Abstract
Purpose
To assess whether the low incidence of severe neutropenia in castrated men with prostate cancer treated with docetaxel is the result of changes in systemic clearance.
Patients and Methods
A total of 10 noncastrated and 20 castrated men with prostate cancer were studied to achieve 80% power (α = .05) to detect at least a 25% change in the clearance of docetaxel. The erythromycin breath test was evaluated to determine hepatic activity of cytochrome P450 3A4 (CYP3A4), the main docetaxel-metabolizing enzyme. Additional studies were performed in rats and transfected cells overexpressing human or rodent transporters.
Results
Docetaxel clearance was increased by approximately 100% in castrated men and was associated with a two-fold reduction in area under the curve (P = .0001), although hepatic activity of CYP3A4 was unchanged (P = .26). In rats, castration was associated with higher uptake of docetaxel in the liver and a concurrent increase in the expression of rOat2 (Slc22a7), an organic anion transporter that regulates, in part, the transfer of docetaxel from the circulation into hepatocytes.
Conclusion
It is recommended that castration- and/or hormone-related changes in the clearance of oncology drugs should be considered as a possible risk factor for treatment failure.
INTRODUCTION
Docetaxel is one of the most widely used cytotoxic chemotherapeutic agents and has currently been approved for the treatment of breast cancer, non–small-cell lung cancer, squamous cell carcinoma of the head and neck, gastric adenocarcinoma, and androgen-independent (hormone-refractory) metastatic prostate cancer. The pharmacokinetic profile of docetaxel is characterized by substantial interindividual variability, with up to 10-fold differences in drug clearance even in patients with normal hepatic function.1 This degree of variability remains largely unexplained,1 yet has important toxicologic and therapeutic ramification. In particular, a previous study documented that a 50% decrease in docetaxel clearance is associated with a greater than 430% increase in the odds of developing severe (grade 3 or 4) neutropenia, the dose-limiting toxicity.2 Furthermore, a reduced area under the curve (AUC) was associated with shorter survival in patients with non–small-cell lung cancer receiving docetaxel chemotherapy.3
When docetaxel is administered as a short intravenous infusion, neutropenia occurs in virtually all patients given a dose between 60 and 100 mg/m2, with severe neutropenia occurring in up to 75% of patients given 60 mg/m2 and in up to 85% of patients given 100 mg/m2.4 A survey of the literature on docetaxel treatment suggests that the incidence of severe neutropenia is substantially reduced in castrated patients with prostate cancer compared with noncastrated patients with prostate cancer or patients with other solid tumors, despite the similarity in total dose administered per course (Table 1).6-9 In view of the established pharmacokinetic-pharmacodynamic relationships of docetaxel, we here evaluated the hypothesis that the castration-dependent decrease in neutropenia in patients with prostate cancer is due to decreased systemic exposure of docetaxel in this population. Our results show that docetaxel clearance is approximately two-fold increased in castrated patients with prostate cancer as a result of increased drug uptake in the liver and that such patients may have altered dose requirements in the context of chemotherapeutic treatment with docetaxel.
Table 1.
Incidence of Severe (grade 3 or 4) Neutropenia in Noncastrated and Castrated Patients With Prostate Cancer
| No. of Patients | Castration State | Dose (mg/m2) | Severe Neutropenia (%) | Author and Reference |
|---|---|---|---|---|
| 102 | Noncastrated | 75 | 88 | Rathkopf et al5 |
| 39 | Noncastrated | 70 | 62 | Hussain et al6 |
| 62 | Noncastrated | 70 | 61 | Taplin et al7 |
| 332 | Castrated | 75 (+ prednisone) | 32 | Tannock et al8 |
| 338 | Castrated | 60-70 (+ estramustine) | 16 | Petrylak et al9 |
PATIENTS AND METHODS
Patients
Using an allocation ratio of 2, group sample sizes of 10 (noncastrated) and 20 (castrated) were required to achieve 80% power to detect at least a 25% change in the mean clearance of docetaxel at a significance level (α) of 0.05. Detailed eligibility criteria have been described previously.5,10–12 Briefly, each patient was diagnosed with a confirmed metastatic prostate cancer, was older than 18 years, and had adequate bone marrow function, renal function, and hepatic function. Castrated patients had histologically proven prostate adenocarcinoma that progressed to an androgen-independent state, and castration was determined by levels of testosterone that were less than 0.5 ng/mL.12 Concurrent use of agents known to alter the pharmacokinetics of docetaxel, including phenytoin, carbamazepine, rifampin, phenobarbital, St John's wort, and ketoconazole, was not allowed. The study was approved by the review board at Johns Hopkins University, in accord with an assurance filed with and approved by the US Department of Health and Human Services, and written informed consent was provided by all patients.
Baseline and Treatment Assessments
In a subset of patients (n = 10 noncastrated and n = 6 castrated), hepatic CYP3A activity at baseline was assessed using the [14C]erythromycin breath test within 1 week before starting docetaxel treatment.13 The parameter C20 minutes, representing the flux of exhaled 14CO2 at 20 minutes, was used as a marker for CYP3A activity. Docetaxel was administered at a dose of 20, 25, or 30 mg/m2 (30-minute infusion given once weekly) or 75 mg/m2 (1-hour infusion given once every 3 weeks). The selection of the administration schedule (weekly or every 3 weeks) was at the discretion of the treating physician. Over the first course of docetaxel administration, serial blood samples were collected for pharmacokinetic studies. These samples were processed and analyzed as described.14 Pharmacokinetic parameters were estimated with noncompartmental methods using WINNonlin version 5.0 (Pharsight, Mountain View, CA). Because the disposition of docetaxel is linear within the range of infusion durations and doses applied,13 the observed AUC in each patient was normalized to a dose of 75 mg/m2 without applying further correction (normalized AUC = observed AUC × [75/actual dose]). Separate blood samples were used at baseline to determine serum levels of various hormones by liquid chromatography–tandem mass spectrometry, with a lower limit of quantitation of 0.1 ng/mL for testosterone, 0.02 ng/mL for dihydrotestosterone, and 0.05 ng/mL for estradiol, androsterone, dehydroepiandrosterone, dehydroepiandrosterone sulfate, estrone, and pregesterone.15 The mean percentage deviation from nominal values (accuracy) and precision (within-run and between-run variability) of quality control samples spiked with known concentrations of the various hormones ranged between 88.3% and 110% and between 1.01% and 13.8%, respectively. The extraction recovery for the hormones ranged between 60.6% and 95.2%.
Animals Experiments
Rats were used in exploratory studies because of the known qualitative and quantitative similarities in docetaxel metabolism between rats and humans16,17 and because rats, unlike mice, express high levels (similar to humans) of OAT2,18,19 a hepatic transporter that we found in the course of our work to be an important contributor to castration-dependent changes in docetaxel clearance. Male Sprague-Dawley rats, postnatal day 17, were purchased from Charles River Laboratories (Wilmington, MA). All animals were maintained on automated 12-hour dark/light cycles and allowed access to food and water ad libitum. Rats were weaned at postnatal day 21, and two to five rats were housed per cage. Half of the rats were left intact, and half underwent surgical castration. All castration surgeries were performed on prepubertal rats, between postnatal day 24 and postnatal day 27, and subsequent experiments were conducted between postnatal day 65 and postnatal day 68. The experiments were initiated by the administration of a single intravenous bolus injection of docetaxel (dose, 10 mg/kg), and terminal blood samples were collected by cardiac puncture at 5, 15, 30, 120, and 360 minutes after drug administration (n = three to four per time point for each condition). Blood samples were collected in 4-mL tubes containing lithium heparin (Franklin Lakes, NJ), centrifuged at 4,000 × g for 10 minutes, and plasma supernatants were stored at or below −20°C until analysis as described.14 Pharmacokinetic parameters in rat liver and plasma were calculated using PK Solutions 2.0 (Summit Research Services, Montrose, CO).
An additional set of castrated and intact rats was used to collect baseline blood samples and livers for transcriptional profiling. Livers were isolated, snap-frozen, and stored at −80°C until processed. A 20- to 30-mg sample was isolated from each frozen liver, and RNA was extracted using an RNeasy minikit (Qiagen, Valencia, CA). RNA samples were analyzed using the Genechip Rat Genome 230v2.0 Array (Affymetrix, Santa Clara, CA), and expression levels were confirmed using real-time reverse-transcriptase polymerase chain reaction with Taqman primers (Applied Biosystems, Carlsbad, CA) for the gene encoding Oat2, Slc22a7, and the housekeeping gene, Gapdh.
In Vitro Accumulation Assays
Human embryonal kidney (HEK293) cells stably transfected with OAT2 or an empty vector were provided by Yuichi Sugiyama, PhD (Tokyo, Japan), and cultured as described.10 Uptake experiments were initiated by addition of a mixture of [3H]docetaxel (Moravek Biochemicals, Brea, CA) and unlabeled docetaxel (Sigma-Aldrich, St Louis, MO) at the desired concentration (0.2 to 1,000 μmol/L) to the culture media in the presence and absence of erythromycin (1 mmol/L). Uptake studies were also performed in Xenopus laevis oocytes injected with water or transporter cRNA (BD Biosciences, Woburn, MA) as described.20
Growth Inhibition Assays
The growth inhibitory potential of docetaxel in vector control cells and OAT2 over-expressing cells was assessed with the method of transcriptional and translational assay21 using 96-well plates seeded with 1.1 × 106 cells that were pretreated with 5 μmol/L of sodium butyrate and allowed to grow for 24 hours. Docetaxel was added to the wells, giving final concentrations ranging from 0.1 to 1,000 μmol/L.
Statistical Considerations
Pharmacokinetic data are presented as a mean value along with standard deviation unless stated otherwise. Statistical significance was evaluated using a t test, Wilcoxon signed-rank test (in case data were not normally distributed), or a one-way analysis of variance followed by a Bonferroni post hoc test, depending on the number of groups considered. In all cases, P < .05 was considered statistically significant. Calculations were performed using Graphpad Prism version 5 (La Jolla, CA).
RESULTS
A total of 30 men with prostate cancer, 10 noncastrated and 20 castrated, received at least one course of docetaxel and were evaluable for pharmacokinetic analysis. With the exception of serum levels of testosterone, patient demographic characteristics were similar between the two patient groups (Appendix Table A1, online only).
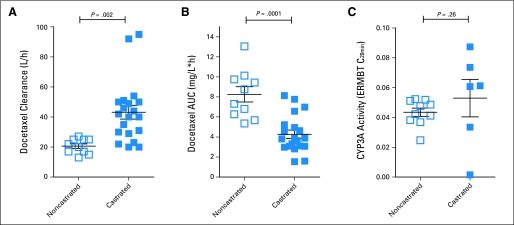
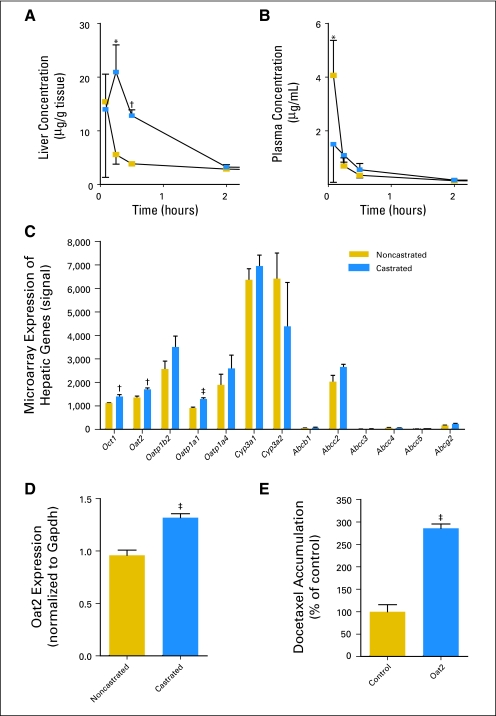
Compared with noncastrated patients, the clearance of docetaxel in castrated patients was approximately 100% increased (Fig 1A) and was associated with an approximately two-fold reduction in AUC (Fig 1B; P = .0001). However, results obtained with an erythromycin breath test indicated that hepatic activity of CYP3A4, the main docetaxel-metabolizing enzyme,1 was not altered in castrated patients, as determined by a Wilcoxon signed-rank test (Fig 1C; P = .26). Next, we considered the possibility that the uptake of docetaxel in the liver is increased in a castrated state. Consistent with this hypothesis, we found that the AUC of docetaxel in liver was significantly higher in castrated rats as compared with intact animals (37.0 v 18.0 μg · h/g; P = .01; Fig 2A) and that this phenomenon was associated with a reduced peak concentration of docetaxel in plasma (1.50 v 4.07 μg/mL; P = .04; Fig 2B).
Fig 1.
Effect of castration on (A) docetaxel clearance, (B) the area under the curve (AUC) of docetaxel normalized to 75 mg/m2, and (C) hepatic CYP3A4 activity as determined by the erythromycin breath test (ERMBT) in noncastrated (open squares, n = 10) and castrated (closed squares, n = 20 for docetaxel studies and n = 6 for ERMBT) patients with prostate cancer. Each square represents an observation in a single patient, and horizontal lines and error bars represent mean and SE, respectively.
Fig 2.
Effect of castration on the concentration-time profile of docetaxel (10 mg/kg, iv) in (A) liver and (B) plasma of noncastrated (gold squares) and castrated (blue squares) Sprague-Dawley rats and on the hepatic expression of solute carriers and adenosine triphosphate binding cassette transporters as measured by (C) microarray or (D) real-time quantitative polymerase chain reaction (n = three to four animals per time point) in noncastrated (gold bars) and castrated (blue bars) Sprague-Dawley rats. (E) Transport of docetaxel in water-injected control (gold bar) Xenopus laevis oocytes or those expressing the rat Oat2 transporter (blue bar, n = 22 observations per group). All data are shown as mean (symbol or bar) and SE (error bar). *P < .05; †P < .01; ‡P < .001.
As a first step toward understanding the mechanism underlying the impact of castration on the liver disposition of docetaxel, we performed microarray analyses on rats liver biopsies. This analysis revealed that castration was associated with a significant increase in the hepatic expression of solute carrier genes encoding the organic cation transporter rOct1 (Slc22a1), the organic anion transporter rOat2 (Slc22a7), and the organic anion transporting polypeptide rOatp1a1 (Slco1a1), whereas the expression of adenosine triphosphate binding cassette transporter genes such as Abcb1a (Mdr1a) and Abcc2 (Mrp2), as well as members of the Cyp3a gene family, remained unchanged (Fig 3C).
Fig 3.
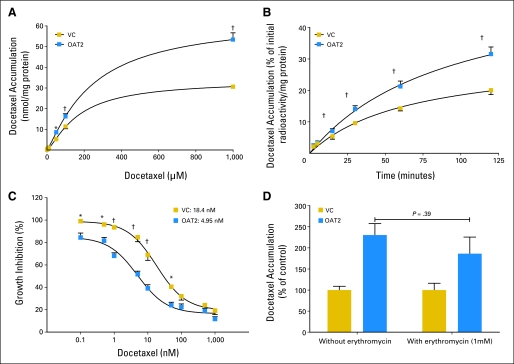
Effect of (A) docetaxel concentration, (B) incubation time, and (D) erythromycin on the transport of docetaxel by the human OAT2 transporter in HEK293 cells and (C) effect of OAT2 on docetaxel-induced growth inhibition. All data are shown as mean (symbol or bar) and SE (error bar) of 5 to 15 observations per group. VC, vector control. *P < .01; †P < .001.
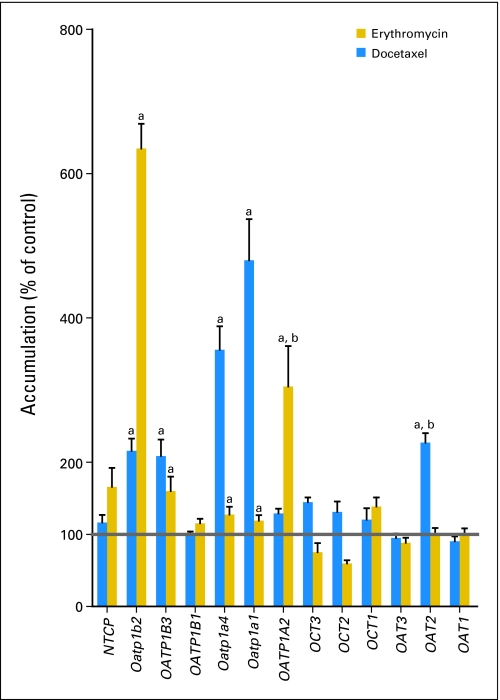
We found that docetaxel is a transported substrate for OAT2 and rOatp1a1, but not for OCT1 (Appendix Fig A1, online only). However, because erythromycin was also a substrate for rOatp1a1, but not for OAT2 (Appendix Fig A1), we focused further specifically on the possibility that increased activity of OAT2 after castration may be the main contributor to the altered pharmacokinetic profile of docetaxel. The increase in hepatic expression of rOat2 observed in the microarray analysis was confirmed with quantitative polymerase chain reaction (Fig 2D). Moreover, rOat2 was capable of taking up docetaxel in an in vitro model (Fig 2E), confirming that docetaxel is a substrate for this transporter. The capability of human OAT2 to transport docetaxel was further confirmed in an in vitro model by demonstrating concentration dependence (Fig 3A) and time dependence of cellular uptake (Fig 3B). We also found that cells overexpressing OAT2 showed a 3.7-fold increase in docetaxel-mediated cytotoxicity compared with control cells (Fig 3C) and that OAT2-mediated uptake of docetaxel in vitro was not influenced by erythromycin (Fig 3D).
DISCUSSION
This study compared the clearance of docetaxel in patients with prostate cancer who underwent castration and those who had intact testicular function to understand the mechanistic basis for the previously noted castration-dependent changes in the incidence of severe neutropenia after docetaxel-based chemotherapy in this patient population. The results of this study indicate that the clearance of docetaxel is substantially increased after castration, probably because of an increase in the hepatocellular uptake of docetaxel.
Because hepatic metabolism is the primary elimination pathway of docetaxel,1,16,22 with urinary excretion accounting for less than 10% of the administered dose,23 we initially hypothesized that the increased clearance in men after castration is the result of increased CYP3A4-mediated metabolism of docetaxel in the liver. However, we found that castration was not associated with significantly elevated hepatic CYP3A4 activity. Although this finding is consistent with a recent report suggesting a lack of phenotypic changes in hepatic CYP3A4 activity in men with prostate cancer before and after start of treatment with luteinizing hormone–releasing hormone agonists such as leuprolide and goserelin,24 it should be noted that in the current study, the small population size is potentially problematic and may cause a type II statistical error. Additional investigation in a larger population will be required to confirm that CYP3A activity is not altered by castration in men with prostate cancer, although evaluation of the erythromycin breath test in patients undergoing rapid androgen cycling also showed no difference between low or high testosterone cycles.5 Collectively, these findings indicate that castration is not causing changes in CYP3A4 activity and also suggest that testosterone, a known inhibitor of CYP3A4-mediated docetaxel hydroxylation in human liver microsomes,25 is unlikely to be directly causative for the observed kinetic alterations of docetaxel after castration.
In the context of prostate cancer, it is of note that docetaxel is typically given in combination with prednisone, an inducer of CYP3A4 expression in human hepatocytes.26 Furthermore, in pediatric patients, prednisone can increase the clearance of etoposide,27 a known CYP3A4 substrate.28 This raises the possibility that the low incidence of severe neutropenia in castrated men with prostate cancer receiving docetaxel is due to the coadministration of prednisone. However, in a subset analysis of the TAX327 study, no significant differences were observed in docetaxel clearance when the drug was administered either alone or in combination with prednisone.29 This suggests that concurrent administration of prednisone does not contribute to the altered clearance of docetaxel in patients with prostate cancer.
Recent experimental data have demonstrated that interpretation of the erythromycin breath test is dependent on the activity of a number of transporters expressed in the liver.30–32 For example, in vitro and in vivo data suggest that the uptake of erythromycin is dependent on active transport by solute carriers, such as OATP1B3,30 and that biliary secretion is regulated by the adenosine triphosphate binding cassette transporters ABCB1 (P-glycoprotein) and ABCC2 (MRP2; cMOAT).33 Taking these studies into consideration, we can assume that castration is unlikely to impact the function of these transporters, as castration-dependent changes would have been seen in the erythromycin breath test results. The uptake of docetaxel in the liver is also, at least in part, transporter-dependent, with a known role for OATP1B3 and OAT2.10,20 These transporters are highly expressed in both the human and rat liver, but not in the mouse,19 and mediate the hepatic uptake of a variety of compounds.18,34,35 Because the erythromycin breath test results strongly suggest that the activities of CYP3A4 and OATP1B3 are not affected by castration, involvement of other docetaxel uptake transporters, such as OAT2, seems likely. Support for this possibility was obtained in rats, where castration was associated with both increased uptake of docetaxel in the liver and also with an increased expression of rOat2 mRNA.
Although to our knowledge an increase in OAT2 expression in livers after castration has not been previously reported, increased expression of rOat2 after castration has been observed previously in the kidney.18 The increase in hepatic rOat2 expression of rats in the current study presents one possible mechanism underlying the increased clearance of docetaxel seen in patients who undergo castration. Indeed, higher levels of OAT2 would theoretically lead to increased uptake of docetaxel into the liver, thus allowing for enhanced CYP3A4-mediated metabolism, and would be reflected in a decreased AUC and increased clearance. Additional investigation is required to unravel the regulatory mechanisms affecting hepatic expression of OAT2 and their dependence on sex hormones.
Collectively, this study suggests that the decreased incidence of neutropenia in castrated patients with prostate cancer after docetaxel-based chemotherapy5–9 is likely due to increased hepatic uptake of docetaxel, leading to a decrease in systemic exposure. This strongly suggests that castrated patients with prostate cancer undergoing docetaxel treatment should be capable of tolerating elevated doses to offset their intrinsically increased ability to clear the drug. Furthermore, the increased clearance found after castration implies that these patients may not be benefiting optimally from standard dosages of docetaxel and may require a dosage increase to prevent undertreatment. It is recommended that information on disease- and/or hormone-related changes in drug clearance and treatment outcome should be considered and assessed prospectively.
Acknowledgment
We thank David Finkelstein for help with analyzing gene expression data, Walter Loos for valuable discussion and preliminary studies in rodents, Yuichi Sugiyama for providing the OAT2 over-expressing HEK293 cells, and Ming Zhao for analysis of hormone levels in patient samples.
Appendix
Fig A1.
Intracellular accumulation of erythromycin and docetaxel in HEK293 cells or Xenopus laevis oocytes overexpressing a variety of solute carriers. Transporters that have significant accumulation of drug, as compared with controls, are denoted by (a). There is substrate overlap in both docetaxel and erythromycin transport by Oatp1a1, Oatp1a4, OATP1B3, and Oatp1b2. However, only docetaxel is a substrate for OAT2, and only erythromycin is a substrate for OATP1A2 (denoted by b). Bars represent mean (% of control) and SE (error bar).
Table A1.
Patient Demographics and Baseline Hormone Levels by Group
| Variable | Noncastrated | Castrated | P |
|---|---|---|---|
| No. of patients | 10 | 20 | — |
| Age, years | .17 | ||
| Median | 64 | 70 | |
| Range | 52-83 | 54-80 | |
| Racial ancestry | |||
| White | 10 | 18 | — |
| African-American | 0 | 2 | — |
| BSA, m2 | .29 | ||
| Median | 2.16 | 2.03 | |
| Range | 1.81-2.29 | 1.73-2.54 | |
| Hormone level (ng/mL) | |||
| Andesterone | BLQ | BLQ | — |
| DHEA* | 0.685 | 0.235 | .07 |
| DHEA sulfate | 1.49 | 3.32 | .93 |
| Dihydrotestosterone† | BLQ | BLQ | — |
| Estrone* | 0.620 | 0.465 | .39 |
| Estradiol* | 0.275 | BLQ | .018 |
| Progesterone | BLQ | BLQ | — |
| Testosterone | 1.20 | BLQ | < .0001 |
NOTE. Values represent median and range (continuous variables) or number of patients (categorical variables).
Abbreviations: BSA, body-surface area; BLQ, below the lower limit of quantitation of the analytical assay; DHEA, dehydroepiandrosterone.
BLQ values in castrated patients: DHEA (seven of 20 patients), estrone (five of 20 patients), estradiol (14 of 20 patients).
Only one patient in each group had values above BLQ.
Footnotes
Supported in part by the American Lebanese Syrian Associated Charities, the United States Public Health Service Cancer Center Support Grant No. 3P30CA021765 (S.D.B.), and the National Institutes of Health Grant No. 3P30CA006973-47 (M.A.R.).
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, May 13-17, 2005, Orlando, FL; and the 109th Annual Meeting of the American Society of Clinical Pharmacology and Therapeutics, April 2-5, 2008, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Michael A. Carducci, sanofi-aventis (C) Stock Ownership: None Honoraria: Michael A. Carducci, sanofi-aventis Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Ryan M. Franke, Michael A. Carducci, Sharyn D. Baker, Alex Sparreboom
Financial support: Michelle A. Rudek, Sharyn D. Baker
Provision of study materials or patients: Michael A. Carducci
Collection and assembly of data: Ryan M. Franke, Michelle A. Rudek, Sharyn D. Baker
Data analysis and interpretation: Ryan M. Franke, Michael A. Carducci, Michelle A. Rudek, Sharyn D. Baker, Alex Sparreboom
Manuscript writing: Ryan M. Franke, Michael A. Carducci, Michelle A. Rudek, Sharyn D. Baker, Alex Sparreboom
Final approval of manuscript: Ryan M. Franke, Michael A. Carducci, Michelle A. Rudek, Sharyn D. Baker, Alex Sparreboom
REFERENCES
- 1.Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel: Recent developments. Clin Pharmacokinet. 2006;45:235–252. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 3.Bruno R, Olivares R, Berille J, et al. Alpha-1-acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9:1077–1082. [PubMed] [Google Scholar]
- 4.sanofi-aventis: Product insert for Taxotere (docetaxel) http://products.sanofi-aventis.us/Taxotere/taxotere.html.
- 5.Rathkopf D, Carducci MA, Morris MJ, et al. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J Clin Oncol. 2008;26:2959–2965. doi: 10.1200/JCO.2007.15.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain A, Dawson N, Amin P, et al. Docetaxel followed by hormone therapy in men experiencing increasing prostate-specific antigen after primary local treatments for prostate cancer. J Clin Oncol. 2005;23:2789–2796. doi: 10.1200/JCO.2005.07.152. [DOI] [PubMed] [Google Scholar]
- 7.Taplin ME, Xie W, Bubley GJ, et al. Docetaxel, estramustine, and 15-month androgen deprivation for men with prostate-specific antigen progression after definitive local therapy for prostate cancer. J Clin Oncol. 2006;24:5408–5413. doi: 10.1200/JCO.2006.06.6589. [DOI] [PubMed] [Google Scholar]
- 8.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 9.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 10.Baker SD, Verweij J, Cusatis GA, et al. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85:155–163. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooker AC, Ten Tije AJ, Carducci MA, et al. Population pharmacokinetic model for docetaxel in patients with varying degrees of liver function: Incorporating cytochrome P4503A activity measurements. Clin Pharmacol Ther. 2008;84:111–118. doi: 10.1038/sj.clpt.6100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinibaldi VJ, Elza-Brown K, Schmidt J, et al. Phase II evaluation of docetaxel plus exisulind in patients with androgen independent prostate carcinoma. Am J Clin Oncol. 2006;29:395–398. doi: 10.1097/01.coc.0000225411.95479.b4. [DOI] [PubMed] [Google Scholar]
- 13.Baker SD, van Schaik RH, Rivory LP, et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res. 2004;10:8341–8350. doi: 10.1158/1078-0432.CCR-04-1371. [DOI] [PubMed] [Google Scholar]
- 14.Baker SD, Zhao M, He P, et al. Simultaneous analysis of docetaxel and the formulation vehicle polysorbate 80 in human plasma by liquid chromatography/tandem mass spectrometry. Anal Biochem. 2004;324:276–284. doi: 10.1016/j.ab.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Baker SD, Yan X, et al. Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids. 2004;69:721–726. doi: 10.1016/j.steroids.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Marre F, Sanderink GJ, de Sousa G, et al. Hepatic biotransformation of docetaxel (Taxotere) in vitro: Involvement of the CYP3A subfamily in humans. Cancer Res. 1996;56:1296–1302. [PubMed] [Google Scholar]
- 17.Vaclavikova R, Soucek P, Svobodova L, et al. Different in vitro metabolism of paclitaxel and docetaxel in humans, rats, pigs, and minipigs. Drug Metab Dispos. 2004;32:666–674. doi: 10.1124/dmd.32.6.666. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi Y, Hirokawa N, Ohshiro N, et al. Differential gene expression of organic anion transporters in male and female rats. Biochem Biophys Res Commun. 2002;290:482–487. doi: 10.1006/bbrc.2001.6180. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y, Ohshiro N, Shibusawa A, et al. Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol. 2002;62:7–14. doi: 10.1124/mol.62.1.7. [DOI] [PubMed] [Google Scholar]
- 20.Smith NF, Acharya MR, Desai N, et al. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–818. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- 21.Hu S, Niu H, Minkin P, et al. Comparison of antitumor effects of multitargeted tyrosine kinase inhibitors in acute myelogenous leukemia. Mol Cancer Ther. 2008;7:1110–1120. doi: 10.1158/1535-7163.MCT-07-2218. [DOI] [PubMed] [Google Scholar]
- 22.Shou M, Martinet M, Korzekwa KR, et al. Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: Enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics. 1998;8:391–401. doi: 10.1097/00008571-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Clarke SJ, Rivory LP. Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet. 1999;36:99–114. doi: 10.2165/00003088-199936020-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hutson PR, Oettel K, Douglas J, et al. Effect of medical castration on CYP3A4 enzyme activity using the erythromycin breath test. Cancer Chemother Pharmacol. 2008;62:373–377. doi: 10.1007/s00280-007-0613-6. [DOI] [PubMed] [Google Scholar]
- 25.Royer I, Monsarrat B, Sonnier M, et al. Metabolism of docetaxel by human cytochromes P450: Interactions with paclitaxel and other antineoplastic drugs. Cancer Res. 1996;56:58–65. [PubMed] [Google Scholar]
- 26.Pichard L, Fabre I, Daujat M, et al. Effect of corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Mol Pharmacol. 1992;41:1047–1055. [PubMed] [Google Scholar]
- 27.Kishi S, Yang W, Boureau B, et al. Effects of prednisone and genetic polymorphisms on etoposide disposition in children with acute lymphoblastic leukemia. Blood. 2004;103:67–72. doi: 10.1182/blood-2003-06-2105. [DOI] [PubMed] [Google Scholar]
- 28.Relling MV, Nemec J, Schuetz EG, et al. O-demethylation of epipodophyllotoxins is catalyzed by human cytochrome P450 3A4. Mol Pharmacol. 1994;45:352–358. [PubMed] [Google Scholar]
- 29.Dagher R, Li N, Abraham S, et al. Approval summary: Docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin Cancer Res. 2004;10:8147–8151. doi: 10.1158/1078-0432.CCR-04-1402. [DOI] [PubMed] [Google Scholar]
- 30.Franke RM, Baker SD, Mathijssen RH, et al. Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin Pharmacol Ther. 2008;84:704–709. doi: 10.1038/clpt.2008.94. [DOI] [PubMed] [Google Scholar]
- 31.Frassetto LA, Poon S, Tsourounis C, et al. Effects of uptake and efflux transporter inhibition on erythromycin breath test results. Clin Pharmacol Ther. 2007;81:828–832. doi: 10.1038/sj.clpt.6100148. [DOI] [PubMed] [Google Scholar]
- 32.Kurnik D, Wood AJ, Wilkinson GR. The erythromycin breath test reflects P-glycoprotein function independently of cytochrome P450 3A activity. Clin Pharmacol Ther. 2006;80:228–234. doi: 10.1016/j.clpt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Franke RM, Baker SD, Schuetz EG, et al. ABCC2 polymorphism as a determinant of the erythromycin breath test. Clin Pharmacol Ther. 2009;85:S67. [Google Scholar]
- 34.König J, Cui Y, Nies AT, et al. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278:G156–G164. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- 35.Sun W, Wu RR, van Poelje PD, et al. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001;283:417–422. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]