 8 h of permanent MCAO significantly decreased the areas of Cresyl violet-stained cells (% of stained areas and average cell sizes) in the cortex and striatum (Fig. 6a, c and Table 4; p < 0.01) and cell numbers in the striatum (Table 4; p < 0.05). Reperfusion markedly accelerated damage to neuronal cells. The percentage of Cresyl violet-stained areas and the total numbers of cells was further decreased relative to the permanent 8-h ischemia group (Fig. 6; p < 0.01, and Table 4; p < 0.05). Administration of FeTMPyP just before reperfusion significantly reversed the decreases of total cell areas and numbers relative to the ischemia-reperfusion group (Fig. 6c, d; p < 0.0 1, and Table 4; p < 0.05).
Fig. 6. Cresyl violet staining and quantification of the Cresyl violet staining in adult rats after focal MCA ischemia.
a and b. Representative figures (10×) in cortex (a) and striatum (b), scale bar = 100 µm; c,d. Quantification of neuronal and glial cells (% of Cresyl violet stained area) in cortex (c) and striatum (d); I = ischemia, R = reperfusion. ## p < 0.01 compared with sham control. ** p < 0.01 compared with permanent ischemia 8 h. && p < 0.01 compared with ischemia-reperfusion group. Reperfusion after 3.5 h MCAO accelerated injury to neuronal and glial cells, as assessed by Cresyl violet staining. Administration of FeTPyP just before reperfusion protected against the injury.
Table 4.
The effects of FeTPyP on average cell sizes and numbers of neural cells in cortex and striatum.
| Groups | Cortex | Striatum | ||
|---|---|---|---|---|
| Average cell Size (µm2/profile) |
Cell numbers/mm2 tissue |
Average cell size(µm2/profile) |
Cell numbers/mm2 tissue |
|
| Sham | 52.7 ± 5.1 | 1586.0 ± 73.2 | 44.4 ± 1.8 | 1618.0 ± 84.1 |
| PMCAO 8 h | 27.2 ± 2.4## | 1491.5 ± 138.2 | 24.7 ± 1.3## | 1248.1 ± 189.5# |
| Isch 3.5 h/R 4.5 h | 24.9 ± 4.6## | 1105.0 ± 271.9*# | 21.4 ± 3.2## | 835.7 ± 203.4*## |
| Isch 3.5 h/R 4.5 h + FeTPyP |
33.2.6 ± 1.6& | 1634.3 ± 156.4& | 26.1 ± 4.0## | 1251.8 ± 244.3& |
PMCAO: permanent middle cerebral artery occlusion. Isch: ischemia. R: reperfusion(n = 4). FeTPyP: peroxynitrite decomposition catalyst.
p < 0.05 and
p < 0.01 compared with sham control,
p < 0.05 compared with permanent ischemia 8 h,
p < 0.05 compared with ischemia-reperfusion group.
Discussion
Our previous study showed that reperfusion after 5 h of ischemia accelerated and potentiated the activations of MMP-9 and MMP-2; further, reperfusion worsened neurovascular injury and hemorrhage transformation (Lu et al. 2008; Lu et al. 2009). In the current study, we initially assessed the effect of a peroxynitrite decomposition catalyst, FeTMPyP, on reperfusion related MMP activation. We found that administration of FeTMPyP just before reperfusion following ischemia inhibited MMP-9 activation and total MMP-2 increases in the cortex and decreased active MMP-9 along with the total amounts of active MMP-9 and active MMP-2 in the striatum. Since FeTMPyP is highly active and relative specific peroxynitrite decomposition catalyst and catalyzes the isomerization of peroxynitrite almost exclusively to nitrate (Salvemini et al. 1998; Cuzzocrea et al. 2001), we speculate that MMP-9 and −2 inhibition produced by FeTMPyP is due, at least in part, to peroxynitrite decomposition. Previous studies have shown that cerebral ischemia followed by reperfusion increases superoxide anions, NO production, and peroxynitrite formation (Kidd et al. 2005; Yang et al. 2008; Jung et al. 2009). Peroxynitrite is a strong oxidizing and nitrating agent (Beckman and Koppenol 1996). In vitro, peroxynitrite oxidizes glutathione and generates S-nitroglutathione (GSNO2), which mediates strong activation of purified human proMMPs (proMMP-1, −8, and −9) through S-glutathiolation (Okamoto et al. 2001). Peroxynitrite is 1,000 times more potent in MMP-8 activation than NO (Okamoto et al. 1997). Peroxynitrite also strongly activates human recombinant 72 kDa MMP-2 by oxidizing a cysteine switch motif in the autoinhibitory domain or by tyrosine nitration (Rajagopalan et al. 1996; Viappiani et al. 2009). In human hepatic stellate cell line LI90 cells, the peroxynitrite donor SIN-1 induces nuclear factor kappa B activation, expression of membrane-type matrix metalloproteinase-1 (EC 3.4.24.80) and the secretion of activated MMP-2 (Migita et al. 2005). Peroxynitrite also inactivates TIMP-1 at lower concentrations (< 250 µM) or even causes TIMP-1 protein fragmentation at high concentrations (>500 µM) (Frears et al. 1996). Because TIMP-1 is one of the naturally-occurring inhibitors of matrix metalloproteinases and inhibits multiple MMP activation (Murphy et al. 1994), TIMP-1 inhibition will increase levels of active MMPs. In isolated hearts, infusion of peroxynitrite results in MMP-2 activation and increases heart injury (Wang et al. 2002). Peroxynitrite formation on microvessels colocalizes with MMP-9 expression after cerebral ischemia and reperfusion (GursoyO-Ozdemir et al. 2004). These reports are in line with our results.
Sharma research group first reported that peroxynitrite decomposition catalysts (FeTMPyP and FeTPPS) decreased infarct volume, brain edema and neurological deficits after transient MCAO and the neuroprotective effect is most likely due to the reduction of peroxynitrite and apoptosis (Thiyagarajan et al. 2004). Current study further shows that administration of FeTMPyP just before reperfusion protects against injuries to basal laminar, endothelial and neural cells. Especially, our results suggest that the neurovascular protection of FeTMPyP may be due, in part, to the MMP-9 and -2 inhibition produced by FeTMPyP. The MMPs degraded matrix proteins of the basal lamina and the extracellular matrix, including collagen, fibronectin and laminin, leading to the disruption of cell-matrix interaction and affecting cell survival (Chen and Strickland 1997; Gasche et al. 1999; Lu et al. 2008). Knockout of MMP-9 or MMP inhibitors prevented degradation of matrix proteins and reduced ischemic brain damage (Asahi et al. 2000). Our results demonstrate that reperfusion after prolonged ischemia increases MMP-9 and -2 activities and leads to neurovascular injury in the cortex. Administration of FeTMPyP just before reperfusion decreased MMP-2 and −9 activities and ameliorated neurovascular injury. However, in the striatum, both permanent and transient MCAO resulted in strong MMP-2 and -9 activations (Fig. 3 e, f), whereas neurovascular injuriy was more severe in the ischemia-reperfusion animals. These results also suggest that multiple mechanisms or proteases are involved in reperfusion injury. Because administration of FeTMPyP prior to reperfusion effectively inhibited MMP-2 and MMP-9 activations and protected against neurovascular injuries, oxidative injury and related MMP activation could play a key role in future therapeutic strategy.
We attempted to quantify nitrotyrosine by immunohistochemistry to confirm that FeTMPyP was effective in decreasing nitrotyrosine-staining neural cells; unfortunately we were unable to obtain reliable results using a commercially available anti-nitrotyrosine antibody. Nevertheless, we present the first evidence here that administration of a peroxynitrite decomposition catalyst, FeTMPyP, prior to reperfusion, after prolonged cerebral ischemia, decreases MMP activity and neurovascular injury. The administration of FeTMPyP at 3 hour after MCAO matches the treatment window of tissue plasminogen activator (tPA) for stroke patients in the clinical setting. This strategy may be useful for future therapies targeted at preventing breakdown of the blood-brain barrier and hemorrhagic transformation.
Acknowledgement
This study was supported by the following NIH Grants; NS44283 (Broderick J and Clark J), NS50569 (Clark J) and NS57367 (Lu A).
Abréviations used
- FeTMPyP
5,10,15,20-tetrakis (2,4,6-trimethyl-3,5-disulfonatophenyl)-porphyrin iron (III)
- MCAO
middle cerebral artery occlusion
- MMPs
Matrix metalloproteinases
- MMP-2
matrix metalloproteinase 2
- MMP-9
matrix metalloproteinase 9
- NO
nitric oxide
- TIMP-1
tissue inhibitors of metalloproteinase-1
- EBA
endothelial barrier antigen
- rCBF
Regional cerebral blood flow
- MABP
Mean arterial pressure
References
- Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- Cuzzocrea S, Misko TP, Costantino G, Mazzon E, Micali A, Caputi AP, Macarthur H, Salvemini D. Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J. 2000;14:1061–1072. doi: 10.1096/fasebj.14.9.1061. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Lindauer U, Them A, Schreiber S, Pfister HW, Koedel U, Reszka R, Freyer D, Villringer A. Global cerebral ischemia in the rat: online monitoring of oxygen free radical production using chemiluminescence in vivo. J Cereb Blood Flow Metab. 1995;15:929–940. doi: 10.1038/jcbfm.1995.118. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frears ER, Zhang Z, Blake DR, O'Connell JP, Winyard PG. Inactivation of tissue inhibitor of metalloproteinase-1 by peroxynitrite. FEBS Lett. 1996;381:21–24. doi: 10.1016/0014-5793(96)00065-8. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Gobbel GT, Chan TY, Chan PH. Nitric oxide- and superoxide-mediated toxicity in cerebral endothelial cells. J Pharmacol Exp Ther. 1997;282:1600–1607. [PubMed] [Google Scholar]
- Gua Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31:1974–1980. doi: 10.1161/01.str.31.8.1974. [DOI] [PubMed] [Google Scholar]
- GursoyO-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Jourquin J, Tremblay E, Decanis N, Charton G, Hanessian S, Chollet AM, Le Diguardher T, Khrestchatisky M, Rivera S. Neuronal activity-dependent increase of net matrix metalloproteinase activity is associated with MMP-9 neurotoxicity after kainate. Eur J Neurosci. 2003;18:1507–1517. doi: 10.1046/j.1460-9568.2003.02876.x. [DOI] [PubMed] [Google Scholar]
- Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd GA, Hong H, Majid A, Kaufman DI, Chen AF. Inhibition of brain GTP cyclohydrolase I and tetrahydrobiopterin attenuates cerebral infarction via reducing inducible NO synthase and peroxynitrite in ischemic stroke. Stroke. 2005;36:2705–2711. doi: 10.1161/01.STR.0000190000.98707.6d. [DOI] [PubMed] [Google Scholar]
- Kumura E, Yoshimine T, Iwatsuki KI, Yamanaka K, Tanaka S, Hayakawa T, Shiga T, Kosaka H. Generation of nitric oxide and superoxide during reperfusion after focal cerebral ischemia in rats. Am J Physiol. 1996;270:C748–C752. doi: 10.1152/ajpcell.1996.270.3.C748. [DOI] [PubMed] [Google Scholar]
- Lin B, Ginsberg MD. Quantitative assessment of the normal cerebral microvasculature by endothelial barrier antigen (EBA) immunohistochemistry: application to focal cerebral ischemia. Brain Res. 2000;865:237–244. doi: 10.1016/s0006-8993(00)02228-9. [DOI] [PubMed] [Google Scholar]
- Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Khatri P, Tomsick T, Sharp FR. Mechanical reperfusion is associated with post-ischemic hemorrhage in rat brain. Exp Neurol. 2009;216:407–412. doi: 10.1016/j.expneurol.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Ran R, Khatri P, Tomsick T, Sharp FR. Reperfusion activates metalloproteinases that contribute to neurovascular injury. Exp Neurol. 2008;210:549–559. doi: 10.1016/j.expneurol.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marla SS, Lee J, Groves JT. Peroxynitrite rapidly permeates phospholipid membranes. Proc Natl Acad Sci U S A. 1997;94:14243–14248. doi: 10.1073/pnas.94.26.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita K, Maeda Y, Abiru S, Komori A, Yokoyama T, Takii Y, Nakamura M, Yatsuhashi H, Eguchi K, Ishibashi H. Peroxynitrite-mediated matrix metalloproteinase-2 activation in human hepatic stellate cells. FEBS Lett. 2005;579:3119–3125. doi: 10.1016/j.febslet.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Murphy G, Willenbrock F, Crabbe T, O'Shea M, Ward R, Atkinson S, O'Connell J, Docherty A. Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci. 1994;732:31–41. doi: 10.1111/j.1749-6632.1994.tb24722.x. [DOI] [PubMed] [Google Scholar]
- Naidu BV, Fraga C, Salzman AL, Szabo C, Verrier ED, Mulligan MS. Critical role of reactive nitrogen species in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2003;22:784–793. doi: 10.1016/s1053-2498(02)00556-9. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Wang ZQ, Stern MK, Currie MG, Misko TP. Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci U S A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanutti G, Pierro A, Smith VV, Klein NJ, Eaton S. Peroxynitrite decomposition catalyst FeTMPyP provides partial protection against intestinal ischemia and reperfusion injury in infant rats. Pediatr Res. 2007;62:43–48. doi: 10.1203/PDR.0b013e31806790c0. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Kaul CL, Sharma SS. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br J Pharmacol. 2004;142:899–911. doi: 10.1038/sj.bjp.0705811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, Leon H, van Mulligen T, Schulz R. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol. 2009;77:826–834. doi: 10.1016/j.bcp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res. 2002;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- Xu J, He L, Ahmed SH, Chen SW, Goldberg MP, Beckman JS, Hsu CY. Oxygen-glucose deprivation induces inducible nitric oxide synthase and nitrotyrosine expression in cerebral endothelial cells. Stroke. 2000;31:1744–1751. doi: 10.1161/01.str.31.7.1744. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ke-Zhou L, Ning GM, Wang ML, Zheng XX. Dynamics of nitric oxide and peroxynitrite during global brain ischemia/reperfusion in rat hippocampus: NO-sensor measurement and modeling study. Neurochem Res. 2008;33:73–80. doi: 10.1007/s11064-007-9414-x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Liu X, Hozeska A, Stagliano N, Riordan W, Lu M, Chopp M. Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb Haemost. 2006;95:166–173. [PubMed] [Google Scholar]