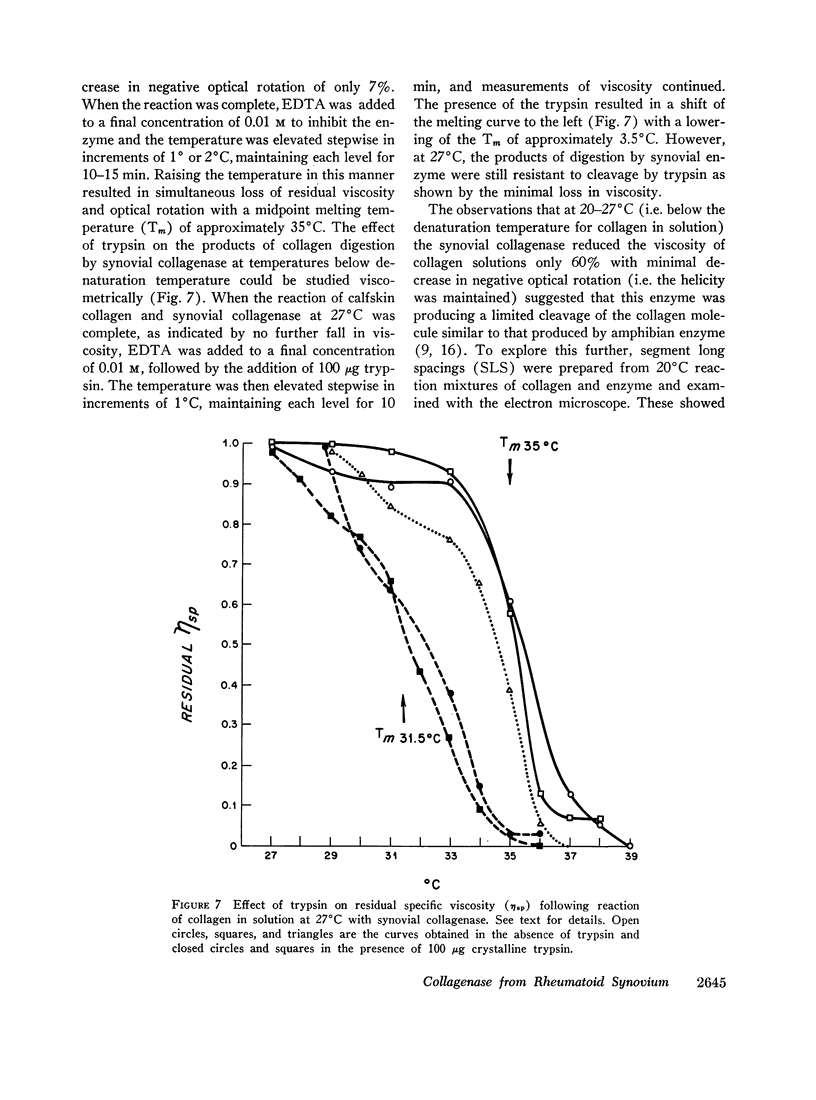
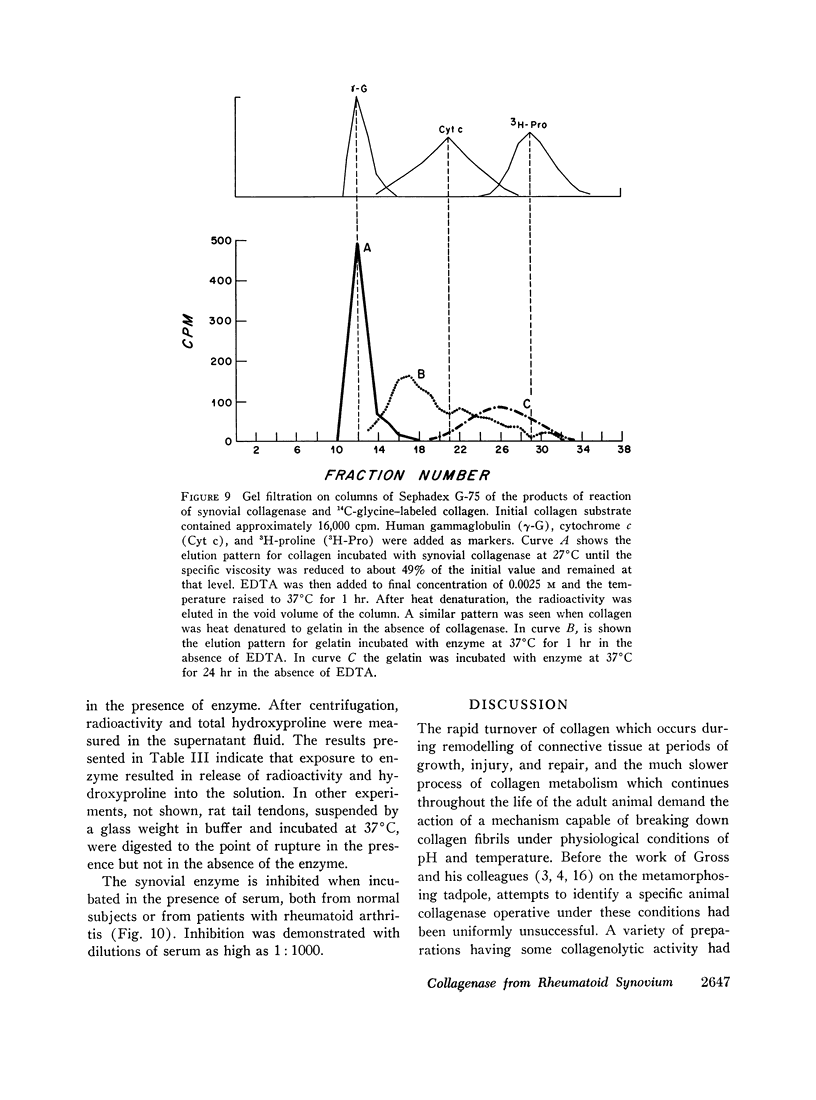
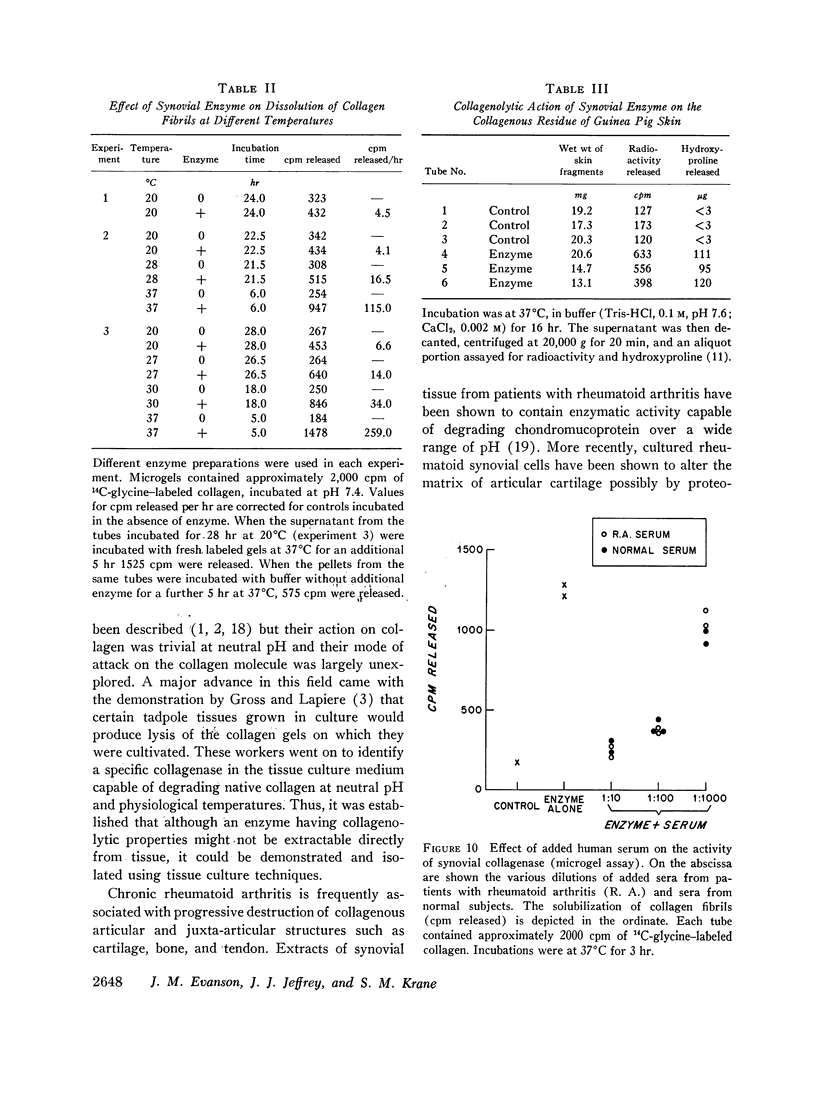
Abstract
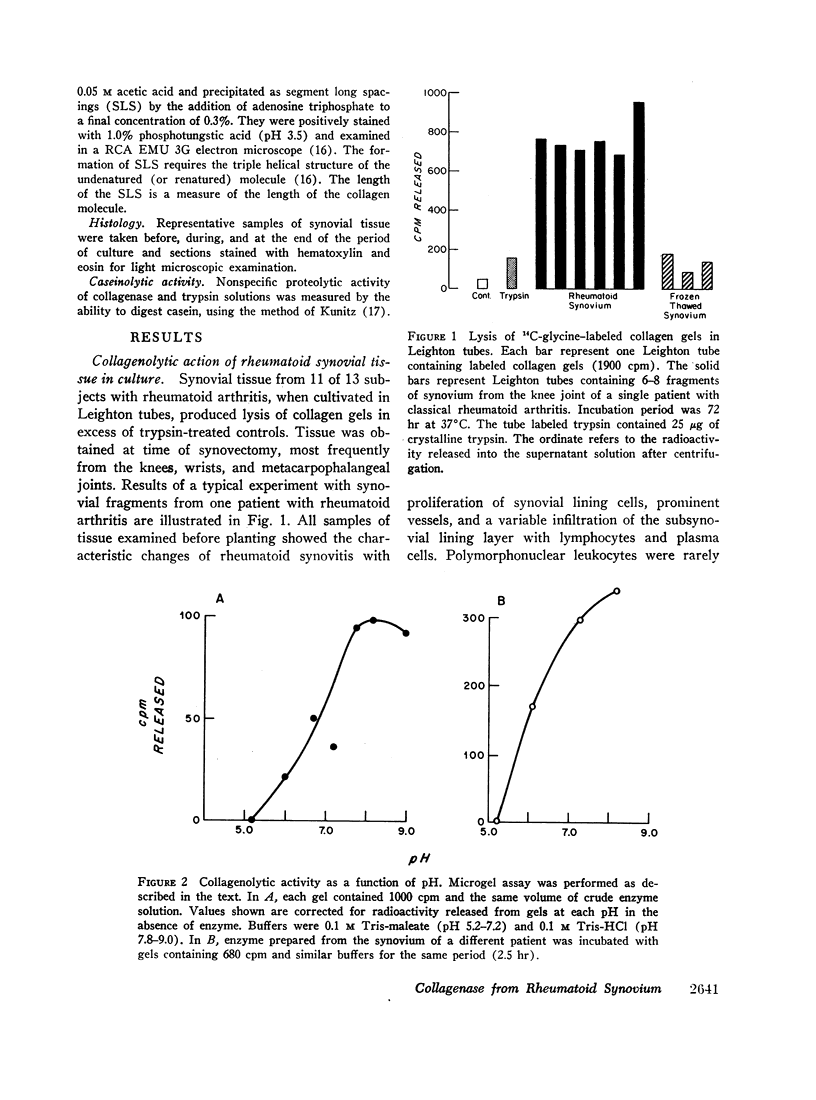
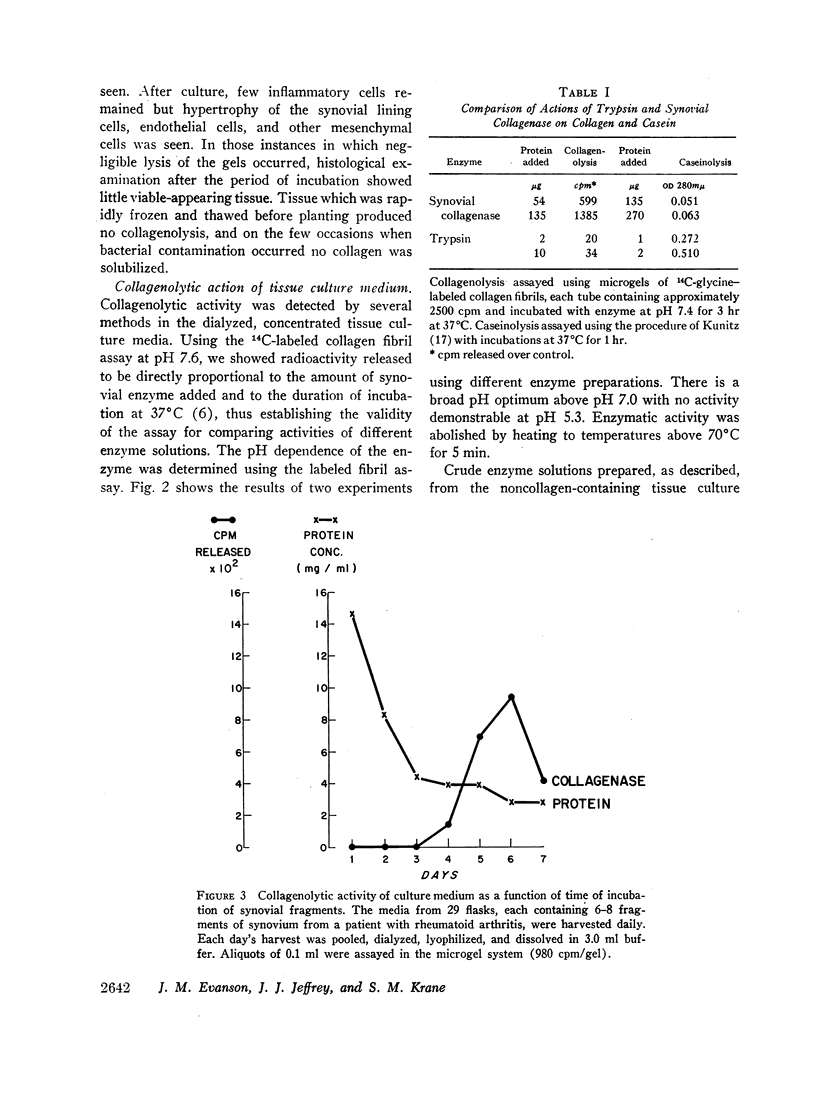
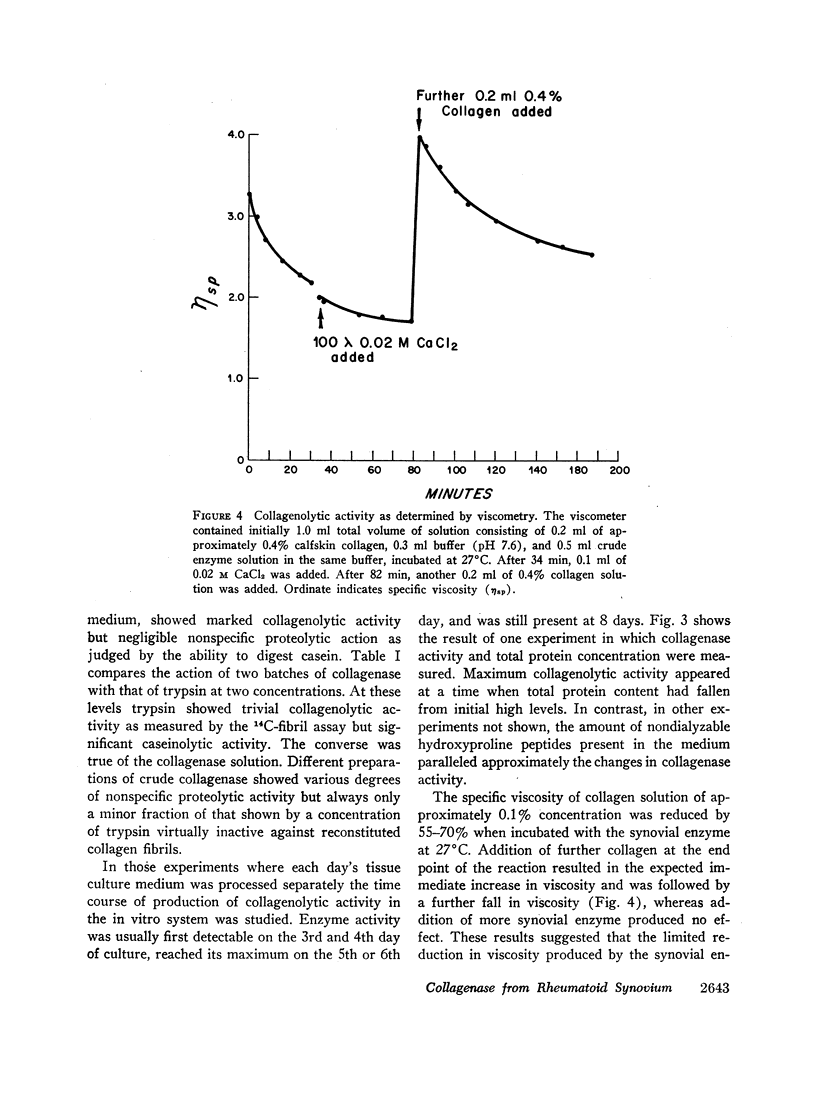
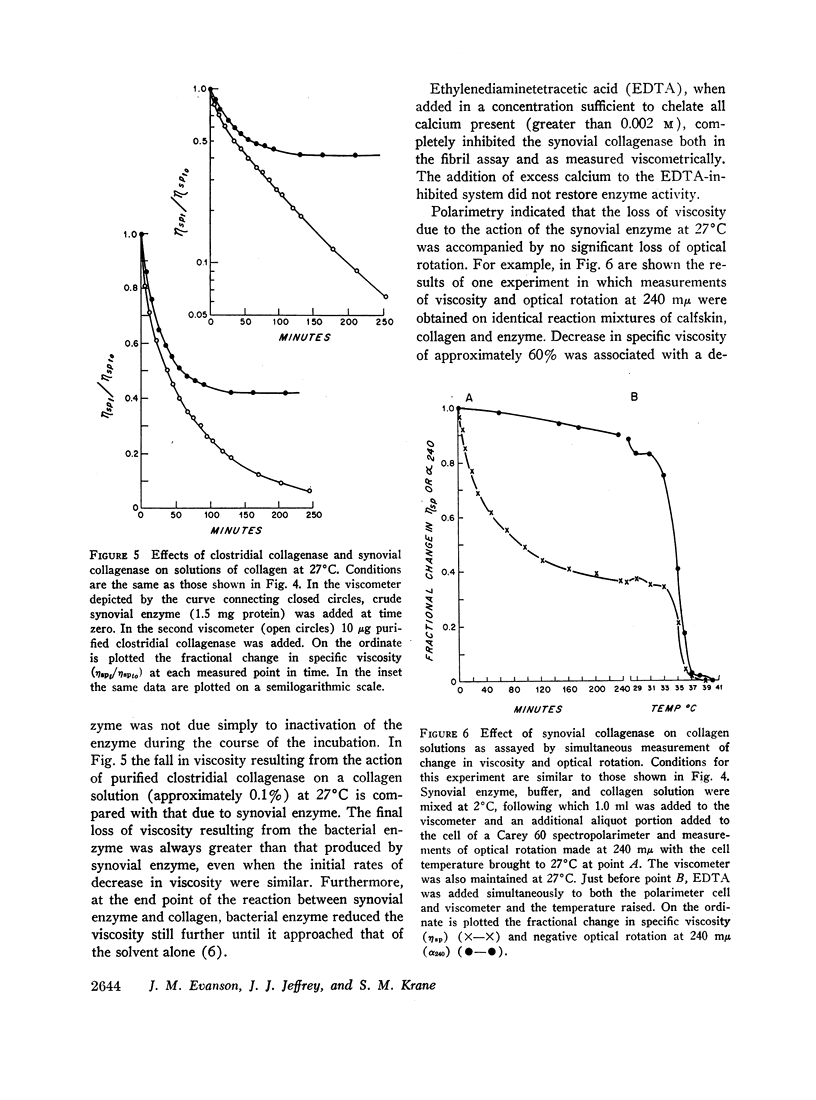
Fragments of synovium from patients with rheumatoid arthritis survive in defined tissue culture medium in the absence of added serum and, after 3-4 days, release into the medium enzyme capable of degrading undenatured collagen. Maximal activity is observed at pH 7-9 but the enzyme is inactive at pH 5. At temperatures of 20° and 27°C, collagen molecules in solution are cleaved into 3/4 and 1/4 length fragments with minimal loss of negative optical rotation, but with loss in specific viscosity of approximately 60%. Above 30°C the fragments begin to denature and denaturation is complete at 37°C. If the enzyme is not inhibited at this stage the large fragments are broken down further to polypeptides of low molecular weight. Reconstituted collagen fibrils and native fibers at 37°C are cleaved to the low molecular weight fragments, although the fibrils are resistant to breakdown at lower temperatures (20°-27°C). It is proposed that the production of such an enzyme by inflamed and proliferating rheumatoid synovium may be responsible for some of the destruction of collagenous structures that accompanies rheumatoid arthritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASTOR C. W., MUIRDEN K. D. COLLAGEN FORMATION IN MONOLAYER CULTURES OF HUMAN FIBROBLASTS. THE EFFECTS OF HYDROCORTISONE. Lab Invest. 1964 Jun;13:560–574. [PubMed] [Google Scholar]
- Eisen A. Z., Jeffrey J. J., Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968 Mar 25;151(3):637–645. doi: 10.1016/0005-2744(68)90010-7. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Human collagenase: identification and characterization of an enzyme from rheumatoid synovium in culture. Science. 1967 Oct 27;158(3800):499–502. doi: 10.1126/science.158.3800.499. [DOI] [PubMed] [Google Scholar]
- FRANKLAND D. M., WYNN C. H. The degradation of acidsoluble collagen by rat-liver preparations. Biochem J. 1962 Nov;85:276–282. doi: 10.1042/bj0850276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer H. M., Gibson W. A., Lazarus G., Stam A. C., Jr Collagenolytic activity of the skin associated with neuromuscular diseases including amyotrophic lateral sclerosis. Lancet. 1966 May 7;1(7445):1007–1009. doi: 10.1016/s0140-6736(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Fullmer H. M., Gibson W. Collagenolytic activity in gingivae of man. Nature. 1966 Feb 12;209(5024):728–729. doi: 10.1038/209728a0. [DOI] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J. Studies on the formation of collagen. I. Properties and fractionation of neutral salt extracts of normal guinea pig connective tissue. J Exp Med. 1958 Feb 1;107(2):247–263. doi: 10.1084/jem.107.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo H. C., Gross J. Collagenolytic activity during mammalian wound repair. Dev Biol. 1967 Apr;15(4):300–317. doi: 10.1016/0012-1606(67)90029-2. [DOI] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman D., Janis R., Smith C. Cartilage matrix depletion by rheumatoid synovial cells in tissue culture. J Exp Med. 1967 Dec 1;126(6):1005–1012. doi: 10.1084/jem.126.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarus G. S., Brown R. S., Daniels J. R., Fullmer H. M. Human granulocyte collagenase. Science. 1968 Mar 29;159(3822):1483–1485. doi: 10.1126/science.159.3822.1483. [DOI] [PubMed] [Google Scholar]
- NAGAI Y., GROSS J., PIEZ K. A. DISC ELECTROPHORESIS OF COLLAGEN COMPONENTS. Ann N Y Acad Sci. 1964 Dec 28;121:494–500. doi: 10.1111/j.1749-6632.1964.tb14221.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Riley W. B., Jr, Peacock E. E., Jr Identification, distribution, and significance of a collagenolytic enzyme in human tissues. Proc Soc Exp Biol Med. 1967 Jan;124(1):207–210. doi: 10.3181/00379727-124-31702. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., GALLOP P. M., KLEIN L., MEILMAN E. Studies on collagen. II. Properties of purified collagenase and its inhibition. J Biol Chem. 1959 Feb;234(2):285–293. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem J. 1962 May;83:304–314. doi: 10.1042/bj0830304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Spilberg I. Breakdown of cartilage proteinpolysaccharide by lysosomes. Arthritis Rheum. 1968 Apr;11(2):162–169. doi: 10.1002/art.1780110206. [DOI] [PubMed] [Google Scholar]
- ZIFF M., GRIBETZ H. J., LOSPALLUTO J. Effect of leukocyte and synovial membrane extracts on cartilage mucoprotein. J Clin Invest. 1960 Feb;39:405–412. doi: 10.1172/JCI104051. [DOI] [PMC free article] [PubMed] [Google Scholar]




