Abstract
Numerous clinical reports have documented an increase in trough blood concentrations of cyclosporine in transplant recipients treated concomitantly with ketoconazole. The objective of this study was to elucidate the mechanism(s) underlying the cyclosporine-ketoconazole interaction using a choledochoureterostomy dog model. Five male beagle dogs received a 4 mg/kg, i.v. bolus dose of cyclosporine either alone or on day seven of a 10-day, 13 mg/kg/day, oral dosing regimen of ketoconazole. Blood samples were collected prior to and at predetermined times for 60 hrs after the cyclosporine dose, while the bile/urine mixture was collected quantitatively for 96 hours after the cyclosporine dose. Ketoconazole decreased the systemic clearance of cyclosporine from 7.0 ml/min/kg to 2.5 ml/min/kg. The terminal disposition rate constant was also decreased significantly from 0.0794 to 0.0354 hrs−1. Ketoconazole caused no significant changes in cyclosporine steady state volume of distribution, or plasma unbound fraction. Ketoconazole did not significantly alter the excretion of cyclosporine and various cyclosporine metabolites in the bile/urine mixture. Inhibition of hepatic drug metabolizing enzymes appears to be the primary reason for the ketoconazole induced elevation in cyclosporine concentration.
INTRODUCTION
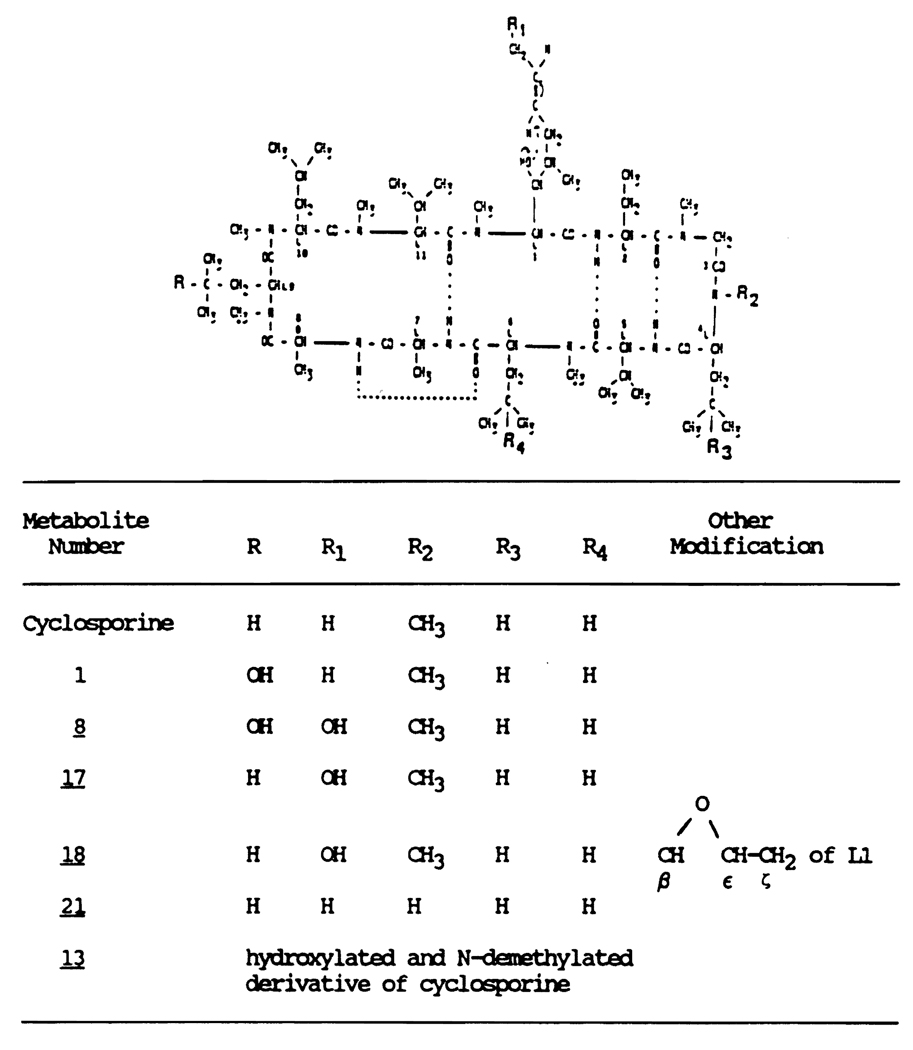
Cyclosporine (CYA) is a unique immunosuppressant used to prevent the rejection of transplanted organs and to treat diseases of autoimmune origin. Cyclosporine is a neutral cyclic polypeptide consisting of 11 amino-acids (Figure 1). Cyclosporine undergoes extensive hepatic metabolism in humans and animals. The biotransformation pathway of CYA is qualitatively similar in all the animal species studied (Beveridge, 1982; Maurer et al.,1984; Venkataramanan, 1988). Of the 17 suspected metabolites of CYA, 11 have been isolated and identified (Maurer et al., 1984). All the identified metabolites have the intact cyclic oligopeptide structure of the parent drug. Structural modifications during metabolism include mono- and dihydroxylation as well as N-demethylation (Figure 1). Less than 1% of the administered dose a CYA is excreted in the bile as parent drug while greater than 44% of the dose appeared as CYA metabolites (Venkataramanan 1985a; Wood et al., 1983). Patients who receive CYA may require antifungal therapy. Ketoconazole is an orally active antifungal drug. Recent clinical reports indicate marked increases in blood trough concentration of CYA along with an increase in serum creatinine in transplant recipients treated concomitantly with ketoconazole (Morgenstern et al., 1982; Ferguson et al., 1982; Dieperink and Moller, 1982; White et al., 1984). However the mechanisms underlying this interaction have not been completely characterized.
Figure 1.
Structure of cyclosporine and its metabolites.
The objective of the present study was to comprehensively examine the effect of ketoconazole on the plasma protein binding, hepatic metabolism, and biliary excretion of CYA.
MATERIALS AND METHODS
Chemicals
Cyclosporine (Sandimmune Injectable - Sandoz) and ketoconazole (Nizoral tablets - Janssen) were purchased from commercial sources. Cyclosporin D and cyclosporin G were gifts from Sandoz, Inc. Other chemicals and solvents were purchased from Fisher Scientific Co. (Fairlawn, NJ) and were of ACS reagent grade and HPLC grade, respectively.
Study Protocol
The study was performed in five male beagle dogs weighing between 13.5–18.0 kg. A choledochoureterostomy was performed in all five dogs approximately six months prior to the present study. The common bile duct was ligated close to the duodenum and connected to the urinary bladder using a plastic stent. In the control phase the dogs received a 4 mg/kg i.v. bolus dose of CYA through a catheter placed in the femoral leg vein. In the treatment phase the dogs received a 13 mg/kg/day oral dose of ketoconazole for 10 days. On day seven of ketoconazole therapy the dogs received a 4 mg/kg, i.v. bolus dose of CYA, one hour after the regular daily dose of ketoconazole. In both phases of the study, the dogs were fasted for 14 hours prior to and for four hours after the CYA dose. A one week washout period was allowed between the two phases. Intravenous CYA solutions were freshly prepared on the day of the study by diluting the commercially available preparation (Sandimmune™ I.V.; Sandoz Inc., Basel, Switzerland) with sterile saline. Ketoconazole was administered orally as a suspension. Commercially available tablets (Nizoral™; Janssen Pharmaceutica Inc., Belgium) were crushed in a mortar and formulated into a suspension with simple syrup.
Blood was sampled before and at 0.25, 0.50, 1.0, 2.0, 4.0, 6.0, 8.0, 12.0, 15.0, 24.0, 36.0, 48.0 and 60.0 hrs after the CYA administration. Blood samples were stored at −20°C pending high pressure liquid chromatographic (HPLC) analysis for intact CYA. An aliquot of the blood sample was centrifuged, plasma collected and stored at −20°C pending HPLC analysis for intact ketoconazole. Bile/urine mixture was collected quantitatively using a metabolic cage in 12 hr intervals, for 96 hrs after the CYA dose. Bile/urine sample volumes were measured, and an aliquot stored at −20°C pending analysis for intact CYA and CYA metabolites. Biochemical indices of liver function was measured in all five dogs during both phases of the study. In vivo plasma protein binding of CYA was determined from erythrocyte partitioning measurements using the procedure described by Trung et al. (1984).
Cyclosporine concentration in blood samples were measured using a specific and sensitive HPLC method (Ptachcinski et al., 1985) while ketoconazole concentrations were measured by the HPLC method of Badcock (1984). The metabolites of CYA were separated using a high pressure liquid chromatographic gradient elution system developed in our laboratory. Six ether extractable metabolites were separated using a solvent system consisting of a linear gradient of acetonitrile and water starting at 35% acetonitrile and increasing to 67% acetonitrile over 65 minutes. The mobile phase was pumped through the column at a flow rate of 1.7 ml/min. The extraction and other chromatographic conditions employed were similar to those employed for the analysis of parent CYA. Since adequate quantities of pure metabolites were not available for construction of standard calibration plots it was not possible to quantitate the absolute amounts of the metabolites. Therefore, peak area ratios of each of the metabolites to the internal standard were calculated for each sample. These values were normalized for differences in the total volume of bile/urine mixture excreted by the five dogs during each phase of the study. These normalized values were subsequently converted to cyclosporine equivalents and used to compare the effect of ketoconazole on the metabolic profile of CYA.
Model independent pharmacokinetic parameters for CYA including the terminal disposition half-life (T1/2), total body clearance (CL), intrinsic clearance (Clu int), and steady state volume of distribution (Vss) were determined according to standard methods (Gibaldi and Perrier, 1982). Differences in model independent pharmacokinetic parameters between the control and treatment groups were evaluated using the Students paired t-test at a significant level of 0.05.
RESULTS
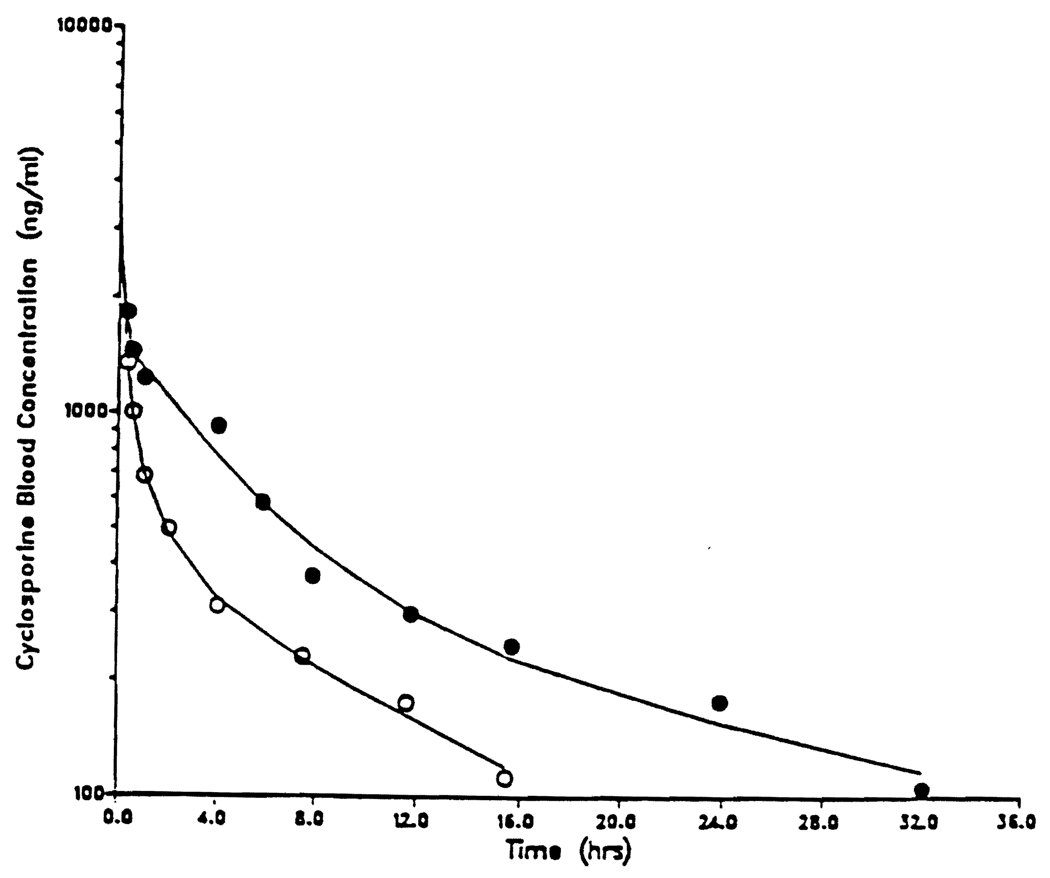
Figure 2 shows the CYA blood concentration time profile before and during ketoconazole treatment in dog number 4, while Table 1 summarizes the effect of ketoconazole on CYA. disposition in five dogs. Ketoconazole caused a significant decrease in the systemic clearance of CYA from 7.0 ml/min/kg to 2.5 ml/min/kg with a proportionate increase in its half-life from 8.7 to 19.5 hours. Ketoconazole also caused a marked decrease in CYA intrinsic clearance from a mean of 67.7 ml/min/kg to 6.7 ml/min/kg. There was no statistically significant change in CYA plasma protein or its steady state volume of distribution.
Figure 2.
Cyclosporine blood concentration versus time profile in dog #4 before (○) and during (○) ketoconazole treatment. Each dog received 4 mg/kg of cyclosporine as an intravenous bolus injection.
Table 1.
Mean cyclosporine pharmacokinetics in five dogs before and during ketoconazole treatment
| Treatment, Mean ± S.D. | |||
|---|---|---|---|
| Parameter (units) |
Cyclosporine Alone |
Cyclosporine + Ketoconazole |
P Value |
| 1) Disposition Half-Life (hrs) | 8.7a | 19.5 | — |
| 2) λ2 (hours−1) | 0.0794 ± 0.033 | 0.0354 ± 0.022 | 0.005 |
| 3) Cumulative Amount Excreted (mg) | 0.19 ± 0.10 | 0.40 ± 0.25 | N.S. |
| 4) Clearance (ml/min/kg) | 7.0 ± 3.6 | 2.5 ± 1.5 | 0.013 |
| 5) Plasma Free Fraction | 0.090 ± 0.05 | 0.16 ± 0.10 | N.S. |
| 6) Intrinsic Clearance (ml/min/mg) | 69.70 ± 10.0 | 6.72 ± 1.8 | 0.001 |
| 7) Steady State Volume of Distribution (L/kg) | 4.0 ± 0.8 | 4.4 ± 1.6 | N.S. |
Harmonic mean
Ketoconazole caused a 17–56% decrease in the levels of three of the six CYA metabolites (Table 2). Simultaneous, and probably compensatory increases were observed in the levels of metabolite numbers 21, 18 and 1. However, none of these changes were statistically significant probably as a consequence of the variability in the data.
Table 2.
Effect of ketoconazole on the metabolic profile of cyclosporine in dogs
| Treatment, Mean ± S.D.b,c | ||
|---|---|---|
| Metablitea Number |
Cyclosporine Alone |
Cyclosporine + Ketoconazoled |
| 21 | 2.4 ± 1.1 | 3.7 ± 1.7 |
| 18 | 0.44 ± 0.2 | 0.55 ± 0.4 |
| 1 | 1.7 ± 0.5 | 2.2 ± 0.7 |
| 17 | 0.92 ± 0.3 | 0.77 ± 0.3 |
| 13 | 2.7 ± 1.2 | 1.5 ± 1.1 |
| 8 | 1.1 ± 0.3 | 0.56 ± 0.5 |
Refer to Figure 1 for detailed structure of the metabolites.
N=5
Values are expressed as cyclosporine equivalents.
None of the parameters are statistically different (p < 0.05).
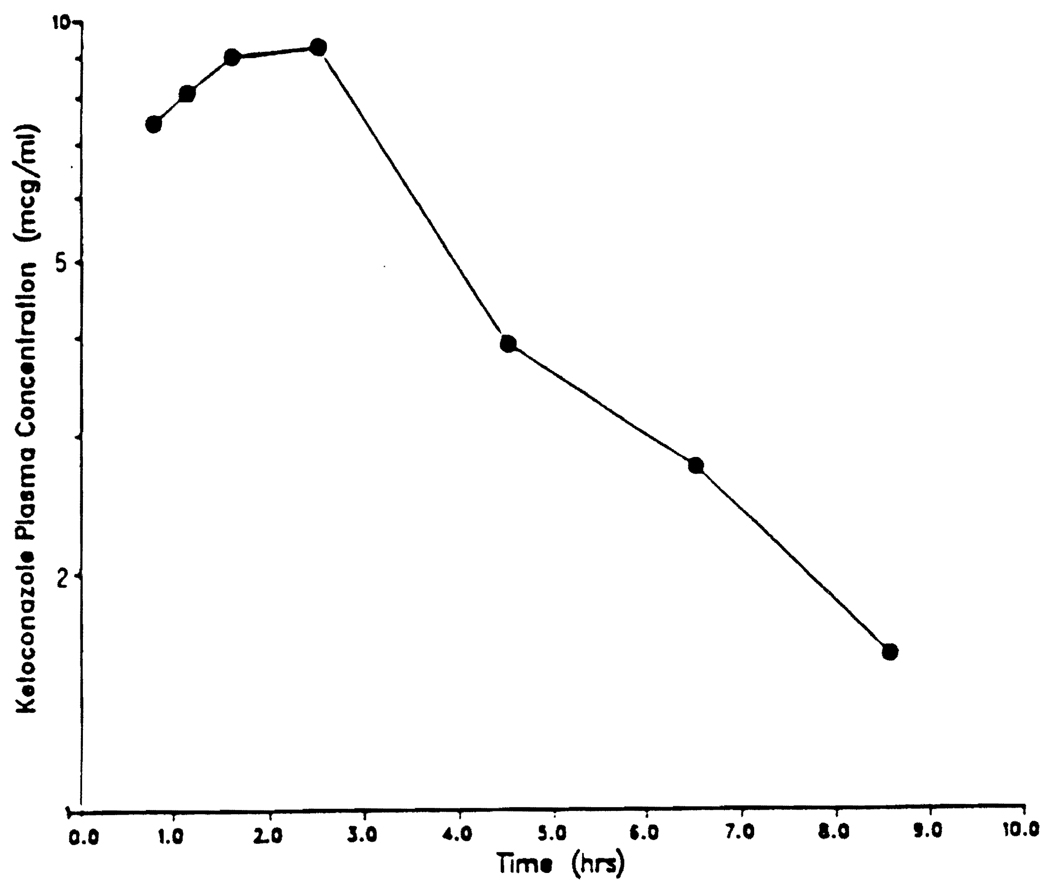
Figure 3 shows the ketoconazole plasma concentration-time profile in dog 1 on day 7 of the treatment period. Peak plasma concentration of 9.3 µg/ml was reached between 2 to 3 hours after the oral dose.
Figure 3.
Ketoconazole plasma concentration-time profile in dog #1 on day 7 of a 10 day 13 mg/kg/day oral dosing regimen.
DISCUSSION
Ketoconazole is a known inhibitor of microsomal enzymes in the rat (Niemegeers et al., 1981; Sheets and Mason, 1984; Meredith et al., 1985) and humans (Brown et al., 1985; Daneshmend et al., 1983). Recent studies in man have shown that Ketoconazole has no effect theophylline clearance whereas the drug decreases chlordiazepoxide plasma clearance leading the authors to conclude that ketoconazole inhibits at least one subset of the hepatic mixed function oxidase system (Brown et al., 1985). Coadministration of ketoconazole increases the serum CYA concentrations in transplant patients (Morgenstern et al., 1982; Dieperink and Moller, 1982; White et al., 1984; Gumbleton et al., 1985). Recently the interaction of CYA and ketoconazole was studied in mice (Anderson and Blaschke, 1986). A significant increase in the half life and a reduction in the clearance of CYA was observed in these animals. Further study by the same group of investigators documents potentiation of the immunosuppressive effect and toxicity of CYA in mice receiving ketoconazole (Anderson et al., 1987). However the study was conducted in two separate groups of animals and the presence of large interindividual variations in the kinetics of CYA could potentially influence the conclusions of the study. Since CYA plasma protein binding was not determined in these animals, the actual mechanism(s) responsible for the observed decrease in the total clearance could not be determined.
In the present study, the same animals were used in the control and treatment phase of the study. Studies were conducted in dogs with choledochoureterostomy. This enables complete collection of bile and the urine for the quantitative determination of CYA and its metabolites. The mean clearance, plasma protein binding, and half life of CYA in these dogs was similar to what has been reported in the literature (Venkataramanan et al., 1987). This indicates that cholodechoureterostomy had no significant effect on the disposition of CYA. The effect of cholodechoureterostomy on the disposition of ketoconazole was not examined in this study. The extent of inhibition of drug metabolizing enzymes is expected to be a function of the concentration of the inhibitor. The mean peak ketoconazole plasma concentrations of 13.1 µg/ml that was observed in the five dogs in this study was comparable to that reported by Baxter et al. (1986) indicating that choledochoureterostomy probably had a minimal effect on the disposition of ketoconazole in these animals.
Ketoconazole treatment resulted in a significant decrease in the intrinsic clearance of CYA in dogs. A study in human liver microsomal incubates has shown that ketoconazole inhibits the metabolism of CYA. A Michaelis-Menten plot of the data indicated a mixed type of inhibition (Mauer, 1985). Pretreatment with ketoconazole probably inhibited the oxidative enzymes responsible for CYA metabolism which in turn accounts for the observed decrease in its intrinsic clearance in the five dogs. Lack of any significant difference in the amount of parent CYA and various metabolites excreted in the bile/urine mixture during the two different phases indicates no selective inhibition of any specific pathways of CYA elimination by ketoconazole in dogs. Earlier studies have suggested that ketoconazole is a potent and specific inhibitor of N-demethylase activity in humans and animals (Brown et al., 1985; Sheets and Mason, 1984; Meredith et al., 1985).
Ketoconazole treatment has been associated with silent and symptomatic hepatic reactions. The effect of ketoconazole on the liver ranges from asymptomatic transient elevations of serum transaminase or alkaline phosphatase to potentially fatal acute necrosis, and may occur at any time during ketoconazole treatment (Lewis et al., 1984). Cyclosporine clearance has been shown to be impaired in patients with liver disease (Venkataramanan et al., 1985b) and in dogs with experimentally induced hepatic dysfunction (Takaya et al., 1987). It is therefore possible that the decrease in CYA clearance during ketoconazole treatment could be partly due to hepatic injury associated with chronic ketoconazole therapy. However, biochemical indices of liver function reveal that ketoconazole treatment caused no change in the functional integrity of the livers of the animals (data not shown). Therefore the decrease in CYA clearance during ketoconazole therapy is primarily due to ketocanazole induced inhibition of the oxidative enzymes responsible for CYA metabolism.
In conclusion, the results of this investigation demonstrate that ketoconazole decreased the systemic clearance of CYA probably as a consequence of an inhibition of CYA metabolizing enzymes. Ketoconazole caused no change in the distribution of CYA in dogs. Since CYA is a low clearance drug in dogs, a decrease in its intrinsic clearance would lead to an increase in the concentration of the unbound, pharmacologically active species. A similar mechanism may also be responsible for the increased nephrotoxicity observed in transplant recipients treated concomitantly with ketoconazole.
REFERENCES
- Anderson JE, Morris RE, Blaschke TF. Ketoconazole potentiates cyclosporine immunosuppression and toxicity in mice. Transplant. Proc. 1987;19:1267–1268. [PubMed] [Google Scholar]
- Anderson JE, Blaschke TF. Ketoconazole inhibits cyclosporine metabolism in vivo in mice. J. pharmacol. Exp. Ther. 1986;236:671–674. [PubMed] [Google Scholar]
- Badcock NR. Microdetermination of ketoconazole in plasma or serum by high performance liquid chromatography. J. Chromatogr. 1984;306:436–440. doi: 10.1016/s0378-4347(00)80912-3. [DOI] [PubMed] [Google Scholar]
- Baxter JG, Brass C, Schentag JJ, Slaughter RL. Pharmacokinetics of ketoconazole administered intravenously to dogs and orally as a tablet and solution to humans and dogs. J. Pharm. Sci. 1986;75:443–447. doi: 10.1002/jps.2600750504. [DOI] [PubMed] [Google Scholar]
- Beveridge T. Pharmacokinetics and metabolism of cyclosporin A. In: White DJG, editor. Cyclosporin A. Oxford, England: Elsevier Biomedical Press; 1982. pp. 36–44. [Google Scholar]
- Brown MW, Maldonado AL, Meredith CG, Speeg KV., Jr 1985 [Google Scholar]
- Daneshmend TK, Warnock DW, Ere MD, Johnson EM, Parker G, Richardson MD, Roberts CJC. Multiple dose pharmacokinetics of ketoconazole and their effects on antipyrine kinetics in man. J. Antimicrob. Chemother. 1983;12:185–188. doi: 10.1093/jac/12.2.185. [DOI] [PubMed] [Google Scholar]
- Dieperink H, Moller J. Ketoconazole and cyclosporine. Lancet. 1982;11:1217. doi: 10.1016/s0140-6736(82)91231-4. [DOI] [PubMed] [Google Scholar]
- Ferguson RM, Sutherland DER, simmons RL, Najarian JS. Ketoconazole cyclosporine metabolism, and renal transplantation. Lancet. 1982;2:882–883. doi: 10.1016/s0140-6736(82)90851-0. [DOI] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D. Pharmacokinetics. 1982. Multicompartment models; pp. 45–112. [Google Scholar]
- Gumbleton M, Brown JE, Hawksworth G, Whiting PH. The possible relationship between hepatic drug metabolism and ketoconazole enhancement of cyclosporine nephrotoxicity. Transplantation. 1985;40:454–455. [PubMed] [Google Scholar]
- Lewis JH, Zimmerman JH, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy - an analysis of 33 cases. Gastroenterology. 1984;86:503–513. [PubMed] [Google Scholar]
- Maurer G. Metabolism of cyclosporine. Transplant. Proc. 1985;17 Suppl 1:19–26. [PubMed] [Google Scholar]
- Maurer G, Loosli HR, Schreier E, Keller B. Disposition of cyclosporine in several animal species and man. Structural elucidation of its metabolies. Drug Metab. Dispos. 1984;13:156–162. [PubMed] [Google Scholar]
- Meredith CG, Maldonado AL, Speeg KV., Jr The effect of ketoconazole on hepatic oxidative drug metabolism in the rat in vivo and in vitro. Drug Metab. Dispos. 1985;13:156–162. [PubMed] [Google Scholar]
- Morgenstern GR, Powles R, Robinson R, McElwain TJ. Cyclosporine interaction with ketoconazole and melphalan. Lancet. 1982;2:1342. doi: 10.1016/s0140-6736(82)91544-6. [DOI] [PubMed] [Google Scholar]
- Niemegeers CJE, Levron JC, Awouters F, Janssen PAJ. Inhibition and induction of microsomal enzymes in the rat. A comparative study of four antimycotics: miconazole, econazole, clotrimazole, and ketoconazole. Arch. Int. Pharmacodyn. 1981;25:26–38. [PubMed] [Google Scholar]
- Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR. Cyclosporine kinetics in renal transplantation. Clin. Pharmacol. Ther. 1985;38:296–300. doi: 10.1038/clpt.1985.174. [DOI] [PubMed] [Google Scholar]
- Sheets JJ, Mason JI. Ketoconazole: a potent inhibitor of cytochrome P-450 dependent drug metabolism in rat liver. Drug Metab. Dispos. 1984;12:603–606. [PubMed] [Google Scholar]
- Takaya S, Zaghloul I, Iwatsuki S, Starzl TE, Noguchi T, Ohmori Y, Burckart GJ, Ptachcinski RJ, Venkataramanan R. Effect of liver dysfunction on cyclosporine pharmacokinetics. Transplant. Proc. 1987;19:1246–1247. [PMC free article] [PubMed] [Google Scholar]
- Trung AH, Sirois G, Dube LM, MoGilveray IJ. Comparison of the erythrocyte partitioning method with two classical methods for estimating free drug fraction in plasma. Biopharm. Drug Dispos. 1984;5:281–290. doi: 10.1002/bdd.2510050310. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R, Wang CP, Habucky K, Ptachcinski RJ, Burckart GJ, Koneru B, Baker R, Todo S, Starzl TE. Species specific cyclosporine metabolism. Transplant. Proc. 1988;20:680–683. [PMC free article] [PubMed] [Google Scholar]
- Venkataramanan R, Tedo S, Zaghloul I, Lynch S, Kam I, Ptachcinski RJ, Burckart GJ, Starzl TE. Comparative pharmacokinetics of cyclosporine and NVa2-cyclosporine in dogs. Transplant. Proc. 1987;19:1265–1266. [PMC free article] [PubMed] [Google Scholar]
- Venkataramanan R, Starzl TE, Yang S, Burckart GJ, Ptachcinski RJ, Shaw BW, Iwatsuki S, Van Thiel DH, Sanghvi A, Seltman H. Biliary excretion of cyclosporine in liver transplant patients. Transplant. Proc. 1985a;17:286–289. [PMC free article] [PubMed] [Google Scholar]
- Venkataramanan R, Burckart GJ, Ptachcinski RJ. Pharmacokinetics and monitoring of cyclosporine following orthotopic liver transplantation. Sem. Liver Dis. 1985b;5:357–368. doi: 10.1055/s-2008-1040633. [DOI] [PubMed] [Google Scholar]
- White DJG, Blatchford NR, Cauwenberg G. Cyclosporine and ketoconazole. Transplantation. 1984;37:214–215. [PubMed] [Google Scholar]
- Wood AJ, Maurer G, Niederberger W, Beveridge T. Cyclosporine–pharmacokinetics, metabolism, and drug interactions. Transplant. Proc. 1983;15:2409–2412. [Google Scholar]