Abstract
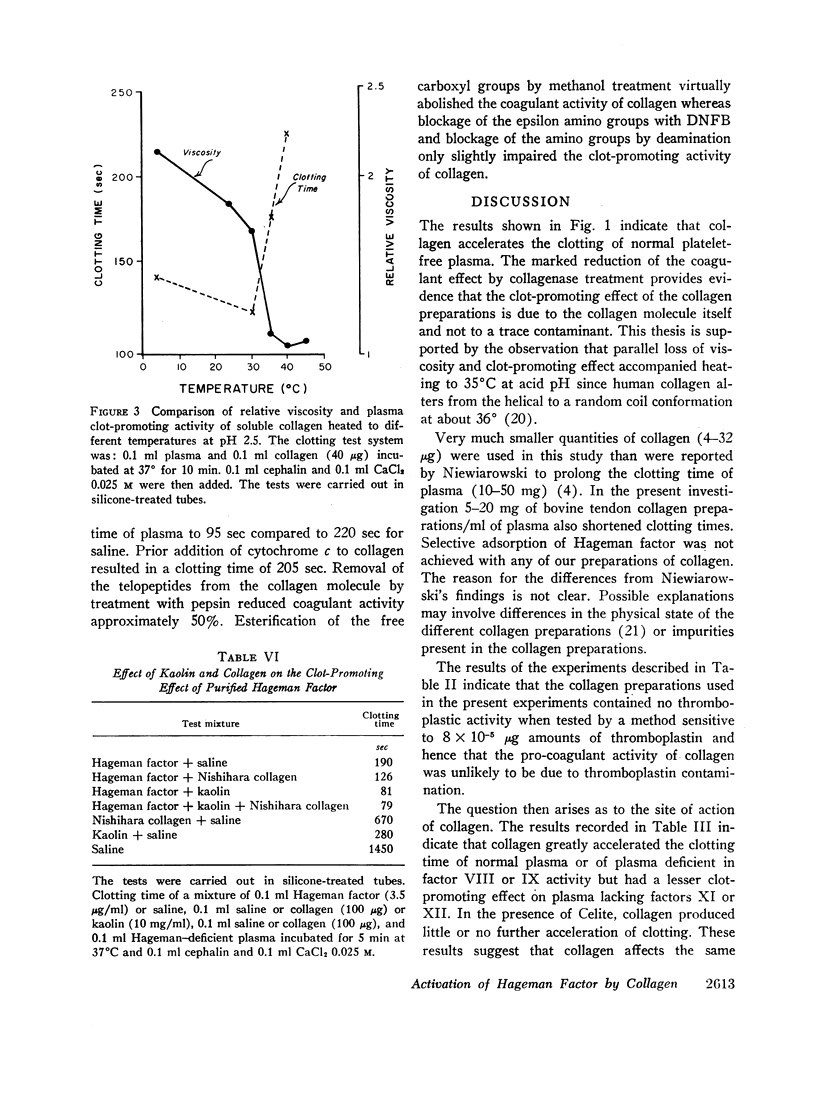
Purified acid-soluble and insoluble human collagen accelerated the clotting of plateletpoor plasma in silicone-treated tubes. The clot-promoting effect did not appear to be due to thromboplastic activity since the collagen preparations did not activate factor X in the presence of factor VII and calcium. Instead, collagen appeared to accelerate clotting by activating Hageman factor (factor XII) on the basis of the following findings: collagen increased the clot-promoting activity of partially purified Hageman factor but exerted no further effect in the presence of kaolin, a known activator of Hageman factor; clot-promoting eluates were obtained from collagen exposed to normal, hemophilic, or PTC-deficient plasma but not from collagen exposed to Hageman or PTA-deficient plasma. The collagen molecule itself appeared to be required for the clot-promoting activity since digestion with collagenase or thermal denaturation at pH 2.5 (about 35°C) resulted in very marked reduction in clot-promoting activity. Since thermal denaturation is associated with transformation of collagen structure from triple helical to random coil form, it is suggested that the native form of collagen is essential for the ability to activate Hageman factor.
Blockage of the free amino groups by treatment with nitrous acid or dinitrofluorobenzene only slightly reduced the clot-promoting activity of collagen. In contrast, since addition of cationic proteins to collagen markedly reduced pro-coagulant activity it is suggested that negatively charged sites on the collagen molecule are critical for Hageman factor activation. This suggestion is supported by the finding that pepsin treatment of collagen, which removes the predominantly negatively charged telopeptides, results in significant decrease in coagulant activity. Esterification of collagen, which neutralizes 80-90% of the free carboxyl groups, reduced coagulant activity by over 90% and it is suggested that the free carboxyl groups of glutamic and aspartic acids provide the negatively charged sites critical for Hageman factor activation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKERMAN S., HERSH R. T. EXTRACTION FROM HUMAN SKIN OF SOLUBLE COLLAGEN MOLECULES CONTAINING ONLY BETA COMPONENTS. Nature. 1964 Jan 11;201:190–191. doi: 10.1038/201190b0. [DOI] [PubMed] [Google Scholar]
- BOWES J. H., MOSS J. A. The reaction of fluorodinitrobenzene with the alpha- and beta-amino groups of collagen. Biochem J. 1953 Dec;55(5):735–741. doi: 10.1042/bj0550735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E., Conio G., Ciferri A., Puett D., Rajagh L. The role of pH, temperature, salt type, and salt concentration on the stability of the crystalline, helical, and randomly coiled forms of collagen. J Biol Chem. 1967 Apr 10;242(7):1361–1369. [PubMed] [Google Scholar]
- Bowes J. H., Kenten R. H. The effect of deamination and esterification on the reactivity of collagen. Biochem J. 1949;44(2):142–152. doi: 10.1042/bj0440142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIS J. Initiation of blood coagulation by glass and related surfaces. J Physiol. 1957 Jun 18;137(1):95–109. doi: 10.1113/jphysiol.1957.sp005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSSEL H. L. THE ACTIVATION AND CONSUMPTION OF FACTOR IX. Thromb Diath Haemorrh. 1964 Dec 31;12:505–509. [PubMed] [Google Scholar]
- Niemetz J., Nossel H. L. Method of purification and properties of anti-XIa (inhibitor of the contact product). Thromb Diath Haemorrh. 1967 May 31;17(3-4):335–348. [PubMed] [Google Scholar]
- Niewiarowski S., Bańkowski E., Fiedoruk T. Adsorption of Hageman factor (Factor XII) on collagen. Experientia. 1964 Jul 15;20(7):367–368. doi: 10.1007/BF02147961. [DOI] [PubMed] [Google Scholar]
- Niewiarowski S., Bańkowski E., Rogowicka I. Studies on the adsorption and activation of the Hageman factor (factor XII) by collagen and elastin. Thromb Diath Haemorrh. 1965 Nov 15;14(3-4):387–400. [PubMed] [Google Scholar]
- Nossel H. L., Rubin H., Drillings M., Hsieh R. Inhibition of Hageman factor activation. J Clin Invest. 1968 May;47(5):1172–1180. doi: 10.1172/JCI105806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., CRUM J. D. ACTIVATION OF HAGEMAN FACTOR BY SOLUTIONS OF ELLAGIC ACID. J Lab Clin Med. 1964 Mar;63:359–377. [PubMed] [Google Scholar]
- RATNOFF O. D., DAVIE E. W. The purification of activated Hageman factor (activated factor XII). Biochemistry. 1962 Nov;1:967–975. doi: 10.1021/bi00912a005. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., ROSENBLUM J. M. Role of Hageman factor in the initiation of clotting by glass; evidence that glass frees Hageman factor from inhibition. Am J Med. 1958 Aug;25(2):160–168. doi: 10.1016/0002-9343(58)90023-8. [DOI] [PubMed] [Google Scholar]
- SPAET T. H., CINTRON J., SPIVACK M. Some properties of the platelet-connective tissue mixed agglutination reaction. Proc Soc Exp Biol Med. 1962 Nov;111:292–295. doi: 10.3181/00379727-111-27771. [DOI] [PubMed] [Google Scholar]
- STEVEN F. S. THE NISHIHARA TECHNIQUE FOR THE SOLUBILIZATION OF COLLAGEN. APPLICATION TO THE PREPARATION OF SOLUBLE COLLAGENS FROM NORMAL AND RHEUMATOID CONNECTIVE TISSUE. Ann Rheum Dis. 1964 Jul;23:300–301. doi: 10.1136/ard.23.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven F. S., Jackson D. S. Purification and amino acid composition of monomeric and polymeric collagens. Biochem J. 1967 Aug;104(2):534–536. doi: 10.1042/bj1040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAALER B. A. Contact activation in the intrinsic blood clotting system; studies on a plasma product formed on contact with glass and similar surfaces. Scand J Clin Lab Invest. 1959;11(Suppl 37):1–133. [PubMed] [Google Scholar]



