Abstract
A clinical trial of intestinal transplantation was initiated at the University of Pittsburgh in May 1990. Eleven children received either a combined liver/small bowel graft (n = 8) or an isolated small bowel graft (n = 3). Induction as well as maintenance immunosuppression was with FK-506 and steroids. Four patients were male, and seven were female; the age range was 6 months to 10.2 years. There were 3 deaths (all in recipients of the combined liver/small bowel graft), which were attributed to graft-versus-host disease (n = 1), posttransplant lymphoproliferative disease (n = 1), and biliary leak (n = 1). Transplantation of the intestine has evolved into a feasible operation, with an overall patient and graft survival rate of 73%. These survivors are free of total parenteral nutrition, and the majority are home. These encouraging results justify further clinical trials.
THE OUTLOOK for patients with intestinal failure has changed dramatically over the last 20 years. Many physicians involved in the care of infant patients who required extensive resections of the intestine can recall when survival was considered at best “unlikely.” Survival was usually limited to patients who had the opportunity to undergo some adaptation of the remaining bowel. Advances in preoperative and postoperative management together with the development of total parenteral nutrition (TPN) have been responsible for this improvement during the acute stage of disease. The long-term prognosis for these patients varies from 65% to 80% (3-year survival) depending on the cause of intestinal failure.1 This may be less favorable in children as compared with adults because of a higher risk for TPN-induced liver dysfunction and venous access complications. The availability of home TPN has further simplified the management, although there are still significant limitations on function as individuals and in society. The expense of such lifetime therapy is estimated at $60,000 to $150,000 per year.
The technical feasibility of intestinal transplantation was pioneered in 1959 by the experimental model of Lillehei et al,2,3 who studied both autografts and allografts in dogs. Transplantation of the small intestine as part of a multivisceral graft was reported experimentally 1 year later by Starzl and Kaupp at the American Surgical Association meeting.4 This was only 1 year after experimental liver transplantation was performed in Chicago and Boston. Numerous attempts at clinical small intestinal transplantation, either alone or with the liver, were attempted between 1964 and 1987.5 Almost all attempts have failed from either graft rejection, sepsis, or technical failure, with loss of graft and, many times, of the patient. Until 1990, there were only two survivors of isolated cadaveric grafts, one in France and the other in Germany.6,7
A trial of small bowel transplantation alone or with the liver was initiated at the University of Pittsburgh in 1990 in both adults and children.8 The longest-surviving child of this series, the recipient of a combined liver-intestinal graft more than 2 years ago,8 has enjoyed a normal life-style free of TPN for essentially all of her posttransplant life.9 Although the present success of intestinal transplantation under FK-506 has been met with enthusiasm, the procedure and postoperative course is complex.
INDICATIONS
Small bowel transplantation is indicated in any patient with permanent intestinal failure who is dependent on TPN for maintenance of nutrition, fluid and electrolyte balance, and normal growth and development. There are many disease states that produce intestinal failure for varying lengths of time. Also, adaptation of the intestine permits recovery in many patients after a period of temporary TPN support. The small number of patients with permanent intestinal failure can go on to have complications related to the long-term use of TPN (eg, catheter infections and venous thrombosis). Multiple hospital admissions are usually required for intravenous (IV) antibiotic therapy and catheter changes. In some patients, thrombosis is so extensive that venous access becomes impossible. Also, TPN-induced liver dysfunction can occur and is manifested by abnormalities in liver chemistries, hepatic cholestasis, steatosis, and eventually cirrhosis with liver failure. 10
The minimum length of intestine necessary for adequate enteral absorption has not been established. Various investigators advocate anywhere between 10 and 20 cm of small intestine with an ileocecal valve, and 40 cm without one. 11,12 Other factors that influence use of the remaining small bowel include the presence of residual ileum (because of its greater potential for adaptation), ileocecal valve (slowing of intestinal transit time), presence of the colon (water absorption), and motility patterns as well as improvement of absorptive function of the remaining intestine.
Table 1 lists the indications for liver/small bowel and isolated small bowel transplantation in 11 children who received transplants between May 1990 and June 1992. The causes of intestinal failure in this group can be divided into two categories: surgical and nonsurgical. Patients with surgical causes are those who present with a small length of bowel after resection for intestinal atresias, or infarctions consequent to volvulus, necrotizing enterocolitis, vascular catastrophes (trauma or thrombosis), and gastroschisis. The length of residual intestine present has been variable. Nonsurgical causes of intestinal failure include motility disorders such as intestinal pseudoob-struction syndromes and absorptive insufficiency as is seen in microvillus inclusion disease.
Table 1.
Indications for Pediatric Small Bowel Transplantation
| No. of Patients |
|
|---|---|
| Necrotizing enterocolitis | 3 |
| Gastroschisis | 2 |
| Intestinal atresia | 1 |
| Midgut volvulus | 3 |
| Intestinal pseudoobstruction | 1 |
| Microvillous inclusion disease | 1 |
| Total | 11 |
Patients presenting with TPN-induced liver disease are candidates for liver/small bowel transplantation. The severity of the liver disease will stipulate the need for a concomitant liver transplant; however, this is not always a straightforward decision. Hyperbilirubinemia and transaminase abnormalities are insensitive guidelines. The presence of fibrosis (or cirrhosis) on liver biopsy, or portal hypertension as manifested by splenomegaly and esophageal varices, are already late manifestations of severe hepatic injury. Choosing the optimum time for transplantation in this type of patient is difficult because the clinical course and life expectancy are variable. The patients are highly susceptible to sudden unpredictable deterioration such as bleeding, sepsis, and encephalopathy.
EVALUATION OF CANDIDATES
There is no test more critical in the preoperative evaluation of a potential small bowel transplant recipient than a thorough history and physical examination. It is necessary to have a complete understanding of the cause of intestinal failure (surgical or nonsurgical) as well as possible associated defects in other organ systems.
Knowledge of all previous operative procedures and present intestinal tract anatomy is critical to the accomplishment of a smooth operative procedure. Also, segments of the remaining intestinal tract may be significantly deformed or functionally inadequate because of either previous surgery or baseline pathology (nonsurgical intestinal failure). This evaluation can be accomplished with standard barium studies of the gastrointestinal tract, motility studies, and absorption studies when appropriate.
Evaluation of hepatic integrity is by standard liver transplant evaluation protocol. Jaundice, which may or may not be present, is not a sensitive indicator in assessing critical liver injury. Tests for hepatocellular reserve using a coagulation profile, albumin level, and ammonia level are standard practice in liver transplant centers around the world. Evidence of portal hypertension includes a history of bleeding esophageal varices, the presence of splenomegaly, ascites, and caput medusae. Diagnostic upper gastrointestinal endoscopy, if not previously performed, can be useful, and it can be performed therapeutically if the patient has an episode of bleeding while under evaluation. Bleeding esophageal varices are treated with sclerotherapy.
Patency of the portal vein is required to assess for adequate drainage of the visceral organs that will remain in the recipient after transplantation (usually the stomach, duodenum, pancreas, and possibly the colon). Patency is documented by Doppler ultrasound. If occlusion is found, it should be confirmed by magnetic resonance imaging or venous phase portography. Occlusion of the portal vein does not contraindicate transplantation because in this instance a multivisceral transplant can be performed after all the native intraabdominal organs are excised.
Nutritional evaluation consists of a thorough history focusing on the present TPN formula and any type of oral diet or supplementation. Tolerance to oral intake is crucial (eating profile and stool output) because many children have not learned or have forgotten to eat. Some patients associate adverse feelings with eating. This can affect posttransplant nutritional management by delaying independence from enteral formula support. Children should be stimulated to eat before transplant, even if no nutritional benefit is gained. Baseline anthropometric measurements as well as laboratory data are collected. Most patients with only intestinal failure are in good overall medical condition and suffer more from recurrent line sepsis and vessel thrombosis. However, patients presenting with liver and intestinal failure may have significant immunologic defects as well as nutritional deficiencies (principally vitamins and trace elements), fluid and electrolyte imbalances, and often obesity. They may present a cushingoid appearance and are susceptible to serious infections, bleeding, and encephalopathy.
All patients are screened with baseline titers for cytomegalovirus (CMV) and Epstein-Barr virus (EBV). Bacterial, fungal, and viral cultures are obtained when clinically indicated. A thorough history of previous infectious complications is critical, particularly if there has been an episode of fungal infection. Table 2 outlines our present protocol for intestinal transplant evaluation.
Table 2.
Investigation of Pediatric Small Bowel Transplant Recipients
| History and physical examination |
| Etiology of intestinal failure |
| Previous surgeries |
| Associated anomalies |
| Routine laboratory data |
| Hemoglobin, leukocyte count, differential count |
| Platelet count, prothrombin time, partial thromboplastin time |
| Bilirubin, alkaline phosphatase, serum glutamic-pyruvic trans- aminase (SGPT), aspartate aminotransferase (AST) |
| Protein electrophoresis |
| α-fetoprotein |
| Urinalysis |
| Blood urea nitrogen (BUN), serum creatinine |
| 24-hour creatinine clearance |
| Nutritional evaluation |
| Weight, height, triceps skinfold, midarm circumference |
| Transferrin, albumin, prealbumin, serum amino acid analysis |
| Vitamins A, D, E, B12, thiamine |
| Triglycerides |
| Radiology |
| Upper and lower gastrointestinal barium studies |
| Liver ultrasound |
| Chest roentgenogram |
| Immunological studies |
| Blood type (ABO) |
| Tissue typing |
| Cross matching |
| Investigations for infection |
| Blood, urine, throat, feces, ascites culture: bacterial, fungal, viral |
| Hepatitis screen |
| Quantitative stool cultures |
| Absorption studies (when indicated) |
| D-Xylose absorption test |
| 72-hour fecal fat test |
| Liver biopsy (when indicated) |
THE DONOR OPERATION
Donors who are suitable for liver donation should also be suitable for small intestinal donation. No functional or anatomic assessment of the intestine is performed. We select a donor who is of similar or smaller size than the recipient because the volume of the peritoneal cavity in the recipient is usually reduced. The ABO blood group should be identical to that of the recipient; the human lymphocyte antigen (HLA) matching is random. Selective bacterial and fungal decontamination is performed in the donor according to the outline in Table 3. No attempt is made to mechanically wash the intestinal contents either before the donor operation or after the organs have been transplanted.
Table 3.
Intestinal Decontamination—Donor and Recipient
| <5 Years |
5 to 12 Years |
>12 Years |
|
|---|---|---|---|
| Amphotericin B (mg) | 100 | 250 | 500 |
| Tobramycin (mg) | 10 | 40 | 80 |
| Polymycin E (mg) | 25 | 50 | 100 |
| Systemic Antibiotics |
|---|
| Cefotaxime 25 mg/kg/dose every 8 h IV |
| Ampicilin 25 mg/kg/dose every 6 h IV |
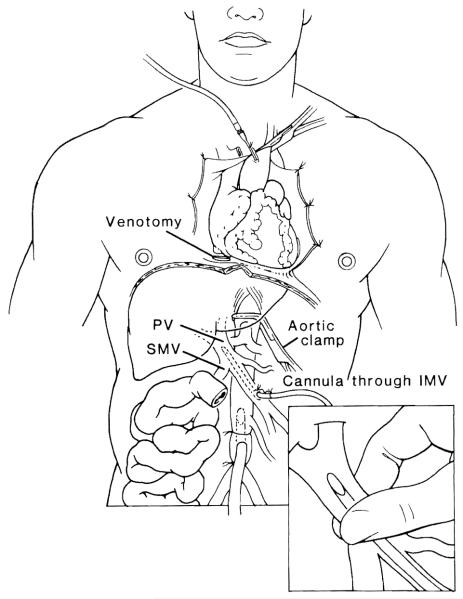
An isolated small bowel or en-bloc liver/small bowel graft usually requires approximately 4 hours of recovery time. The harvesting technique involves a hilar dissection similar to that performed in an isolated liver graft.13 The ascending and transverse colon are mobilized, and the small intestine is divided with a stapler at the ligament of Treitz and just proximal to the ileocecal valve, leaving the enteric contents undisturbed. A duodenopancreatectomy exposes the portal and superior mesenteric veins. The aorta is encircled below the diaphragm for later cross-clamping when the circulation is arrested. The distal infrarenal aorta is also encircled, and a cannula is inserted at this point for the infusion of the preservation fluid. After the proximal aorta is clamped, in situ arterial perfusion is initiated using chilled University of Wisconsin (UW) solution, and the venous bed is decompressed via a suprahepatic vena caval venotomy. The amount of fluid volume infused is variable and should be guided by blanching of the organs. Because the volume for the nonhepatic viscera is usually less, the liver can be perfused more thoroughly by cannulation of the inferior mesenteric vein or the splenic vein14 (Fig 1). Alteration of the graft lymphoreticular tissue (with the use of antilymphocyte globulin [ALG], OKT3, or irradiation) is not performed. 15 The liver and small bowel from the ligament of Treitz to the ileocecal valve are harvested en-bloc and used for liver/small bowel candidates. For cases in which only a small bowel graft is required, the graft can be separated on the back table and the liver used for another recipient.
Fig 1.
Procurement of multivisceral graft. Inset shows secondary perfusion of the liver through inferior mesenteric vein (IMV). PV, portal vein; SMV, superior mesenteric vein. (By permission of SURGERY. Gynecology & Obstetrics.14)
THE RECIPIENT OPERATION
The recipient is brought to the operating room once news from the donor team confirms adequacy of the donor organs. Because the graft is generally brought en-bloc either as a multivisceral or a liver/small bowel graft, the final decision regarding the patient’s organ needs can be made at the time of exploration. Patients presenting with jaundice and biopsy-confirmed cirrhosis, or severe fibrosis and cholestatic liver disease in which significant portal hypertension is confirmed at exploration, will receive an en-bloc liver/small bowel graft. Patients who do not present the above findings and have only chemical abnormalities (noted on liver-function tests) with no evidence of portal hypertension usually require an isolated small bowel graft only.
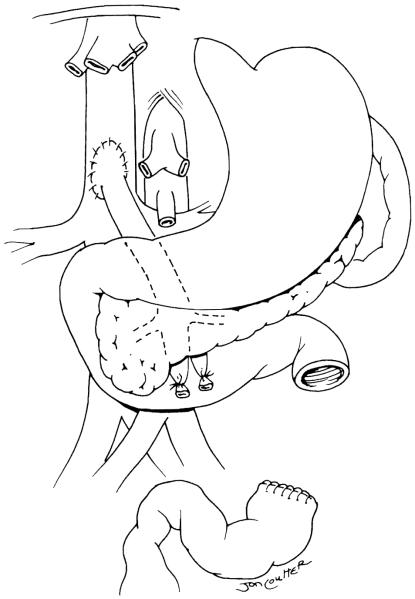
When an en-bloc liver/small bowel graft is transplanted, the abdomen is entered through previous incisions, and after takedown of adhesions the hepatic hilus is exposed. The liver is devascularized by ligating the hepatic arterial branches and the common bile duct, thus exposing the portal vein from the confluence to its bifurcation. Subsequently the portal vein is tied and sectioned, and the liver is removed in a piggyback fashion by sequentially ligating the veins draining directly into the retrohepatic inferior vena cava and then clamping the hepatic veins and removing the liver (Fig 2). A temporary portocaval shunt is used to allow decompression of the remaining splanchnic organs (stomach, duodenum, pancreas, and spleen).16 The amount of residual intestine varies from very little in patients who have had previous resections to the entire intestine in those who present with malabsorption or pseudoobstruction. We always attempt to preserve the colon and ileocecal valve. Any residual small intestine found is preserved; in patients with malabsorption or intestinal pseudoobstruction, the small intestine and colon are removed.
Fig 2.
Recipient. The liver has been removed and a portocaval shunt performed. This relieves the portal hypertension and permits completion of hemostasis. The shunt may be left permanently, or taken down and a native porto-to-donor portal shunt performed. (Reprinted with permission.9)
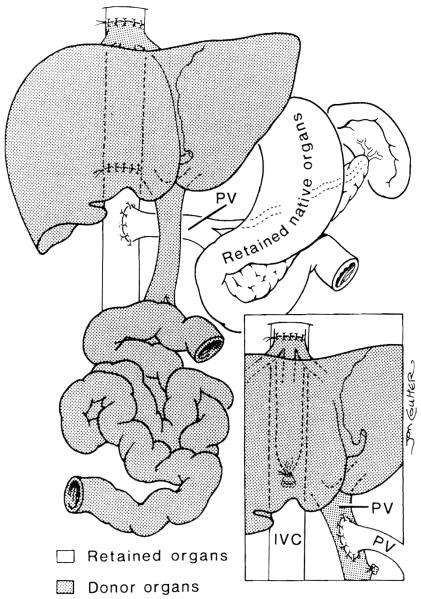
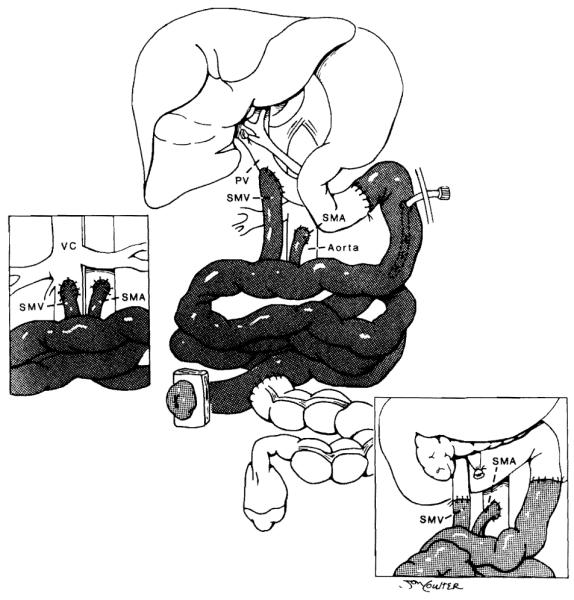
After hemostasis is accomplished, the liver/small bowel graft is brought to the operative field. The small bowel graft, with or without the liver, encompasses its entire length. The exact method of revascularization depends on the operative findings of the recipient, which can be distorted by multiple previous abdominal procedures. When the liver is removed in a piggyback fashion, the venous drainage is into the hepatic veins of the recipient. If the retrohepatic vena cava of the host is removed with the specimen, then this segment of vena cava is replaced with the graft (Fig 3). In this situation the use of veno-venous bypass is necessary to channel blood from the splanchnic system and lower body back to the heart.17 Arterialization is performed using a carrel patch of the donor aorta, which includes celiac and superior mesenteric arteries. This is anastomosed directly to the recipient aorta either above or below the renal arteries. An interposition graft of donor thoracic or abdominal aorta can also be sutured to this carrel patch, then subsequently to the recipient aorta. When the small intestine is transplanted alone, it is based on the superior mesenteric artery (with or without an aortic carrel patch) and a skeletonized segment of superior mesenteric vein or portal vein. Arterialization is from the recipient infrarenal aorta.8 Venous drainage has usually been accomplished either directly to the stump of remaining recipient superior mesenteric vein or to the recipient’s portal vein at the level of the hepatic hilus in an end-to-side fashion (piggyback technique).18 Drainage into the inferior vena cava has been performed during retransplantation in an adult recipient of a small bowel graft only (Fig 4).
Fig 3.
Venous drainage of the combined graft without and with (inset) preservation of the inferior vena cava (IVC). Inset also shows native porto-to-donor portal shunt. PV, portal vein. (By permission of SURGERY, Gynecology & Obstetrics.14)
Fig 4.
Venous drainage of isolated small bowel graft into native superior mesenteric vein (SMV), portal vein (PV), or vena cava (VC). (Reprinted with permission.8)
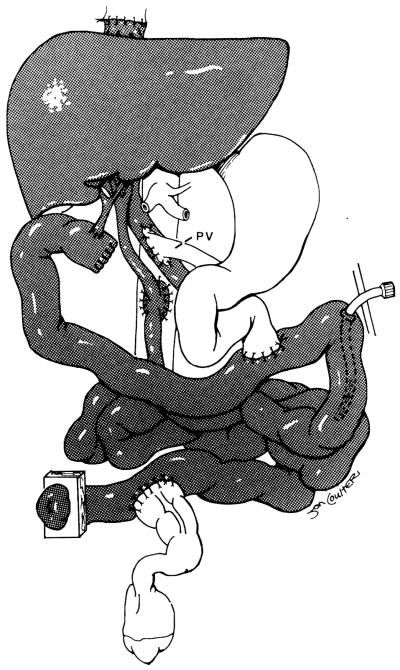
After the vascular anastomosis is completed, blood is allowed to perfuse the organs; however, the venous drainage clamp is not released until the organs are completely perfused. Bleeding is allowed to occur from the superior mesenteric vein (in an isolated small bowel graft) or from the infrahepatic vena cava (in the liver/small bowel graft). This permits drainage of the preservation solutions. After the subsequent removal of the venous drainage clamp, there may be some peaked T waves that can usually be easily treated with intravenous calcium and bicarbonate solutions. Once hemostasis is achieved, a donor cholestectomy is performed, and reconstruction of the gastrointestinal tract is accomplished with a conventional technique. In the initial two cases, both ends of the intestinal graft were exteriorized by a “chimney” method anastomosing the recipient intestines to the side of the graft near the chimney enterostomy.9 In the subsequent cases the proximal chimney was eliminated, and a tube jejunostomy was used to drain the proximal intestine. This tube is subsequently used for enteral nutrition. The biliary reconstruction is required only in liver/small bowel recipients and is performed with a Roux-en-Y choledochojejunostomy at the most proximal end of the transplanted jejunum (Fig 5).
Fig 5.
Composite graft showing biliary reconstruction to the most proximal end of transplanted jejunum, proximal enteric anastomosis, jejunostomy drainage tube, and then distal enteric anastomosis. (Reprinted with permission.8)
IMMUNOSUPPRESSION
The prospects for progress in intestinal grafting have improved dramatically with the use of FK-506 immunosuppression. To avoid nephrotoxic levels, FK-506 (0.1 to 0.15 mg/kg/d) is given by continuous intravenous (IV) infusion immediately after graft revascularization. Steady levels are targeted at between 1 and 2 ng/mL. Levels are measured daily until discharge and then subsequently 2 to 3 times per week for the first 3 months and at longer intervals thereafter. Oral FK-506 is started once intestinal motility is present, and integrity of the intestinal anastomosis is confirmed by contrast barium studies. Because FK-506 absorption is independent of biliary enterohepatic circulation,19 maintenance of adequate levels by oral dosage alone is possible early in the postoperative course. Steroid therapy in the isolated small bowel recipients consists of 1 g of IV hydrocortisone given intraoperatively, followed by methylprednisolone starting at a dose of 100 mg/d and then rapidly tapering over 5 days to a dose of 10 mg/d. Recipients of liver/small bowel grafts received only baseline steroid therapy—20 mg/d for patients weighing more than 10 kg, and 10 mg/d for patients weighing less than 10 kg. Prostaglandin E1 is administered at 0.003 to 0.009 μg/kg/min intraoperatively and then continued for 5 days. This is given for its immunosuppressive properties and the beneficial effect seen with FK-506 nephrotoxicity.20
Episodes of graft rejection (liver and/or small bowel) are treated initially with steroid bolus therapy using IV hydrocortisone or oral prednisone. Optimization of FK-506 trough levels should be performed by either increasing the baseline oral FK-506 dose or using supplemental IV FK-506. Rejection of the small bowel can alter FK-506 absorption. Steroid recycle therapy (similar to induction tapering doses) is used in cases of more severe rejection or when bolus therapy is inadequate. Use of OKT3 was not required in any of these cases; however, it has been the next line of therapy in our adult small bowel recipients when rejection has progressed on a steroid recycle. Azathioprine was used to supplement baseline immunosuppression in cases of severe rejection (two patients), and where reduction of the FK-506 dose was necessary because of nephrotoxicity (two patients).
Long-term immunosuppressive management has entailed reduction of FK-506 dosage (independent of the level) if the patient is clinically well and has a normally functioning graft. At present, only two patients are using additional steroid and azathioprine. All other patients are on monotherapy with FK-506.
INFECTION CONTROL
All donors receive selective bacterial and fungal decontamination as outlined in Table 3. The number of doses given is variable and depends on the local organ procurement circumstances; however, an initial dose at the time of acceptance of the donor followed by a second dose just before transfer to the operating room is ideal. Mechanical preparation is not performed. The recipient receives the same selective decontamination regimen. Because some candidates are hospitalized for varying periods before transplant, random screening of the intestinal flora should be performed and treated accordingly. Intestinal decontamination is continued for a minimum of 6 weeks postoperatively and should be reinstituted during episodes of rejection.
Broad-spectrum IV antibiotics (ampicillin and cefotaxime) are administered to both donor and recipient. The recipient may have a history of nosocomial infections just before transplant (bacterial and fungal), which need to be addressed appropriately. Prophylactic antibiotics are given for 5 days.
POSTOPERATIVE CARE AND RESULTS
Patients receiving a combined liver/small bowel graft usually have had significant liver failure and require the same level of intensive care as a liver-transplant recipient. They require respiratory support for at least 48 hours, and are susceptible to significant fluid shifts. Accurate management of fluid and electrolytes is critical to avoid pulmonary edema or renal failure. Similarly, the management of FK-506 infusions and any antibiotic regimen requiring peak and trough levels (such as aminoglycosides and vancomycin) should be meticulously gauged.
Chest roentgenograms are checked daily while the patient is in the intensive-care unit, and when indicated thereafter (eg, respiratory failure, fever workup, pneumonia). Daily determinations of renal function (blood urea nitrogen [BUN], creatinine), hematology (white blood cell count [WBC], platelets, hemoglobin levels), electrolytes, calcium, phosphate, magnesium, amylase, lipase, and hepatic function (bilirubin, transaminase, and alkaline phosphatase levels) are performed.
Monitoring of the intestinal graft includes a combination of clinical, endoscopic, histological, radiological, bacteriologic, and metabolic evaluations.21 The character of the intestinal graft stoma is assessed on a daily basis for color, friability, and stomal output (color, consistency, presence of blood, presence of reducing substances as tested by pH and clinitest). Endoscopic evaluation through the ileostomy with mucosal biopsies (minimum of five samples) is performed twice a week for the first month and whenever clinically indicated thereafter.
Most patients with intestinal allograft rejection presented a combination of fever, abdominal pain and distension, nausea or vomiting, and an initial increase in stomal output. In cases of severe rejection, graft ileus and absence of stomal output can occur, as well as intestinal bleeding from mucosal sloughing. A septic-shock picture can be seen with or without the presence of bacterial translocation (concomitant presence of the same infectious organism in both blood and stool). This has been documented on multiple occasions and represents one of the few transplant situations in which treatment of sepsis entails not only antibiotic coverage but also, more importantly, additional immunosuppression. Intestinal decontamination should continue during episodes of rejection; stomal cultures must be assessed for overgrowth (≥ 108 is considered significant).
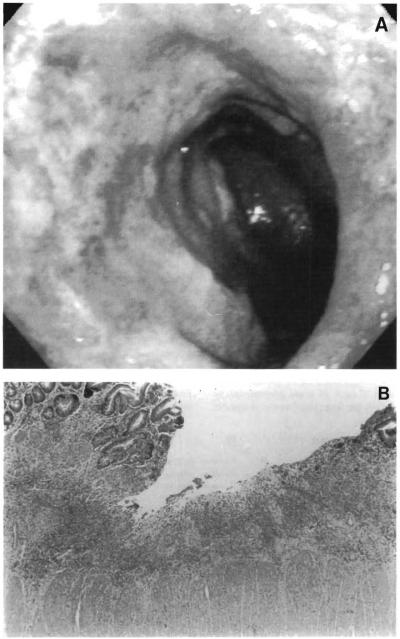
Endoscopically acute rejection episodes are documented by an ischemic or dusky mucosa with focal ulcerations. A nodular mucosal pattern with diffuse ulcerations or sloughing of large areas of mucosa can be seen in cases of severe rejection (Fig 6A). Absence of peristalsis can be determined at endoscopy and confirmed by barium studies.
Fig 6.
(A) Endoscopic photograph displaying exfoliation of mucosa caused by rejection in a stiff aperistaltic intestine. (B) Photomicrograph or transplanted intestinal mucosa showing evidence or severe rejection .
The histological criteria for the diagnosis of acute intestinal allograft rejection include mononuclear cell infiltrates, villous blunting, and cryptitis. Complete mucosal and crypt destruction are seen in patients who have severe rejection (Fig 6B). The reader is referred to a more complete analysis of intestinal transplant pathology in this group of children and adults from this institution.22
The results reported herein pertain to 11 children who received intestinal transplants. Eight transplants were in combination with the liver, and three were solitary. There were seven girls and four boys, with ages ranging from 6 months to 10 years. The specifics of these patients and their outcomes are detailed in Table 4.
Table 4.
Clinical Characteristics
| Patient No. |
Age at Transplant (mo) |
Primary Disease | Previous Surgery | Type of Transplant |
Survival (mo) |
|---|---|---|---|---|---|
| 1 | 38 | Necrotizing enterocolitis | SB resection, jejunostomy | LSB | 30 |
| 2 | 52 | Gastroschisis | SB resection | LSB | 26 |
| 3 | 33 | Necrotizing enterocolitis | SB resection | LSB | 14 |
| 4 | 6 | Intestinal atresia | Total enterocolectomy | LSB | .7 |
| 5 | 13 | Midgut volvulus | SB + right colectomy, duodenos- tomy |
LSB | 17 |
| 6 | 19 | Midgut volvulus | SB resection | LSB | 14 |
| 7 | 31 | Microvillous inclusion | None | SB | 13 |
| 8 | 14 | Midgut volvulus | SB resection | SB | 10 |
| 9 | 123 | Intestinal pseudo obstruction | SB resection | SB | 10 |
| 10 | 18 | Necrotizing enterocolitis | SB resection | LSB | 2.5 |
| 11 | 48 | Gastroschisis | SB resection | LSB | 6 |
Abbreviations: SB, small bowel; LSB, liver/small bowel.
Two of the three patients who received small bowel grafts experienced at least one episode of rejection; one of these patients had only one rejection episode. The other had six episodes of rejection; however, all were treated successfully with bolus steroid therapy and optimization of FK-506 trough levels. None of these episodes was severe.
Of the eight liver/small bowel recipients, five patients experienced at least one episode of rejection of the intestinal graft (3.4 episodes per patient), and four patients had at least one episode of rejection of the liver graft (5.8 episodes per patient). Two patients had no episodes of rejection, and only one patient had two episodes of concomitant rejection of the liver and small bowel components of the graft. Of these patients, two had severe rejection of the intestinal graft with mucosal sloughing, requiring TPN support during treatment. The diagnosis and incidence of liver graft rejection in patients with a combined graft were similar to those experienced by a control group of recipients who received only a liver.23 No incidence of chronic rejection was seen in these pediatric patients; however, it has occurred in an adult recipient of an isolated small bowel graft.
Patients who received the combined liver/small bowel transplant required a longer intensive care unit stay (mean, 37 days) than patients who received an isolated small bowel transplant (mean, 8 days) as well as a longer total hospital stay (mean, 4.5 months versus mean, 2.3 months for an isolated small bowel transplant). This can be explained by the fact that candidates for a liver/small bowel graft generally have end-stage liver disease with many of its associated complications. In addition, the procedure requires significantly more operative time and blood transfusions. The incidence of infection and technical complications is also significantly higher.
The only acceptable standard of success with intestinal transplantation is independence from TPN. Therefore, the functional assessment of the transplanted small bowel is critical and can be divided into three phases. Phase 1 begins with perfusion of the intestinal graft and ends when initial stomal output occurs postoperatively (usually between days 5 and 8). The intestinal graft usually perfuses rapidly; however, there may be segments of initial venous congestion and spasm that require careful manipulation and positioning of the graft as well as irrigation with warm saline solutions. Peristalsis may be present; however, more often than not the intestine remains aperistaltic. This is reflected postoperatively by a significant period of ileus. Ischemic damage to the graft may occur during this time and be manifested by congestion, edema, and aperistalsis. Mucosal sloughing and bleeding may also occur. Phase 2 encompasses the period when rejection episodes are most commonly encountered (after the first week) and treated in the manner as described in previous sections. Intestinal motility begins to recover at this time, and the passage of stool through the ileostomy signals the moment to begin enteral feeding. During phases 1 and 2, adequacy of perfusion and control of rejection are the goals permitting recovery of the intestinal graft, and they are best reflected by the presence of peristalsis and stomal outputs. This can be confirmed by a gastrointestinal barium study showing adequacy of the intestinal anastomosis, peristalsis, and the contour of the mucosal surface. Some degree of mild mucosal edema can be seen at this stage. Phase 3 begins at this time.
Although minimal amounts of enteral formulas can be infused through the jejunostomy before phase 3, progress in weaning of TPN solutions is not accomplished until after phase 3 has begun. Nutritional management during this time of adaptation has consisted of balanced TPN solutions using dextrose, crystalline amino acids, and fat emulsions sufficient to provide 100 kcal/kg/d administered via the central vein. Enteral feedings are begun using standard formulas (Tolerex [Sandoz Nutrition, Minneapolis Falls, MN], Peptamen [Clintec Nutrition Co, Deerfield, IL], Compleat-B [Sandoz Nutrition, Minneapolis Falls, MN]) and advanced as tolerated. Elemental formulas have not been well tolerated, and our present standard is the use of dipeptide formulas such as Peptamen. Continuous feedings are provided by a nasogastric or nasoduodenal tubes, gastrostomy tubes (with extension tube past the pylorus), or jejunostomy tube (into the transplanted bowel). Daily stomal outputs are measured for volume, pH, presence of reducing substances, and quantitative bacterial cultures.
Weight at operation and at latest follow-up as well as the length of time required for complete adaptation to enteral feedings (independence from TPN) are measured. Changes in height are assessed in patients less than 18 years of age. Steady weight gain on enteral feeding alone was a major criterion for hospital discharge. Significant laboratory data include a total serum protein level, albumin level, transferrin level, and vitamin levels.
All surviving patients are presently off TPN. Recipients of liver/small bowel grafts usually require a mean of 80 days to become independent of TPN, whereas the recipients of small bowel grafts are weaned from TPN at approximately 30 days posttransplant. Because children have either not learned to eat or have acquired an aversion to food, enteral supplementation is required. Only one child is presently maintained on oral intake alone.
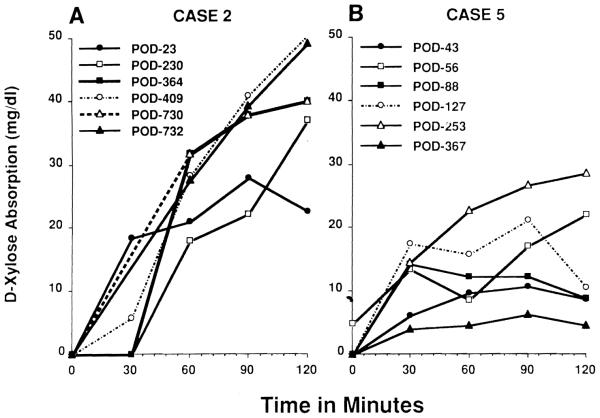
Functional studies included the absorption of D-xylose, and FK-506 as well as fecal fat excretion. Abnormalities in absorption, increased stomal output, or dysmotility (rapid or slow transit) prompted aggressive immunologic workup. Satisfactory absorption curves of D-xylose were documented for all patients at some point during the postoperative course. Peak values ranged from 15 to 20 ng/dL. Results improved with time as the transplanted bowel recovered normal motility and enteral feedings were advanced (Fig 7A). Abnormal results occurred during episodes of rejection (both acute and chronic) and reflected both absorptive dysfunction as well as dysmotility and hypersecretion that may accompany rejection (Fig 7B).
Fig 7.
(A) D-xylose absorption curves for patient no. 2, showing progressive improvement up to 732 days posttransplant. (B) Patient no. 5 had initial improvement followed by deterioration correlating with severe rejection and then the development of posttransplant lymphoproliferative disease.
The excretion of fat in the stool was abnormal in almost all patients and tended to be more so in the early postoperative course. Fat absorption has improved with time and has normalized in two patients. No child has presented clinical steatorrhea; however, one adult small bowel transplant recipient with chronic rejection and pancreatitis had significant steatorrhea. One child with significant fat excretion in the stool had consistent bacterial overgrowth.
Oral FK-506 was initiated at 7 to 14 days posttransplant and usually overlapped with IV FK-506. Adequate absorption was reflected by maintenance of satisfactory blood trough levels of IV therapy, which occurred between 7 and 46 days postoperatively (mean, 27.9 days) in the liver/small bowel recipients and between 19 and 44 days (mean, 28.2 days) in the small bowel only recipients.
Total protein and albumin levels have improved and have been maintained in all children postoperatively. The degree of improvement and maintenance has been similar for recipients of an isolated small bowel and liver/small bowel grafts.
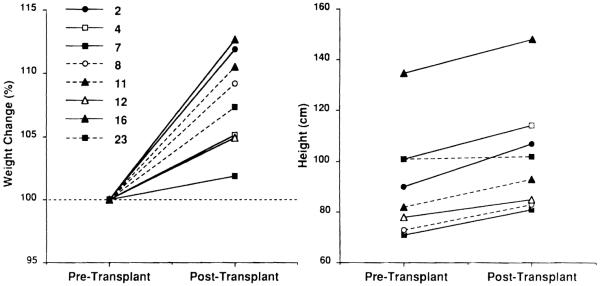
Weight has increased steadily in all children, with percentile increments of 2% to 10% as compared with pretransplant weight (Fig 8A). More importantly, growth (a predictable attribute of health in children) has also occurred at a satisfactory rate. Normal growth rates have been observed for all children24 (Fig 8B).
Fig 8.
Weight and height changes after small bowel transplantation in eight children.
Stomal outputs tended to be high during the initial postoperative period. Any change in volume (either more or less) prompted an aggressive search for rejection. Bacterial overgrowth in the presence of high stomal output was treated with oral antibiotics; however its relevance is still under study.
Paregoric, loperamide, Imodium, pectin, somatostatin, or oral antibiotics were used as appropriate when high stomal outputs occurred. Sodium bicarbonate was added to the formula or given IV if metabolic acidosis was present. Initial aversion to food has been the rule in most children, and they require a prolonged period of adaptation and well-being to learn the joy of eating.
Radiological evaluations performed early after transplantation were valuable in assessing the mucosal pattern, which was normal in most patients. Transit times varied from 30 minutes to 5 hours (mean, 2 hours). Some evidence of mucosal edema has been seen in the presence of intestinal graft rejection. In cases of severe rejection with exfoliation of the mucosa, there was ablation of normal mucosal pattern and dysmotility. There were significant abnormalities of the native (recipient) proximal gastrointestinal tract, characterized by delayed gastric emptying in one child and a severely hypotonic and dilated duodenum in another.
There were three deaths, all recipients of the combined liver/small bowel graft. One patient had an immunodeficiency pretransplant characterized by low levels of immunoglobulin G and M, as well as abnormal T- and B-cell function. Pneumocysystis carinii pneumonia was diagnosed in the patient on the second postoperative day, and subsequently a leak from the proximal intestinal anastomosis was observed. Immunosuppression was reduced drastically. Erythema of the abdominal wall developed, and multiple skin biopsies did not show graft-versus-host disease (GVHD) until the 21st postoperative day, when apoptosis was noted. Reinstitution of therapeutic immunosuppression was ineffective, and the patient died of multisystem organ failure. We attribute such severe GVHD to inadequate immunosuppression in the face of a surgical complication, and an inherent immunodeficiency disorder. This complication has not occurred in any other recipient of an intestinal graft.
One patient suffered from paralysis of the right hemidiaphragm secondary to phrenic nerve injury. This necessitated prolonged ventilatory support and tracheostomy. He later experienced multiple episodes of rejection that were treated with steroid boluses, azathioprine, and an increase in baseline immunosuppression (FK-506 and steroids). The patient had pleomorphic lymphoproliferative disease of the intestinal allograft, and he died of sepsis and liver failure 13 months after transplantation. Another patient had a leak from the choledochojejunostomy, with sepsis and subsequently severe rejection with mucosal sloughing of the intestinal allograft. He died of sepsis 72 days after transplantation.
In this population there was a high incidence of infectious complications, which included bacterial, fungal, and viral organisms. Translocation of organisms has occurred in the early postoperative course and can be an early indicator of small bowel graft rejection. This translocation has included both bacterial (Enterococcus, Staphylococcus aureus) and fungal agents (Candida albicans). Viral infections included CMV, adenovirus, and EBV.25 Posttransplant lymphoproliferative disease (PTLD) occurred in two patients, both recipients of liver/small bowel grafts. Both patients had multifocal disease and were treated with IV acyclovir and withholding of immunosuppression. One patient died of this complication and was described above. The other child survived the PTLD and later presented rejection of allograft. This was treated successfully with steroids and reinstitution of FK-506 immunosuppression. The patient is alive & well, and was off TPN 9 months after successful treatment (16 months after transplantation).
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Howard L, Heaphey L, Fleming R, et al. Four years of North American registry home parenteral nutrition outcome data and their implications for patient management. J Parenter Enter Nutr. 1991;15:384–393. doi: 10.1177/0148607191015004384. [DOI] [PubMed] [Google Scholar]
- 2.Lillehei RC, Goott B, Miller FA. The physiological response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543–560. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillehei RC, Idezuki Y, Feemster JA, et al. Transplantation of the stomach, intestine and pancreas: Experimental and clinical observations. Surgery. 1967;62:721–741. [PubMed] [Google Scholar]
- 4.Starzl TE, Kaupp HA., Jr Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Grant D. Intestinal transplantation: Current status. Transplant Proc. 1989;21:2869–2871. [PubMed] [Google Scholar]
- 6.Goulet O, Revillon Y, Jan D, et al. Small-bowel transplantation in children. Transplant Proc. 1990;22:2499. [PubMed] [Google Scholar]
- 7.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: Report of a case. Clin Transplant. 1989;3:89. [Google Scholar]
- 8.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–224. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzakis AG, Todo S, Reyes J, et al. Liver and small bowel transplantation for short gut syndrome in child. Transplant Sci. 1991;1:27–33. [Google Scholar]
- 10.Steiger E, Srp F. Morbidity and mortality related to home parenteral nutrition in patients with gut failure. Am J Surg. 1983;145:102–105. doi: 10.1016/0002-9610(83)90174-5. [DOI] [PubMed] [Google Scholar]
- 11.Grosfeld J, Rescoria FJ, West KW. Short bowel syndrome in infancy and childhood: Analysis of survival in 60 patients. Am J Surg. 1986;151:41–46. doi: 10.1016/0002-9610(86)90009-7. [DOI] [PubMed] [Google Scholar]
- 12.Wilmore DW. Factors correlating with a successful outcome following extensive intestinal resection in newborn infants. J Pediatr. 1972;80:88–95. doi: 10.1016/s0022-3476(72)80459-1. [DOI] [PubMed] [Google Scholar]
- 13.Starzl TE, Hakala TR, Shaw BW, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Todo S, Tzakis AG, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 15.Casavilla A, Selby R, Abu-Elmagd K, et al. Logistic and technique for combined hepatic-intestinal retrieval. Ann Surg. 1992;216:605–609. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzakis AG, Reyes J, Nour B, et al. Temporary end-to-side portocaval shunt in orthotopic liver transplantation. Surg Gynecol Obstet. 1993;176:181–182. [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzakis AG, Todo S, Reyes J, et al. Piggyback orthotopic intestinal transplantation. Surg Gynecol Obstet. 1993;176:297–298. [PMC free article] [PubMed] [Google Scholar]
- 19.Venkataramanan R, Jain A, Warty VW, et al. Pharmacokinetics of FK-506 following oral administration: A comparison of FK-506 and cyclosporine. Transplant Proc. 1991;23:931–933. [PMC free article] [PubMed] [Google Scholar]
- 20.Takaya S, Iwaki Y, Starzl TE. Liver transplantation in positive cytotoxic crossmatch cases using FK-506, high dose steroids, and prostaglandin E1. Transplantation. 1992;54:927–929. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Elmagd K, Tzakis A, Todo S, et al. Monitoring and treatment of intestinal allograft rejection in humans. Transplant Proc. 1993;25:1202–1203. [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K, Abu-Elmagd K, Todo S, et al. Pathology of human small intestinal transplantation: Alone or in combination with the liver. Hepatology. in press. [Google Scholar]
- 23.Jain AB, Fung JJ, Todo S, et al. Incidence and treatment of rejection episodes in primary orthotopic liver transplantation under FK-506. Transplant Proc. 1991;23:928–930. [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes J, Tzakis AG, Todo S, et al. Nutritional management of intestinal transplant recipients. Transplant Proc. 1993;25:1200–1201. [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes J, Abu-Elmagd K, Tzakis A, et al. Infectious complications after human small bowel transplantation. Transplant Proc. 1992;24:1249–1250. [PMC free article] [PubMed] [Google Scholar]