Abstract
Purpose
To compare response rates for two schedules of irinotecan with vincristine in patients with rhabdomyosarcoma at first relapse or disease progression.
Patients and Methods
Patients with first relapse or progression of rhabdomyosarcoma and an unfavorable prognosis were randomly assigned to one of two treatment schedules of irinotecan with vincristine: regimen 1A included irinotecan 20 mg/m2/d intravenously for 5 days at weeks 1, 2, 4, and 5 with vincristine 1.5 mg/m2 administered intravenously on day 1 of weeks 1, 2, 4, and 5; regimen 1B included irinotecan 50 mg/m2/d intravenously for 5 days at weeks 1 and 4 with vincristine as in regimen 1A. Disease response was assessed at week 6. Those with responsive disease continued to receive 44 weeks of multiagent chemotherapy that incorporated the assigned irinotecan-vincristine regimen.
Results
Ninety-two eligible patients were randomly assigned (1A, 45; 1B, 47). Response could be assessed in 89 patients (1A, 42; 1B, 47). There were five complete responses and six partial responses on regimen 1A (response rate, 26%; 95% CI, 16% to 42%) and 17 partial responses on regimen 1B (response rate, 37%; 95% CI, 25% to 51%; P = .36). Neutropenia was less common on regimen 1A (P = .04). One-year failure-free and overall survival rates for regimen 1A were 37% (95% CI, 23% to 51%) and 55% (95% CI, 39% to 69%), respectively, and for 1B, they were 38% (95% CI, 25% to 53%) and 60% (95% CI, 44% to 72%).
Conclusion
There was no difference in the response rates between the two irinotecan-vincristine schedules. We recommend the shorter, more convenient regimen (1B) for further investigation.
INTRODUCTION
Patients diagnosed with rhabdomyosarcoma (RMS) who relapse have a poor prognosis.1,2 This is especially true for patients with alveolar histology; stage 2 or 3 and clinical group II or III embryonal histology; or those with stage 1 or clinical group I embryonal tumors who were initially treated with vincristine, dactinomycin, and cyclophosphamide (VAC). For these patients, the 5-year postrelapse survival rate is approximately 10% compared with that for a relatively more favorable group comprising those with botryoid histology and stage 1 or clinical group I embryonal tumors treated with vincristine and dactinomycin in whom survival is approximately 50%.2 The Soft Tissue Sarcoma Committee of the Children's Oncology Group (COG) chose to evaluate the activity of two schedules of irinotecan in a group of patients with an unfavorable prognosis and measurable disease.
Irinotecan (CPT-11; 7-ethyl-10-(4-[1-piperidino-]-1-piperidino)-carbonyloxy-camptothecin) is a synthetic, water soluble analog of camptothecin that stabilizes topoisomerase-I DNA adducts resulting in single-strand DNA breaks and cytotoxicity, mainly in the S phase of the cell cycle.3 Xenografts derived from untreated patients with RMS have been shown to be highly responsive to irinotecan.4 In addition, RMS xenografts that were resistant to vincristine, melphalan, and topotecan responded to irinotecan. The antitumor activity of irinotecan in RMS and other tumor xenograft models is highly schedule dependent, and prolonged exposures result in improved tumor responsiveness.5-8 In addition, vincristine shows synergism with the camptothecins.9,10 Clinical trials with irinotecan have used both a short and a prolonged schedule in phase I studies conducted by the Pediatric Oncology Group11 and St. Jude Children's Research Hospital12 (daily for 5 days v daily for 5 days, 2 days off, and then an additional 5 days, respectively). The respective dose-limiting toxicities were myelosuppression and diarrhea. Since the administration schedule of irinotecan appeared to be an important in vivo determinant of antitumor activity, we conducted a highly innovative, randomized phase II window trial to directly compare two schedules of irinotecan together with vincristine in patients with RMS at first relapse or disease progression.
PATIENTS AND METHODS
Patients eligible for COG-ARST0121 had biopsy-proven RMS, undifferentiated sarcoma or ectomesenchymoma, and were younger than 21 years of age at the time of initial diagnosis; experienced first relapse or disease progression; and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2 and a life expectancy of at least 2 months. Adequate organ function was required as defined by a hemoglobin > 10 g/dL (transfusion allowed); absolute neutrophil count > 750/μL; platelet count > 75,000/μL; serum creatinine < 1.5 × normal for age or creatinine clearance/radioisotope glomerular filtration rate > 70 mL/min/1.73 m2; serum bilirubin < 1.5 × normal for age; serum alanine transaminase < 2.5 × normal for age; and left ventricular shortening fraction of > 27% by echocardiogram or ejection fraction of > 50% by gated radionuclide scan. Any CNS toxicity had to be grade < 2, and adequate control of any seizure disorder with anticonvulsants was required. Patients who had received more than one prior chemotherapy treatment regimen, those with prior exposure to anthracyclines, ischemic heart disease, myeloablative chemotherapy followed by hematopoietic stem-cell rescue, disease impinging on or within the brain and spinal cord, and those who were pregnant/lactating were excluded. Written informed consent was required from all participants and/or their parents/legal guardians after all institutional, US Food and Drug Administration, and National Cancer Institute (NCI) requirements for human studies were met.
Clinical Trial Design
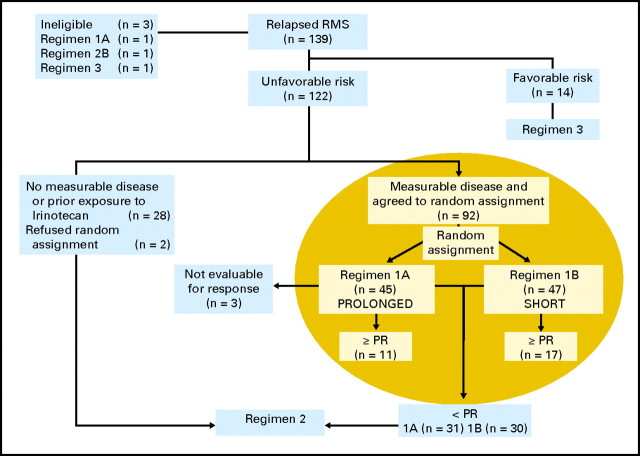
The overall experimental design of the ARST0121 trial is shown in Figure 1. Patients with an unfavorable prognosis (alveolar histology at initial diagnosis; stage 1 clinical group I embryonal histology at initial diagnosis with distant recurrence; or stage 2, 3, or 4 and clinical group II, III, or IV embryonal histology at initial diagnosis) and measurable disease who consented were randomly assigned to one of two treatment schedules of irinotecan with vincristine: regimen 1A included irinotecan 20 mg/m2/d intravenously for 5 days at weeks 1, 2, 4, and 5 with vincristine 1.5 mg/m2 administered intravenously on day 1 of weeks 1, 2, 4, and 5; regimen 1B included irinotecan 50 mg/m2/d intravenously for 5 days at weeks 1 and 4 with vincristine as in regimen 1A. Disease response was assessed by the treating institution using the NCI Response Evaluation Criteria in Solid Tumors (RECIST) at week 6. Those with responsive disease (complete response [CR] or partial response [PR]) continued to receive 44 weeks of multiagent chemotherapy that incorporated the assigned irinotecan-vincristine regimen. The schedule, route, and doses of chemotherapy agents are depicted in Table 1. Those who were ineligible for and who refused random assignment and those who demonstrated less than a PR to 6 weeks of irinotecan-vincristine window therapy were nonrandomly assigned to 31 weeks of multiagent chemotherapy including doxorubicin-cyclophosphamide together with tirapazamine 330 mg/m2 intravenously on day 1 alternating with etoposide-ifosfamide (regimen 2). Patients with a favorable prognosis (botryoid histology at initial diagnosis, any stage or group, and embryonal tumors that were stage 1 or clinical group I at initial diagnosis, not treated with VAC, and who recurred either locally or regionally) at the time of relapse were assigned to 31 weeks of multiagent therapy identical to regimen 2 but without tirapazamine (regimen 3). The outcomes for patients treated with regimens 2 and 3 will be reported separately.
Fig 1.
Scheme and details of Children's Oncology Group-ARST0121 study. The highlighted area represents the patients described in this analysis. RMS, rhabdomyosarcoma; PR, partial response.
Table 1.
Treatment Regimen for Randomly Assigned Patients
| Chemotherapy | Regimen 1A | Regimen 1B |
|---|---|---|
| Irinotecan (IV) | 20 mg/m2 for 5 days on weeks 1, 2, 4, 5, 13, 14, 25, 26, 34, 35, 46, 47, 49, 50 | 50 mg/m2 for 5 days on weeks 1, 4, 13, 25, 34, 46, 49 |
| Vincristine (IV), 1.5 mg/m2* | Weeks 1, 2, 4, 5, 13, 14, 25, 26, 34, 35, 46, 47, 49, 50 | Identical to 1A |
| Doxorubicin (IV), 75 mg/m2 | Weeks 7, 16, 28, 37, 40 | Identical to 1A |
| Cyclophosphamide (IV), 1.2 g/m2 | Weeks 7, 16, 28, 37, 40 | Identical to 1A |
| Etoposide (IV), 100 mg/m2/d × 5) | Weeks 10, 19, 22, 28, 31, 37 | Identical to 1A |
| Ifosfamide (IV), 1.8 g/m2/d × 5) | Weeks 10, 19, 22, 28, 31, 37 | Identical to 1A |
Abbreviation: IV, intravenous.
Maximum dose, 2 mg.
Statistical Analysis
This analysis compares the response rate, toxicities, failure-free survival (FFS), and overall survival (OS) of patients treated on regimens 1A and 1B. The study was powered to detect a 25% improvement in the response rate to regimen 1A compared with 1B (α = .1, 1-β = .9, one-sided test favoring regimen 1A since the only difference of clinical importance was an improved response with the more prolonged but inconvenient schedule of regimen 1A). A sample size of 51 patients per arm (102 randomly assigned patients) was required to detect a significant improvement in response rate. CR was defined as disappearance of all target lesions, PR as a ≥ 30% decrease in the dimension used to define the target lesion, progressive disease (PD) as a ≥ 20% increase in the measurement used to define the target lesion, and stable disease as insufficient tumor shrinkage or increase to be considered PR or PD. Toxicities were reported using the NCI Common Toxicity Criteria. Fisher's exact test was used to compare the difference in proportions for baseline patient characteristics and treatment response between regimens 1A and 1B. The estimation of survival was performed using the Kaplan- Meier method, and the curves were compared using the log-rank test. OS was defined as the time from enrollment to death from any cause. FFS was defined as the time of enrollment to disease progression or death. A P value < .05 was considered statistically significant.
RESULTS
Patient Characteristics
COG-ARST0121 enrolled 139 patients between June 2002 and October 2006 (Fig 1). Ninety-three patients were enrolled and randomly assigned between the prolonged (regimen 1A) and the short (regimen 1B) schedules of irinotecan together with vincristine, and one was deemed ineligible on regimen 1A because there was no informed consent available. Characteristics of patients, including age, histology, primary site, size of the largest lesion, and whether the recurrence was local, regional or distant, were similar for those treated on regimens 1A and 1B (Table 2). However, there was a larger proportion of males on regimen 1B compared with 1A (70% v 40%; P = .004; Table 2). Recurrences were local in 25 patients; regional nodal in seven; distant metastatic in 36; combined local and regional nodal in five; combined local and distant metastatic in 10; and combined local, regional nodal, and distant metastatic in two. Types of recurrences were well distributed between the two regimens. Data on site of recurrence was not available for three patients treated on regimen 1A and for four treated on regimen 1B.
Table 2.
Patient Characteristics
| Characteristic | Regimen 1A (n = 45) | Regimen 1B (n = 47) | P |
|---|---|---|---|
| Age, years | |||
| < 10 | 24 | 24 | N/S |
| ≥ 10 | 21 | 23 | |
| Sex | |||
| Male | 18 | 33 | .004 |
| Female | 27 | 14 | |
| Histology | |||
| Alveolar | 22 | 27 | N/S |
| Embryonal | 15 | 16 | |
| Other | 8 | 4 | |
| Primary site (at original diagnosis)* | |||
| Favorable | 6 | 6 | N/S |
| Unfavorable | 39 | 41 | |
| Size (largest target lesion), cm | |||
| ≥ 5 | 12 | 16 | N/S |
| < 5 | 33 | 31 | |
| Recurrence† | |||
| Local | 22 | 20 | N/S |
| Regional lymph nodes | 10 | 11 | |
| Bone marrow/bone | 11 | 9 | |
| Other distant sites | 20 | 23 |
Abbreviation: N/S, not significant.
Favorable sites: nonparameningeal head and neck, nonbladder/prostate genitourinary and biliary; unfavorable sites: all others.
Numbers indicate distribution of sites of recurrence. Patients may have had more than one site of recurrence.
Toxicity
Toxicity was evaluated for the first 6 weeks of therapy on regimens 1A and 1B. There were no unexpected toxicities or deaths during this time. Fifty percent of patients on regimen 1A and 66% on 1B experienced grade ≥ 3 toxicity in the first 6 weeks of therapy. When comparing several important toxicities between regimens 1A and 1B, we did not observe statistically significant differences in diarrhea (22% v 13%; P = .23), anemia (39% v 28%; P = .31), or need for packed red cell transfusion (31% v 21%; P = .21). Although neutropenia was less common on regimen 1A (16% v 34%; P = .04), there was no difference in the incidence of febrile neutropenia between the two regimens (4% v 13%; P = .27). Two percent of patients had grade ≥ 3 thrombocytopenia on both regimens.
Response
Response at week 6 could be assessed in 89 of 92 randomly assigned patients (42 on regimen 1A and 47 on regimen 1B). Three patients on regimen 1A were not evaluable (one withdrew consent, one did not complete window therapy, and one had a response that could not be assessed because of a metal artifact on the radiographic scan). Response data are provided in Table 3. The overall objective response rate (CR + PR) to the phase II window was 31.5%. There was no statistically significant difference between the response rate for regimen 1A (26%; 95% CI, 16% to 42%) and regimen 1B (36%; 95% CI, 25% to 51%; P = .36). Of note, there were no CRs on regimen 1B. There were 10 responses (four CR, six PR) seen in 21 patients with alveolar RMS on regimen 1A. A similar response rate was noted on regimen 1B in which 13 responses were seen in 27 patients with alveolar RMS. Patients with alveolar RMS had a significantly higher response rate when compared with patients with embryonal or other RMS when treated with regimen 1A (48% v 5%; P = .01) versus regimen 1B (48% v 20%; P = .08).
Table 3.
Response Rate of Patients for the Two Schedules of Irinotecan Combined With Vincristine
| Regimen | No. of Patients With Complete Response | % | 95% CI | No. of Patients With Partial Response | % | 95% CI | No. of Patients With Stable Disease | % | 95% CI | No. of Patients With Progressive Disease | % | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A (prolonged; n = 42) | 5 | 12 | 5 to 25 | 6 | 14 | 7 to 28 | 12 | 29 | 17 to 44 | 19 | 45 | 31 to 60 |
| 1B (short; n = 47) | 0 | 17 | 36 | 24 to 50 | 14 | 30 | 19 to 44 | 16 | 34 | 22 to 48 |
Survival
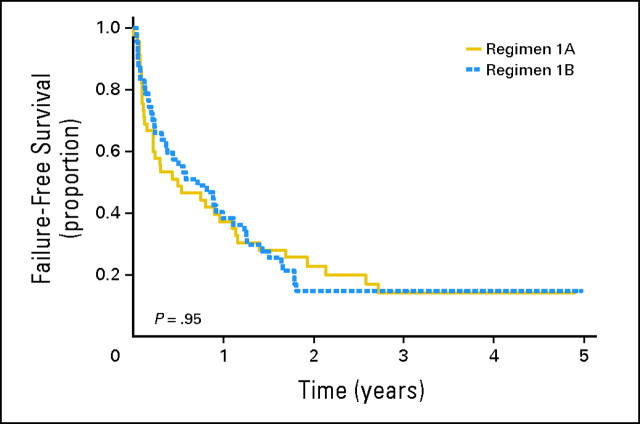
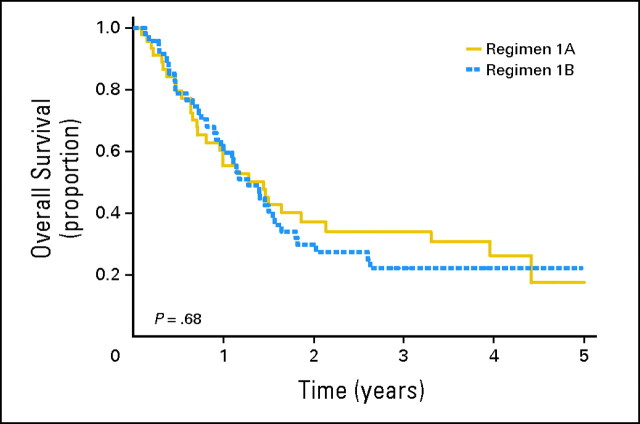
The 1- and 3-year FFS rates for the randomly assigned cohort were 38% (95% CI, 28% to 48%) and 15% (95% CI, 8% to 23%), respectively. Figure 2 depicts the FFS of patients treated on regimens 1A and 1B. FFS was similar between the two regimens (P = .95). The respective 1-year FFS rates on regimens 1A and 1B were 37% (95% CI, 23% to 51%) and 38% (95% CI, 25% to 52%); these declined to 14% (95% CI, 5% to 27%) and 15% (95% CI, 7% to 26%) at 3 years. The median time to disease progression was 0.5 years on regimen 1A and 0.7 years on regimen 1B. The respective 1- and 3- year OS rates for patients treated in the phase II window were 57% (95% CI, 47% to 67%) and 28% (95% CI, 18% to 37%). Figure 3 displays the OS on regimens 1A and 1B. The 1-year OS rates on regimens 1A and 1B were 55% (95% CI, 39% to 68%) and 60% (95% CI, 44% to 72%); these decreased to 34% (95% CI, 20% to 49%) and 22% (95% CI, 11% to 35%), respectively, at 3 years (P = .68). The median survival times were 1.4 years for regimen 1A and 1.3 years for regimen 1B.
Fig 2.
Probability of failure-free survival by randomly assigned regimen.
Fig 3.
Probability of overall survival by randomly assigned regimen.
DISCUSSION
Irinotecan was identified as an active agent for RMS on the basis of significant preclinical work.4,5 Investigators have demonstrated that prolonged exposure to irinotecan results in improved tumor responses in human colon cancer and RMS xenografts.5 Other investigators showed similar results with prolonged irinotecan exposure in ovarian, soft tissue sarcoma, neuroblastoma, and medulloblastoma models.6,7 Prolonged and short schedules, as well as single infusions of irinotecan alone or in combination with other agents, have been investigated in the traditional phase II setting in various pediatric target tumors such as neuroblastoma, Ewing sarcoma, Wilms tumor, and certain malignant brain tumors.13-21 Although preclinical models suggested that a prolonged administration schedule of irinotecan would be more effective than a short schedule, the prolonged schedule is less convenient for patients. No trial to date has compared the prolonged and short schedule. Our study was designed to determine whether the prolonged schedule was superior to the shorter, more convenient schedule. In addition, we tested the two schedules of irinotecan in patients with a historically poor outcome, reasoning that activity in recurrent tumors would predict a higher likelihood of improving survival rates in newly diagnosed patients.
The COG Soft Tissue Sarcoma Committee has conducted several up-front, phase II window trials in patients with previously untreated high-risk RMS.13 To the best of our knowledge, this is the first reported randomized phase II window clinical trial conducted in children and adolescents with RMS at first relapse or disease progression. A relatively large number of patients participated on this study. The toxicity experienced on this trial was similar to that in previous studies,11,12 in that patients treated with the short schedule were more likely to experience myelosuppression when compared with those treated with the prolonged schedule who were somewhat more likely to experience diarrhea. The response rate of 31.5% to irinotecan-vincristine was modest, particularly compared with the 70% response rate seen in an upfront phase II window trial in previously untreated patients with metastatic RMS.10 Responses to phase II therapy may, in part, be dependent on prior therapy. The phase II study of topotecan as a single agent showed no responses in 22 patients with recurrent RMS,22 while the combination of topotecan and cyclophosphamide showed a 67% response rate (10 of 15 patients with recurrent RMS).23
Our trial revealed no differences in response rates between the two schedules of irinotecan, disproving the preclinical prediction of superior activity with a prolonged schedule. One can speculate on why the preclinical data did not translate similarly in this clinical trial. First, vincristine is one of the most active agents against RMS and has been used to treat all newly diagnosed patients with RMS in every Intergroup Rhabdomyosarcoma Study (IRS) and COG study.24-29 When irinotecan was tested alone in a phase II window using the prolonged schedule in previously untreated high-risk patients with RMS, the response rate was 42% and was associated with 32% of patients experiencing PD.10 However the response rate increased to 70% when vincristine was added, with only 8% of patients experiencing PD.10 Perhaps the addition of vincristine to irinotecan abrogated any difference in response rate that would have been observed had the two schedules been compared using irinotecan as a single agent. Second, although the mouse RMS xenograft has accurately identified agents with clinical activity, like all preclinical systems, there are limitations when predicting response and outcome in patients, especially when agents are used in combination. Indeed, topotecan was identified as an active agent in the mouse RMS xenograft and even had significant activity in patients with high-risk and recurrent RMS when combined with cyclophosphamide.5,30,31 However, when topotecan and cyclophosphamide were combined with vincristine and given as cycles alternating with standard VAC therapy, topotecan and cyclophosphamide failed to improve the outcome of newly diagnosed patients with intermediate-risk RMS in a recently concluded COG trial.29 Third, there is a possibility that studying the two schedules in the relapse setting did not allow us to see the difference, because response rates are much lower than those in newly diagnosed patients. The better response rate in patients with alveolar RMS on regimen 1A is interesting. However, the significance of this finding is unclear since our study was not designed to compare response based on histology.
Patients who responded to the irinotecan-vincristine window continued to receive this therapy combined with other chemotherapy agents on this trial. Despite the additional therapy, the median survival time of these patients was similar to that in the historical report.2 This highlights the fact that relapsed disease has a uniformly poor prognosis with present treatment strategies. Therefore, to improve survival in RMS, novel strategies are required either to improve the outcome for relapsed patients or to decrease the relapse rate in newly diagnosed patients. On the basis of the results of this trial, we have chosen the shorter, more convenient schedule of irinotecan for future front-line COG RMS clinical investigations.
Footnotes
Supported by Children's Oncology Group National Institutes of Health Chair's Grant No. U10 CA98543 and the Statistics & Data Center Grant No. U10 CA9841 (J.R.A.).
Presented at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00025363.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Philip P. Breitfeld, David O. Walterhouse, Sarah S. Donaldson, Charles N. Paidas, James R. Anderson, William H. Meyer, Douglas S. Hawkins
Administrative support: William H. Meyer
Collection and assembly of data: Leo Mascarenhas, Philip P. Breitfeld, David M. Parham, James R. Anderson, Douglas S. Hawkins
Data analysis and interpretation: Leo Mascarenhas, Elizabeth R. Lyden, David O. Walterhouse, Charles N. Paidas, James R. Anderson, William H. Meyer, Douglas S. Hawkins
Manuscript writing: Leo Mascarenhas, Elizabeth R. Lyden, Philip P. Breitfeld, David O. Walterhouse, Sarah S. Donaldson, Charles N. Paidas, David M. Parham, James R. Anderson, William H. Meyer, Douglas S. Hawkins
Final approval of manuscript: Leo Mascarenhas, Elizabeth R. Lyden, Philip P. Breitfeld, David O. Walterhouse, Sarah S. Donaldson, Charles N. Paidas, David M. Parham, James R. Anderson, William H. Meyer, Douglas S. Hawkins
REFERENCES
- 1.Raney RB, Jr, Crist WM, Maurer HM, et al. Prognosis of children with soft tissue sarcoma who relapse after achieving a complete response: A report from the Intergroup Rhabdomyosarcoma Study I. Cancer. 1983;52:44–50. doi: 10.1002/1097-0142(19830701)52:1<44::aid-cncr2820520110>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Pappo AS, Anderson JR, Crist WM, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 1999;17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487. [DOI] [PubMed] [Google Scholar]
- 3.Creemers GJ, Lund B, Verweij J. Topoisomerase I inhibitors: Topotecan and irenotecan. Cancer Treat Rev. 1994;20:73–96. doi: 10.1016/0305-7372(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 4.Houghton PJ, Cheshire PJ, Hallman JC, et al. Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: Lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Res. 1993;53:2823–2829. [PubMed] [Google Scholar]
- 5.Houghton PJ, Cheshire PJ, Hallman JD, 2nd, et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol. 1995;36:393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- 6.Jansen WJ, Kolfschoten GM, Erkelens CA, et al. Anti-tumor activity of CPT-11 in experimental human ovarian cancer and human soft-tissue sarcoma. Int J Cancer. 1997;73:891–896. doi: 10.1002/(sici)1097-0215(19971210)73:6<891::aid-ijc22>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Vassal G, Boland I, Santos A, et al. Potent therapeutic activity of irinotecan (CPT-11) and its schedule dependency in medulloblastoma xenografts in nude mice. Int J Cancer. 1997;73:156–163. doi: 10.1002/(sici)1097-0215(19970926)73:1<156::aid-ijc24>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Vassal G, Terrier-Lacombe MJ, Bissery MC, et al. Therapeutic activity of CPT-11, a DNA-topoisomerase I inhibitor, against peripheral primitive neuroectodermal tumour and neuroblastoma xenografts. Br J Cancer. 1996;74:537–545. doi: 10.1038/bjc.1996.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson J, George EO, Poquette CA, et al. Synergy of topotecan in combination with vincristine for treatment of pediatric solid tumor xenografts. Clin Cancer Res. 1999;5:3617–3631. [PubMed] [Google Scholar]
- 10.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: The Children's Oncology Group. J Clin Oncol. 2007;25:362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 11.Blaney S, Berg SL, Pratt C, et al. A phase I study of irinotecan in pediatric patients: A Pediatric Oncology Group study. Clin Cancer Res. 2001;7:32–37. [PubMed] [Google Scholar]
- 12.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;17:1815–1824. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 13.Soft Tissue Sarcoma Committee of the Children's Oncology Group. Lager JJ, Lyden ER, et al. Pooled analysis of phase II window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2006;24:3415–3422. doi: 10.1200/JCO.2005.01.9497. [DOI] [PubMed] [Google Scholar]
- 14.Bomgaars LR, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol. 2007;25:4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 15.Vassal G, Giammarile F, Brooks M, et al. A phase II study of irinotecan in children with relapsed or refractory neuroblastoma: A European cooperation of the Société Française d'Oncologie Pédiatrique (SFOP) and the United Kingdom Children Cancer Study Group (UKCCSG) Eur J Cancer. 2008;44:2453–2460. doi: 10.1016/j.ejca.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Kushner BH, Kramer K, Modak S, et al. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol. 2006;24:5271–5276. doi: 10.1200/JCO.2006.06.7272. [DOI] [PubMed] [Google Scholar]
- 17.Osone S, Hosoi H, Tsuchiya K, et al. Low-dose protracted irinotecan as a palliative chemotherapy for advanced neuroblastoma. J Pediatr Hematol Oncol. 2008;30:853–856. doi: 10.1097/MPH.0b013e318180bb71. [DOI] [PubMed] [Google Scholar]
- 18.Turner CD, Gururangan S, Eastwood J, et al. Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: The Duke experience. Neuro Oncol. 2002;4:102–108. doi: 10.1093/neuonc/4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner LM, McAllister N, Goldsby RE, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48:132–139. doi: 10.1002/pbc.20697. [DOI] [PubMed] [Google Scholar]
- 20.Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: The Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029–1034. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 21.Shitara T, Shimada A, Hanada R, et al. Irinotecan for children with relapsed solid tumors. Pediatr Hematol Oncol. 2006;23:103–110. doi: 10.1080/08880010500457152. [DOI] [PubMed] [Google Scholar]
- 22.Nitschke R, Parkhurst J, Sullivan J, et al. Topotecan in pediatric patients with recurrent and progressive solid tumors: A Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol. 1998;20:315–318. doi: 10.1097/00043426-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Saylors RL, 3rd, Stine KC, Sullivan J, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: A Pediatric Oncology Group phase II study. J Clin Oncol. 2001;19:3463–3469. doi: 10.1200/JCO.2001.19.15.3463. [DOI] [PubMed] [Google Scholar]
- 24.Maurer HM, Moon T, Donaldson M, et al. The intergroup rhabdomyosarcoma study: A preliminary report. Cancer. 1977;40:2015–2026. doi: 10.1002/1097-0142(197711)40:5<2015::aid-cncr2820400505>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 27.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 28.Raney RB, Maurer HM, Anderson JR, et al. The Intergroup Rhabdomyosarcoma Study Group (IRSG): Major lessons from the IRS-I through IRS-IV studies as background for the current IRS-V treatment protocols. Sarcoma. 2001;5:9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group Study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappo AS, Lyden E, Breneman J, et al. Up-front window trial of topotecan in previously untreated children and adolescents with metastatic rhabdomyosarcoma: An Intergroup Rhabdomyosarcoma study. J Clin Oncol. 2001;19:213–219. doi: 10.1200/JCO.2001.19.1.213. [DOI] [PubMed] [Google Scholar]
- 31.Walterhouse DO, Lyden ER, Breitfeld PP, et al. Efficacy of topotecan and cyclophosphamide given in a phase II window trial in children with newly diagnosed metastatic rhabdomyosarcoma: A Children's Oncology Group study. J Clin Oncol. 2004;22:1398–1403. doi: 10.1200/JCO.2004.05.184. [DOI] [PubMed] [Google Scholar]