FK 506 (Tacrolimus, Prograf™) has been recently approved by the Food and Drug Administration (FDA) for baseline immunosuppression following orthotopic liver transplantation (OLT). The drug was first used clinically in 1989,1 and 1 year later at the Thirteenth International Congress of Transplantation Society, San Francisco, CA, 1990, extensive reports were given from our center describing the potential value of this drug in clinical transplantation.2–5 We have so far treated 1497 primary liver transplant recipients with FK 506 and an additional 512 patients who were converted to FK 506 from cyclosporine (CyA). This report will focus on the first 1000 consecutive primary OLTs in the series. The parameters examined were patient and graft survival, causes of death and retransplant, effect of age, United Network for Organ Sharing (UNOS) status, level of maintenance immunosuppression, and adverse events.
MATERIALS AND METHODS
Cases accrued between August 1989 and December 1992. There were 600 male and 400 female patients. Mean age was 42.6 ± 20.2 years. One fifth of all patients were older than 60 years and 7.5% were younger than 2 years. Mean follow-up was 39.5 (range 19 to 59) months. The age group distribution and indications for liver transplantation are shown in Tables 1 and 2, respectively. One hundred fifty-nine patients (15.9%) were UNOS class 1 and 2 (out of hospital with limited working capacity), 382 patients (38.2%) were class 3 (in the hospital), 328 (32.8%) were class 4 (in intensive care unit [ICU], and 131 (13.1%) were UNOS 4 STAT (life expectancy <2 days in the ICU). Two hundred seventy donors were younger than 18 years (mean 9 ± 2), 347 were 18 or older but younger than 35 (mean 25 ± 2) years, 242 were 35 or older but younger than 50 (mean 42 ± 4) Years, and 141 were older than 50 (mean 57 ± 4) years of age at the time of primary transplant.
Table 1.
Distribution of Patients as Per Age and UNOS
| Age (y) | Mean Age | n (%) | |
|---|---|---|---|
| Infants | 2 | 0.95 | 75 (7.5) |
| Children | 2 ≤18 | 9.2 | 91 (9.1) |
| Adults | 18 ≤60 | 45.2 | 630 (63.0) |
| Seniors | 60 | 64.8 | 204 (20.4) |
Table 2.
Indications for Transplants
| n (%) | |
|---|---|
| Nonalcoholic cirrhosis | 341 (34.1) |
| Alcoholic cirrhosis | 188 (18.8) |
| Biliary atresia | 86 (8.6) |
| Primary biliary cirrhosis | 83 (8.3) |
| Primary sclerosing cholangitis | 60 (6.0) |
| Primary hepatic malignancy | 79 (7.9) |
| Acute fulminant failure | 34 (3.4) |
| Miscellaneous | 129 (12.9) |
| Total | 1000 |
Immunosuppression
FK 506 was begun IV on the day of operation and converted to oral dosing when bowel function returned. The current IV dose is 0.05 mg/kg/d for adults and seniors, 0.1 mg/kg/d for children and infants, less than one half of what was given in 1989 and early 1990. The beginning oral doses averaged 0.2 ± 0.1 mg/kg/d. However, dose adjustments were made continuously, guided by plasma trough levels of FK 506 (targeted at 1.0 ng/ml) and above all by the balance of rejection control, signs of nephrotoxicity, neurotoxicity, and diabetogenicity. In the initial part of the trial, 63 patients received 1 g methylprednisone followed by a 5-day methylprednisolone burst, beginning at 200 mg on day 1, and ending with a maintenance dose of 20 mg/d. In the middle part of the trial, this steroid bolus was omitted. Children received 1 g hydrocortisone and infants received 500 mg hydrocortisone instead of methylprednisone.
RESULTS
Retransplantation
A total of 1147 transplants were performed on the 1000, patients during the period of study. A single retransplantation was performed on 118 (11.8%) of the patients, 13 (1.3%) required three, and 1 (who is alive) underwent 4 transplants. The retransplantation rate was 9.3% for infants, 12.1% for children, 14.3% for adults, and 11.8% for seniors. The overall rate was 13.2%. The indications for retransplantation are shown in Table 3.
Table 3.
Indications for Retransplantation
| n (%) | |
|---|---|
| Primary nonfunction | 53 (4.6) |
| Vascular complications | 45 (3.9) |
| Ischemic injury | 16 (1.4) |
| Rejection | 13 (1.1) |
| Biliary complication | 6 (0.5) |
| Recurrence of disease | 5 (0.5) |
| Miscellaneous | 9 (0.8) |
| Total | 147 12.8 |
Survival
Patients
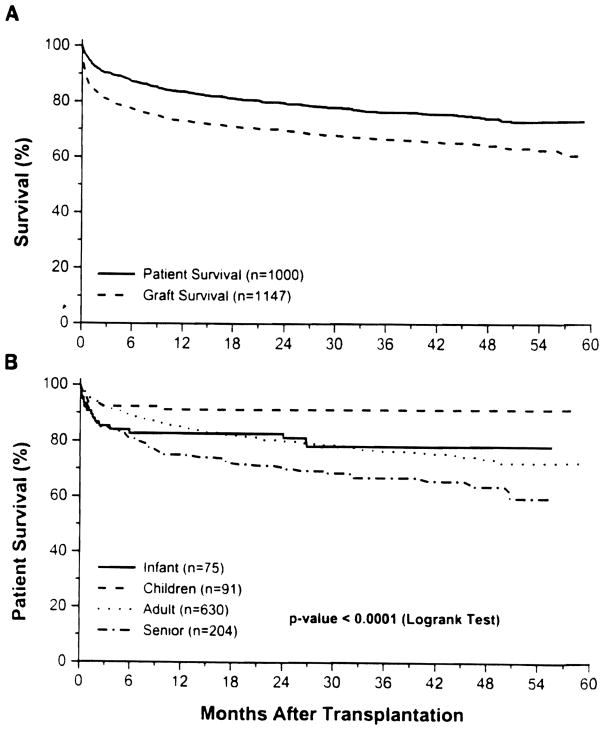
The overall actual patient survival at 1 year was 83.1 % and the 4-year actuarial survival rate was 73.4% (Fig 1A). Differences in patient survival for different age groups was statistically significant (P < .0001 by log rank test: Fig 1B). The best results were with patients older than 2 but who were 18 years or younger with actual 3-month and 12-month survival rates of 92.3% and 91.2% respectively. Taking the adult age group as the reference, the risk of mortality was 1.55 (lower 1.17, upper 2.07, P = .003) for seniors, 0.34 (lower 0.17, upper 0.7, P = .003) for children, and 0.89 (lower 0.53, upper 1.5, P = .67) for infants. Similarly, taking UNOS status 3 group as a reference, the relative risk of mortality at 95% confidence interval, for UNOS 4 was 1.56 (lower 1.15, upper 2.12, P = .004) and for UNOS 4 STAT was 1.71 (lower 1.18, upper 2.5; P = .005). However, there was no significant difference for UNOS status 1 and 2 taking UNOS 3 as a reference group.
Fig 1.
(A) Patient and graft survival over the 5-year post-transplantation period. (B) Patient survival by age group of the recipients at the time of first transplant.
Grafts
The overall actual graft survival rate for 1 year was 72.2% and actuarial 4-year graft survival rate was 64.1 %. The effect of the UNOS urgency status and age factors was similar to that on patient survival.
Causes of Death
A total of 245 patients died in the follow-up period of 19 to 59 months. Their causes of death are shown in Table 4. Infection was the most common cause of death.
Table 4.
Causes of Death (n = 245)
| n (%) | |
|---|---|
| Infection | 93 (38) |
| Bacterial | 60 (24) |
| Fungal | 21 (9) |
| Viral | 4 (2) |
| Other | 8 (3) |
| Cardiopulmonary | 36 (15) |
| Malignancy | 29 (12) |
| Primary nonfunction | 23 (9) |
| Technical | 8 (3) |
| Multisystem failure | 8 (3) |
| Intracranial | 6 (2) |
| Miscellaneous | 42 (17) |
Maintenance Immunosuppression
FK 506
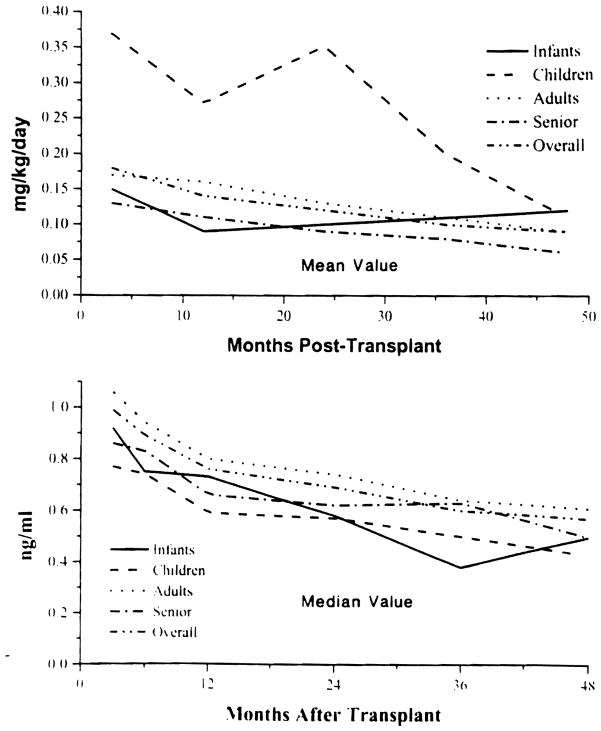
The FK 506 doses steadily declined with time in all age groups (except in infants) (Fig 2, top), along with a decrease in average plasma trough levels (Fig 2, bottom).
Fig 2.
Mean FK 506 dose (mg/kg/d) by age (top) and median FK 506 plasma trough level by age (bottom).
Prednisone
By 3 months, 53% of surviving patients were steroid free and at 4 years this had increased to 69%. For those on steroids at these milestones, the doses were low, only 10%, and 6% of the patients requiring more than 10 mg/d at 3 months and 4 years, respectively. Infants and children were weaned off steroids much faster than adults and seniors (Fig 3).
Fig 3.
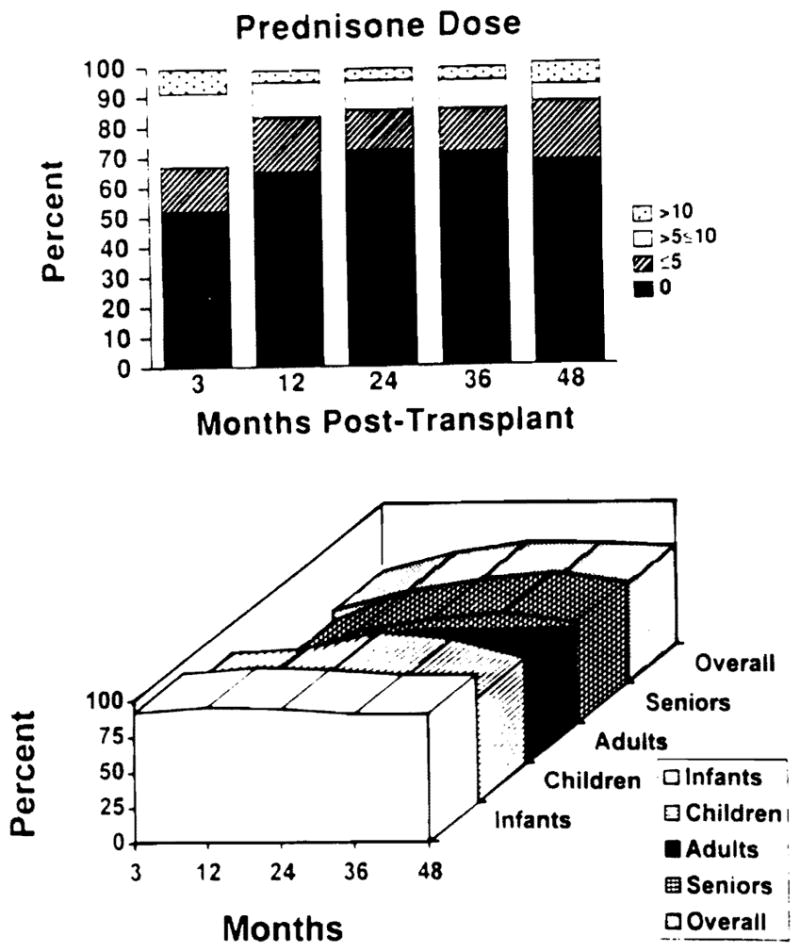
(Top) Steroid dose varying from 0, ≤5, >5, ≤10, >10 at various time points. (Bottom) Freedom of steroids for various age groups at various time points from the time of transplant.
Organ Function
Liver AIlograft
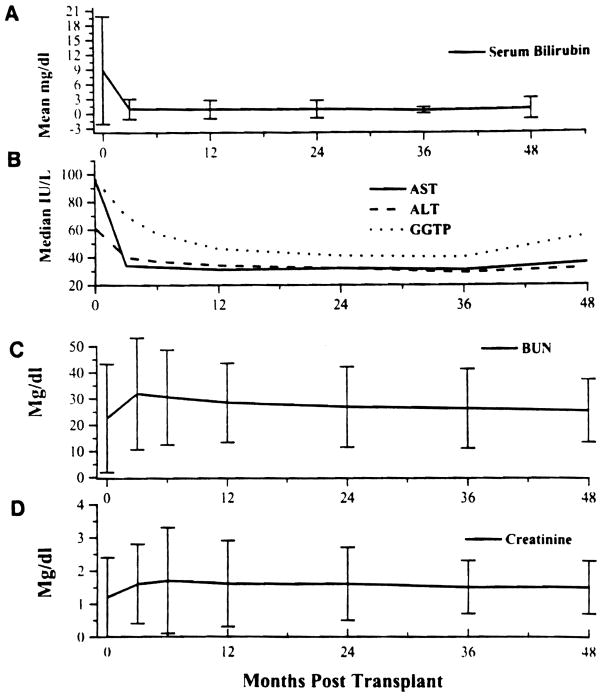
Liver function tests are summarized in Fig 4A, B. The slight upward shift in aspartate transaminase (AST), alanine transaminase (ALT), and gamma glutamyl transpeptidase (GGTP) at 4 years was related to recurrence of hepatitis in some of the patients.
Fig 4.
(A) Liver function bilirubin (mean value) and (B) ALT, AST, and GGTP (median value) posttransplant. (C) Renal function, BUN (mean ± S.D.), and (D) serum creatinine (mean ± S.D.) posttransplant.
Kidney Function
Mean serum creatinine and blood urea nitrogen (BUN) levels were elevated at 3 months, but were stable thereafter during the entire follow-up period (Fig 4C, D).
Rejection
The first 123 adults and seniors have now completed 4 years of follow-up, allowing the precise study of rejection accrual: first 3 months 42%, 3 to 12 months 18%, 12 to 24 months 17%, 24 to 36 months 4%, and 36 to 48 months 3%. Rejection after 3 months was mild and easily reversible.
Only 6 (0.5%) of the 1147 grafts were lost due to acute rejection in the entire study, 3 in the learning phase of the trial. Interestingly, the majority of these patients had a strongly positive lymphocytic crossmatch, an adverse factor also previously noted in CyA-treated liver recipients.6
Adverse Events
Renal
Nephrotoxicity, the subject of a vast literature review, was already well characterized by 1990.2–4, 7 Only more subtle manifestations are considered here. Hyperkalemia was the most common adverse event and was seen in 41.6%, 46.0%, 46.9%, and 44.6% of patients at 1, 2, 3, and 4 years posttransplant respectively, which was easily controlled with 0.1 to 0.2 mg/d of fludrocortisone. Hypertension requiring one (usually) or more antihypertensive medications was observed in 28.7%, 33.6%, 37.0%, and 38.5% of patients at 1, 2, 3, and 4 years, respectively. This was not the true incidence of de novo hypertension, for it included many patients who were hypertensive preoperatively.
Metabolic
The incidence of insulin-dependent diabetes was 16.2%, 13.0%, 12.8%, and 14.4% at 1, 2, 3, and 4 years, respectively, including an estimated 5% of patients who were already on insulin before transplantation. Median serum cholesterol concentrations were 149, 147, 159, and 158 mg/dL at 1, 2, 3, and 4 years, respectively.
Neurologic
Seizure activity or other major neurologic complications occurred in 7.06% of patients, which were almost always dose related and reversible with reductions in immunosuppression.
Infections
Significant bacterial, viral, or fungal infections were recorded at a 36%, 26%, 22%, and 10% incidence, respectively, during the 4-year follow-up.
Post Transplant Lymphoproliferative Disorder (PTLD)
The total incidence over the first 4 years was 3.95%, which was highest in children. More than one half of these cases were infectious mononucleosis syndrome which responded to reduction in immunosuppression. The spectrum of this syndrome has been extensively reported8 and is not drug specific.
DISCUSSION
The reports from Pittsburgh of 19891,9,10 and those from the 13th International Congress of the Transplantation Society held in San Francisco2–5, 7 have been confirmed in a recently published analysis of the European multicenter randomized trial comparing FK 506 and CyA for OLT.11 The advantages of this drug over CyA include a reduction in acute, refractory acute, and chronic rejection episodes and the ability to achieve this objective with smaller requirements of corticosteroids. No side effects uniquely attributable to FK 506 have emerged. Patient survival and graft survival rates in the European trial were 5.5%, and 4.9%, respectively, which were better than that in the alternative treatment group. Results from the American multicenter trial have not yet been published, but early data suggest that FK 506 has at least equivalent efficacy to CyA.
In our current study, there was an unusually large number of patients in the high urgency UNOS categories 3 and 4 and of recipients (20.4%) above the age of 60 years. Furthermore, many of the more seriously ill as well as the older patients had been rejected as transplant candidates at other centers and most as well as infants and children were excluded from the American multicenter trial. These groups are thought to be selectively helped by FK 506 because of the ability to minimize steroid use. Included also in our study were 34 (3.4%)patients with acute fulminant failure and 79 (7.9%) with primary hepatic malignancy. Because of the deepening organ shortage, 16% of the adults and seniors received their first organ grafts from donors over the age of 50 years (mean 57 ± 6), adding to the hazard as reported separately at this meeting by Marino et al. 12
Freedom from hirsutism, gingival hyperplasia, and steroid stigmata contributed to a good quality of life. Nephrotoxicity was the most serious side effect during the learning phase,13 but with downward dose adjustments, this was not significantly different than with previous immunosuppressive regimens nor was a trend observed. The complications of infections. PTLD, diabetes, and neurologic syndromes also were comparable to past regimens. Hypertension and hypercholesterolemia were less frequent and severe.
Acknowledgments
The authors acknowledge Sheetal Jain, Vishal Jain, Claudine Martin, and Sandi Mitchell for their enormous help with data collection and analysis.
Supported by project grant DK 29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Starzl TE, Todo S, Fung JJ, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Fung JJ, Tzakis AJ, et al. Transplant Proc. 1991;23:914. [PubMed] [Google Scholar]
- 3.Todo S, Fung JJ, Demetris AF, et al. Transplant Proc. 1991;23:1397. [PubMed] [Google Scholar]
- 4.Fung JJ, Todo S, Tzakis A, et al. Transplant Proc. 1991;23:14. [PMC free article] [PubMed] [Google Scholar]
- 5.Jain AB, Fung JJ, Todo S. Transplant Proc. 1991;23:928. [PMC free article] [PubMed] [Google Scholar]
- 6.Takaya S, Bronsther O, Iwaki Y. Transplantation. 1992;53:400. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCauley J, Takaya S, Fung JJ, et al. Transplant Proc. 1991;23:1444. [PMC free article] [PubMed] [Google Scholar]
- 8.Nalesnik MA. Springer Semin Immunopathol. 1991;13:199. doi: 10.1007/BF00201469. [DOI] [PubMed] [Google Scholar]
- 9.Starzl TE, Fung J, Jordan M, et al. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Fung JJ, St kearzl TE, et al. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European FK 506 multicentre liver study group. Lancet. 1994;344:423. [PubMed] [Google Scholar]
- 12.Marino IR, Doyle H, Doria C, et al. Transplant Proc. doi: 10.1016/s0041-1345(96)00199-6. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alessiani M, Kusne S, Martin FM, et al. Transplant Proc. 1991;23:1501. [PMC free article] [PubMed] [Google Scholar]