Abstract
Background and objectives: The multicenter Stuivenberg Hospital Acute Renal Failure 4 study investigated outcome in patients with acute kidney injury (AKI) stratified according to disease severity by the Stuivenberg Hospital Acute Renal Failure score. Patients in need of renal replacement therapy (RRT) received intermittent RRT or continuous RRT. This study investigated long-term mortality, renal function, comorbidity, and quality of life.
Design, setting, participants, & measurements: All AKI hospital survivors were included. Mortality at 1 and 2 years of follow-up was traced for all patients. Between 1 and 2 years after hospital discharge, survivors were visited at home to determine morbidity (renal function), comorbidity (Charlson comorbidity index [CCI]), and quality of life (Medical Outcome Survey SF-36).
Results: The baseline population consisted of 595 AKI patients. Mortality rates were 23.0 and 7.6%, respectively, during the first and second year after discharge. Total mortality increased from 50.7% at discharge to 65.7% 2 years after AKI and was not related to disease severity or treatment modality offered during hospitalization. Two hundred four survivors could be visited at home. Mean serum creatinine did not differ between discharge and follow-up. CCI was only related with age. SF-36 scores were negatively correlated with CCI, age, and body mass index, but not with disease severity, renal function, or dialysis modality.
Conclusions: Long-term outcome of AKI consists of a high additional mortality unrelated to treatment modality offered during hospitalization, varying evolution of renal recovery, and many comorbidities, but a mental health at the same level as the general population.
Acute kidney injury (AKI) is common among critically ill patients admitted to the intensive care unit (ICU), up to 25% depending on the population studied (1–4). Despite the advances in renal replacement therapy (RRT) and supportive measures, the mortality rate remains very high, with an in-hospital mortality rate of 50% and higher in particular populations (2,5,6). Although most studies use hospital mortality as their major endpoint, little is known about the long-term outcome after AKI. Previous studies investigating long-term outcome of AKI had small sample sizes, were single center retrospective studies, made no distinction in treatment modality, or dealt with only few of the long-term outcome aspects (7–12). In their meta-analysis reviewing 48 AKI outcome studies, Coca et al. (13) made the important conclusion that even mild AKI is associated with long-term adverse outcomes and highlighted the need for further multicenter, prospective trials.
Not only long-term survival, but also quality of life, renal recovery, comorbidity, and costs of care have become important outcome parameters. Both in general intensive care management and in specific treatment of AKI, it has been recommended that these outcome measures should be incorporated in future research (14,15). Given the poor outcome of AKI and the current trends in limitation of health care resources, questions arise about the allocation of resources to this population. Investigating long-term outcome with quality of life and renal recovery are necessary to answer these questions.
In previous Stuivenberg Hospital Acute Renal Failure (SHARF) studies, a new scoring system for all patients with AKI admitted to the ICU has been introduced. The SHARF score for hospital mortality of patients with AKI was developed in a single center study (16). In a second phase, the SHARF score was tested in a multicenter study. After adaptation based on multivariate analysis, the SHARF score was tested and proved to be useful in different settings for comparing groups of patients and centers (17).
This multicenter SHARF4 study investigated different treatment options (conservative versus RRT) in patients with AKI stratified according to severity of disease by SHARF score. Patients in need for RRT (50%) were randomized to intermittent (IRRT) or continuous RRT (CRRT) (18). The survivors of this study were used for the SHARF4 follow-up to investigate long-term mortality, renal function, comorbidity, and quality of life.
Patients and Methods
SHARF4 Study
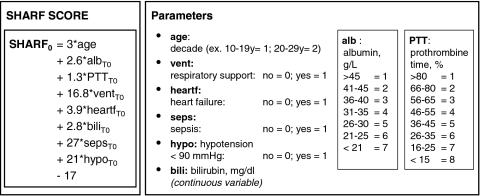
The multicenter prospective SHARF4 study included all adult patients consecutively admitted to the ICU of the participating centers with AKI defined as a serum creatinine >2 mg/dl (177 μM). Participating hospitals, patients, data collection, and RRT are described in detail previously (18). In all included patients, disease severity was defined by calculating the SHARF score (17) (see Appendix), and patients were stratified in one of the SHARF severity classes accordingly (SHARF <30, 30 to 60, >60). The decision to treat conservatively or to start RRT was at the discretion of the responsible physician, taking into account the rules of good clinical practice in this field. Patients in need for RRT were assigned to daily IRRT (intermittent hemodialysis for 4 to 6 hours daily) or CRRT (continuous veno-venous hemofiltration).
Short-term outcome parameters studied were mortality, ICU and hospital length of stay, and renal function at hospital discharge.
Follow-up Study
The study population of the SHARF4 study consisted of 1303 patients. Half of the patients died during their hospital stay. All patients of the SHARF4 study who survived hospitalization and who were admitted to one of the five participating hospitals that recruited patients until the end of the SHARF4 study were included in this follow-up study (n = 595).
At 1 and 2 years after discharge, mortality was checked in the National Registry.
After informed consent, a visit at home was performed by trained medical students and study nurses. Weight and height were assessed, and two questionnaires were completed. To determine comorbidity and quality of life, we used the Charlson Comorbidity index (CCI) (19) and the Medical Outcome Survey Short-Form 36 (SF-36) (20).
The CCI consists of 19 comorbidities with a specific weight and higher scores correlating with increased illness. We used the CCI to score comorbid disease of each patient, excluding the additional weighting for patient age.
The SF-36 is a generic measure of health status composed of 36 questions that are summarized with different weights in two overall scales: the physical component summary (PCS) and the mental component summary (MCS) (20,21). The SF-36 PCS and MCS scores were calculated using the published methods (21). These norm-based scoring algorithms are based on a general U.S. population in 1998 and have a mean of 50 ± 10 (SD). A higher score indicates a better health-relating quality of life (20,21).
Follow-up serum creatinine values were obtained from the general practitioner or the participating hospitals. Renal function at hospital discharge and at follow-up was investigated using the calculated creatinine clearance according the Cockroft and Gault formula (22) and corrected for body surface area. Stages of chronic kidney disease were defined according the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF K/DOQI) guidelines (23).
Statistical Analyses
Data were analyzed using the SPSS statistical package, version 12.0. Descriptive, univariate analysis was performed on all characteristics to investigate differences between mean and proportions using Student's t test and χ2 test. Outcome variables studied were the dichotome variable mortality and the continuous variables SF-36 and CCI.
In the multivariate analysis, independent factors were added if they showed significance in the univariate analysis. Binary logistic regression analysis was performed on mortality, and SF-36 and CCI were analyzed by multiple regression. Survival 1 and 2 years after hospital discharge was studied using the method of life table analysis with the baseline assigned at hospital discharge. The significance level was set at P < 0.05.
Ethical Considerations
The protocol of the SHARF4 and this follow-up study has been approved initially by the Ethics Committee of the Stuivenberg Hospital in Antwerp and followed by the Ethics Committee of each participating center. A written informed consent was received from each patient or his representative in case the patient was unconscious or ventilated during hospitalization.
Results
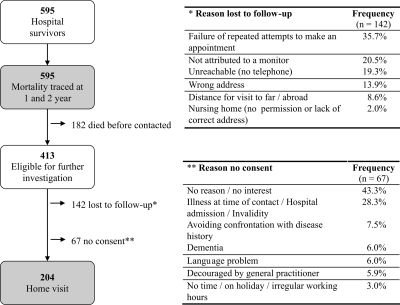
The baseline population studied consisted of 595 patients that survived hospitalization after AKI (Figure 1). Patient characteristics at hospital discharge are given in Table 1. Mean age of hospital survivors was 64.1 years (range, 16 to 97 years), and 63.9% were male.
Figure 1.
Study population flow chart. Baseline population consisted of 595 hospital survivors. The investigated population was composed of 204 patients that received a home visit.
Table 1.
Patient characteristics and clinical parameters at hospital discharge: description of hospital survivors and comparison of the long-term survivors studied for morbidity, comorbidity, and quality of life with the rest of the population
| Hospital Survivors (n = 595) | Long-Term Survivors Studied (n = 204) | Long-Term Survivors Not Studied (n = 209) | P Value of Difference | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age: mean (range) | 64.1 (16 to 97) | 61.1 (15 to 90) | 60.8 (19 to 96) | 0.848 |
| Male sex (%) | 63.9 | 58.9 | 64.1 | 0.278 |
| BMI: mean (range) | 26.3 (14.5 to 55.1) | 26.3 (14.7 to 55.1) | 26.8 (14.5 to 50.8) | 0.387 |
| Clinical characteristics | ||||
| Ventilation (%) | 47.4 | 49.0 | 46.4 | 0.600 |
| Sepsis (%) | 40.0 | 41.5 | 36.4 | 0.287 |
| Setting of AKI (% medical) | 71.5 | 74.0 | 65.7 | 0.068 |
| Type of AKI (%) | 0.026 | |||
| Pre-renal | 49.7 | 44.8 | 53.4 | |
| Renal | 42.4 | 46.8 | 42.7 | |
| Post-renal | 0.8 | 0.0 | 1.0 | |
| Acute on chronic disease | 7.0 | 8.4 | 2.9 | |
| Specific renal causes of AKI (%) | 0.981 | |||
| Acute tubular necrosis | 84.4 | 86.3 | 87.5 | |
| Acute glomerulonephritis | 5.9 | 6.3 | 5.7 | |
| Acute interstitial nephritis | 4.7 | 4.2 | 4.5 | |
| Systemic disease | 3.9 | 3.2 | 2.3 | |
| Severity scores | ||||
| SHARF score 0 h: mean (±SD) | 51.1 (26.8) | 50.2 (26.5) | 50.4 (27.4) | 0.942 |
| APACHE II 0 h: mean (±SD) | 21.0 (8.8) | 22.0 (9.1) | 20.5 (9.0) | 0.111 |
| SOFA 0 h: mean (±SD) | 7.8 (3.4) | 7.8 (3.3) | 7.9 (3.5) | 0.728 |
| SOFA 24 h: mean (±SD) | 7.3 (3.8) | 7.2 (3.5) | 7.6 (3.9) | 0.306 |
| SOFA 48 h: mean (±SD) | 6.9 (3.7) | 7.0 (3.6) | 6.8 (3.8) | 0.667 |
| Treatment (%) | 0.037 | |||
| Conservative | 57.1 | 52.5 | 63.6 | |
| IRRT | 28.4 | 33.3 | 22.5 | |
| CRRT | 14.5 | 14.2 | 13.9 | |
| ICU and hospital stay | ||||
| Days hospitalization: mean (range) | 42.8 (1 to 339) | 41.8 (1 to 189) | 41.9 (1 to 339) | 0.976 |
| Late admission to ICU (%) | 42.8 | 37.3 | 44.7 | 0.132 |
| Renal outcome | ||||
| Creatinine clearance at discharge | 61.0 (31.4) | 59.5 (27.8) | 66.0 (33.5) | 0.064 |
| Mean (±SD) ml/min | ||||
| ESRD at discharge (%) | 13.1 | 13.2 | 10.5 | 0.395 |
To convert creatinine clearance in ml/min to ml/s, multiply by 0.01667. AKI, acute kidney injury; RRT, renal replacement therapy; IRRT, intermittent renal replacement therapy; ICU, intensive care unit.
Mortality
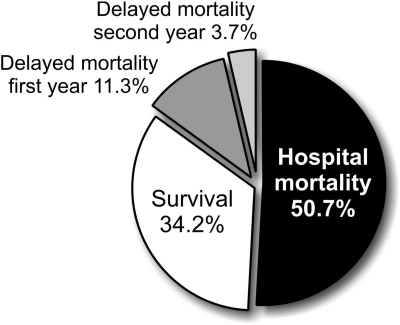
Mortality could be traced for all 595 patients at 1 and 2 years after hospital discharge. One hundred thirty-seven of the 595 hospital survivors (23.0%) died within 1 year and 45 (7.6%) died during the second year after discharge. Total mortality, including hospital mortality, increased from 50.7 to 65.7% 2 years after AKI (Figure 2).
Figure 2.
Mortality in AKI patients admitted to the intensive care unit. Mortality could be traced for all patients at 1 and 2 years after discharge. Delayed mortality = mortality during the first/second year after release.
Characteristics of the long-term survivors at hospital discharge are described in Table 2. Comparing survivors at 2 years with nonsurvivors showed that the nonsurvivors were older than the survivors (68.7 [range, 27 to 93 years] versus 60.9 years [range, 15 to 96 years]; P < 0.001) and were proportionally more male (70.2 versus 61.2%; P = 0.036). Survival analysis showed a 1-month survival of 92.6%, a 1-year survival of 76.7%, and a survival at 2 years of 69.2% for the total group. Mean creatinine clearance at discharge was significantly lower in the nonsurvivors versus survivors (55.7 ± 31.1 versus 63.0 ± 31.3 ml/min; P = 0.030; expressed in SI units 0.93 ± 0.52 versus 1.05 ± 0.52 ml/s). Binary logistic regression analysis detected only age and gender as independent predictors of long-term mortality.
Table 2.
Baseline characteristics of long-term survivors and nonsurvivors
| Survivors (n = 413) | Nonsurvivors (n = 182) | P Value of Difference | |
|---|---|---|---|
| Patient characteristics | |||
| Age: mean (range) | 60.9 (15 to 96) | 68.7 (27 to 93) | <0.001 |
| Male sex (%) | 61.2 | 70.2 | 0.036 |
| BMI: mean (±SD) | 26.5 (5.6) | 25.9 (4.3) | 0.253 |
| Severity scores | |||
| SHARF score 0 h: mean (±SD) | 50.8 (26.7) | 51.9 (27.2) | 0.658 |
| APACHE II 0 h: mean (±SD) | 21.1 (9.1) | 20.8 (8.1) | 0.779 |
| SOFA 0 h: mean (±SD) | 7.8 (3.4) | 7.6 (3.5) | 0.414 |
| Treatment (%) | 0.980 | ||
| Conservative | 57.1 | 57.1 | |
| IRRT | 28.6 | 28.0 | |
| CRRT | 14.3 | 14.8 | |
| ICU and hospital stay | |||
| Days ICU: mean (range) | 13.4 (1 to 106) | 13.6 (1 to 88) | 0.909 |
| Days hospitalization: mean (range) | 41.5 (1 to 339) | 45.6 (1 to 272) | 0.285 |
| Renal outcome | |||
| Creatinine clearance at discharge: mean (±SD) ml/min | 63.0 (31.3) | 55.7 (31.1) | 0.030a |
| NKF K/DOQI categories at discharge: (%) | 0.049a | ||
| >60 ml/min | 41.5 | 32.0 | |
| 30 to 60 ml/min | 34.7 | 34.7 | |
| <30 ml/min | 23.8 | 33.3 | |
| ESRD at discharge (%) | 11.9 | 15.9 | 0.175 |
RRT, renal replacement therapy; IRRT, intermittent renal replacement therapy; ICU, intensive care unit.
Binary logistic regression detected only age and gender as independent predictors of long-term mortality.
As shown in Table 2, the disease severity observed during hospitalization (SHARF, Acute Physiology and Chronic Health Evaluation [APACHE II], Sequential Organ Failure Assessment [SOFA]) was not related to the 2-year mortality. Additionally, the length of stay in the ICU and hospital, late ICU admission, type of AKI, and cause of AKI did not show significant differences between survivors and nonsurvivors.
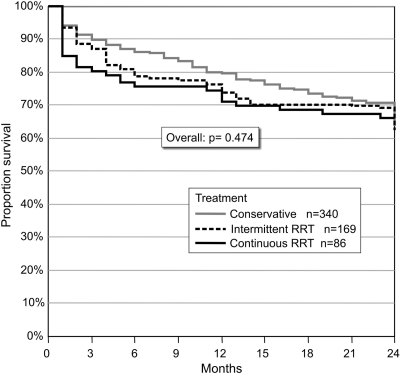
No difference in 2-year survival was observed in treatment modality (conservative treatment, IRRT, CRRT) offered during hospital stay (Figure 3). Additional correction for disease severity using the SHARF, APACHE II, and SOFA scores did not change this result. Moreover, separate survival analysis comparing the conservative treated AKI with the total group of RRT and a subanalysis comparing the two types of RRT did not show a significant difference in long-term outcome according to treatment modality.(all P > 0.05)
Figure 3.
Two-year survival according to treatment option. A general comparison showed no difference in long-term survival according to different treatment options.
Morbidity, Comorbidity, and Quality of Life
Of the 595 hospital survivors, 182 patients died before contacted. From the 413 long-term survivors considered for further study, 142 patients were lost to follow-up (mostly no contact and/or no appointment possible) and 67 patients did not consent (Figure 1). Two hundred four patients were studied with home visit at a mean of 20.3 ± 7.3 (SD) months after hospital discharge. The baseline data of the long-term survivors with and without home visit only differed regarding the type of AKI and treatment modality (Table 1).
Serum creatinine values were compared between discharge and follow-up in 153 of the 204 patients. Mean serum creatinine of this population was 1.4 ± 0.7 mg/dl (124 ± 62 μM) at discharge and 1.4 ± 0.6 mg/dl (124 ± 53 μM) after 1 year (P = 0.717). Creatinine clearance at discharge (61.1 ± 27.5 ml/min; 1.02 ± 0.46 ml/s) did not differ significantly from follow-up (61.9 ± 28.4 ml/min; 1.03 ± 0.47 ml/s; P = 0.663).
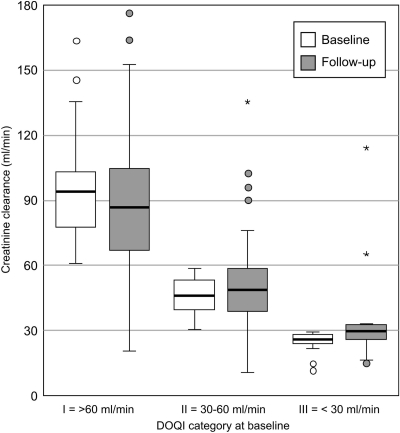
According to the NKF K/DOQI classification of renal failure, one half of the patients stayed in the same category, one quarter decreased, and one quarter increased. Figure 4 shows for the total population that, within each class, there is an evolution toward a larger spread in both a positive and negative direction. At hospital discharge, 27 patients were diagnosed with ESRD. During the follow-up period, 13 patients became dialysis-independent, whereas 7 patients needed chronic dialysis treatment.
Figure 4.
Creatinine clearance at baseline and follow-up according to NKF K/DOQI categories at baseline. The spread within each category increases toward follow-up, both in the positive and in the negative direction.
CCI at follow-up showed a median of 2 (range, 0 to 15). Peripheral vascular disease (24.5%), peptic ulcer disease (21.6%), diabetes (19.1%), and myocardial infarction (16.7%) were the most common comorbidities. CCI did not show any significant relationship with clinical parameters noted during hospitalization (different scores for severity of disease, renal function, treatment modalities, sepsis, ventilation, body mass index, late admission to the ICU, and length of stay in the hospital), except for age (r = 0.165, P < 0.05).
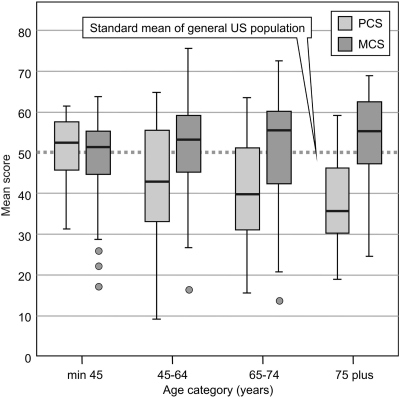
The SF-36 was completed in all 204 patients by self-administration or with the assistance of a trained interviewer in case of difficulties with reading. The mean PCS was 42.1 ± 12.8 and the mean MCS had a value of 51.0 ± 11.6. PCS and MCS were negatively correlated with CCI (r = −0.401, P < 0.01 and r = −0.149, P < 0.05, respectively). Of all of the clinical parameters during hospitalization, only age and body mass index correlated with health-related quality of life. Age showed a significant negative correlation of −0.282 with PCS (P < 0.01) and a significant positive correlation of 0.174 with MCS (P < 0.05). Body mass index was negatively correlated with PCS (r = −0.223, P < 0.01). Figure 5 represents the distribution of the scores by different age groups and shows that the PCS declines with age and the MCS generally remains stable or even increases with age.
Figure 5.
Medical Outcome Survey Short-Form 36 mean physical component summary and mental component summary scores by age groups. The scores were normed based on the general U.S. population with a standard mean of 50 ± 10 (SD).
The SF-36 summary scores did not show any significant relationship with the different scores for severity of disease, renal function, treatment modalities, and length of stay in the hospital. Binary logistic regression analysis for comparison of PCS values <35 and >35 was applied to identify factors associated with poor health-related quality of life. Body mass index and age categories seemed to be significantly related (P = 0.032 and P = 0.047, respectively), indicating a lower physically health-related quality of life with higher body mass index and older age.
Discussion
Several interesting findings emerged from this multicenter prospective follow-up study.
Through a complete reporting of 1- and 2-year survival, we found comparable outcomes for intermittent and continuous RRT. Moreover, the inclusion of a large number of conservative treated patients allowed the observation that 2-year mortality is comparable between the conservatively treated group versus those patients treated with RRT. Apart from mortality, we assessed renal function after hospital discharge as advised in a recent review of RRT in AKI patients (24). Furthermore, our long-term follow-up study included survival, morbidity, and quality of life.
Mortality
Our results confirmed the poor prognosis of AKI with a hospital mortality rate of 50.7% in the original cohort and a high additional mortality rate of 11.3 and 3.4%, respectively, during the first and second year after hospital discharge. The same observation was made in a 1-year follow-up of the previous SHARF II study that investigated mortality and renal function after hospitalization for AKI with a hospital mortality rate of 51% and an additional mortality rate during the first year of 11% (25). Comparison with published data of other long-term follow-up studies of AKI is difficult because they differ in the populations studied and length of observation period. Åhlström et al. (26) reported both a 1- and 5-year high mortality rate of 60 and 70%. Korkeila et al. (7) observed a mortality of 55% at 6 months that rose to 65% at 5 years. Gopal et al. (8) and Morgera et al. (11) reported both a higher hospital mortality and mortality at follow-up. Both of these studies, however, included only patients treated with CRRT, possibly indicating more severe illness.
Comorbid conditions during hospitalization for AKI, such as sepsis, ventilation, late ICU admission, and other characteristics of severity of disease expressed by different scoring systems, have an important impact on survival in the ICU and hospital (5,7,27,28). In contrast, these parameters were not predictive for long-term prognosis in this cohort study. Also, the SHARF score was not predictive for long-term prognosis.
The most important decline in survival occurred during the first month (7.4%). It might be that patients are discharged from the hospital too soon or that there is a lack of follow-up immediately after discharge. Possibly, preventive measures should aim at follow-up during the first year after discharge with special attention to the first month.
Although it has been shown by us (18) and others (15,24) that the method of RRT has no influence on short-term outcome, we can confirm that, in this multicenter, prospective trial, there is also no difference in the treatment modalities regarding long-term outcome of AKI.
Renal Function
In this study, we observed a wide variation in the evolution of renal function after discharge. During the follow-up period, 13 patients (of 27 with ESRD at discharge) became dialysis independent and 7 additional patients developed ESRD. Of the population that was visited at home, 10.3% was in need of chronic RRT. This result is in line with observations made by other groups. In the study of Bhandari et al. (29), ∼16% of the AKI survivors were dialysis dependent at discharge, with only a small portion recovering at 18 months after AKI. Morgera et al. (11) reported that 10% of their patients required RRT between 6 months and 5 years after discharge.
Comorbidity
Comorbidity of patients in this study has been assessed by the CCI, which has been proven to be efficient to predict survival in several ESRD populations (30–32). We found a median CCI score of 2 (0 to 15), which is comparable with the findings in other AKI follow-up studies (9,10). Because we assessed the CCI at follow-up and not at hospital discharge, we could not use the CCI to predict long-term survival. However, determination of comorbidity stayed important in evaluating other outcome parameters. As reported by van Manen et al. (33), CCI adjusts for the potential confounding effect of comorbidity in evaluation studies with health status as an outcome. As expected, CCI showed a negative correlation with SF-36 scores (both PCS and MCS).
Quality of Life
The SF-36 has been validated both in the ICU (34,35) and in chronic hemodialysis (36) populations.
We found in relation to age that PCS declined and MCS remained stable in comparison with the U.S. population. Moreover, we confirmed the lower PCS of our AKI population by comparing our results with the general population values of our neighbor countries (37).
The mean MCS was comparable to the general population values. The decline of PCS with age and the MCS remaining stable or even increasing (Figure 5) have also been described in general population samples of nine Western countries (37), which explains that the decline is a normal trend in general populations, although in our study population, the decrease of PCS was more pronounced.
Apart from age, other parameters such as severity of disease and clinical parameters during hospitalization did not show any significant relationship with the SF-36 summary scores.
Therefore, this observation confirmed the statement of Maynard et al. (10) that health-related quality of life is difficult to predict from data available at the time of acute illness in AKI patients.
Limitations
The most important limitation of our study is the limited number of subjects that could be studied at home for morbidity, comorbidity, and quality of life. This study might have overestimated the health-related quality of life and underestimated the burden of comorbidities because reasons such as illness, hospital admission, avoiding confrontation with their disease, and dementia played a major role in the consent process. However, this selection bias concerned CCI and SF-36 as outcome parameters but not mortality, which could be checked in all of the hospital survivors at 1 and 2 years after discharge. Despite its limitations, this study adds to our knowledge, because all data are derived from a multicenter prospective study, and represents the largest cohort with investigation of the long-term outcome of AKI with mortality, renal function, comorbidity, and health-related quality of life. A second limitation in our study concerns the lack of data of health-related quality of life and comorbidity before admission or at discharge. Therefore, individual evolution or predictive value of both the SF-36 and the CCI could not be assessed. Next, we did not investigate the causes of death during follow-up or their relationship with AKI. Finally, because this is the first multicenter prospective trial investigating the long-term outcome of AKI comparing treatment modalities, our results need to be confirmed in larger trials.
Conclusion
In conclusion, the long-term survival of patients with AKI admitted to the ICU is poor.
This mortality rate is not related to disease severity or treatment modality offered during hospitalization. Comparison of renal function between discharge and follow-up shows no change during follow-up. A considerable part of the hospital survivors stayed in need of chronic RRT and had important comorbidities. The physical component health-related quality of life of the SF-36 score in the survivors was lower compared with age-matched general populations, whereas the mental health-related quality of life was found to be the same as in the general population.
Appendix.
SHARF score.
Disclosures
None.
Acknowledgments
This work was supported by the nephrological research resources of AZ Stuivenberg Hospital. The members of the SHARF study group were as follows—Coordinating Center: R. L. Lins, M. M. Elseviers, S. Van Bastelaere, and A. Van Berendoncks; Steering Committee: P. Damas, J. Devriendt, M. M. Elseviers, E. Hoste, R. L. Lins, M. Malbrain, and P. Van der Niepen; Data Collection: T. De Keyser, J. W. De Neve, V. Lins, T. Mellaerts, S. Van Bastelaere, and A. Van Berendoncks; Data Analysis and Statistics: M. M. Elseviers and A. Van Berendoncks; Participating Centers: University Hospital Vrije Universiteit Brussel, P. Van der Niepen, D. Verbeelen, I. Hubloue; ZNA Stuivenberg Hospital, R. Daelemans, M. Malbrain, J. Leys, R. L. Lins; University Hospital Gent, E. Hoste, R. Lameire, W. Van Biesen; University Hospital Liège, P. Damas, B. Dubois, J. M. Krzesinski; Brugmann University Hospital, Brussel, J. Devriendt, M. Dratwa, R. Wens.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Bellomo R, Ronco C, Kellum JA, Metha RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup: Acute renal failure: Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F: Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26: 915–921, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Liano F, Pascual J: Epidemiology of acute renal failure: a prospective, multicenter, community-based study. The Madrid Acute Renal Failure Study Group. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ: Acute renal failure in intensive care units: Causes, outcome, and prognostic factors of hospital mortality: A prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med 24: 192–198, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W: Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30: 2051–2058, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Korkeila M, Ruokonen E, Takala J: Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med 26: 1824–1831, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Gopal I, Bhonagiri S, Ronco C, Bellomo R: Out of hospital outcome and quality of life in survivors of combined acute multiple organ and renal failure treated with continuous venovenous hemofiltration/hemodiafiltration. Intensive Care Med 23: 766–772, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe W, Simpson M, Khan IH, Prescott GJ, Simpson K, Smith WC, MacLeod AM; Scottish Renal Registry: Acute renal failure requiring renal replacement therapy: Incidence and outcome. QJM 95: 579–583, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, Whittle J, Chelluri L, Arnold R: Quality of life and dialysis decisions in critically ill patients with acute renal failure. Intensive Care Med 29: 1589–1593, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH: Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis 40: 275–279, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Ponte B, Felipe C, Muriel A, Tenorio MT, Liano F: Long-term functional evolution after an acute kidney injury: A 10-year study. Nephrol Dial Transplant 23: 3859–3866, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angus DC, Carlet J: 2002 Brussels Roundtable Participants: Surviving intensive care: A report from the 2002 Brussels Roundtable. Intensive Care Med 29: 368–377, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Tonelli M, Manns B, Feller-Kopman D: Acute renal failure in intensive care unit: A systematic review of the impact of dialytic modality on mortality and renal recovery. Am J Kidney Dis 40: 875–885, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Lins RL, Elseviers M, Daelemans R, Zachée P, Zachée P, Gheuens E, Lens S, De Broe ME: Prognostic value of a new scoring system for hospital mortality in acute renal failure. Clin Nephrol 53: 10–17, 2000 [PubMed] [Google Scholar]
- 17.Lins RL, Elseviers MM, Daelemans R, Arnouts P, Billiouw JM, Couttenye M, Gheuens E, Rogiers P, Rutsaert R, Van der Niepen P, De Broe ME: Re-evaluation and modification of the Stuivenberg Hospital Acute Renal Failure (SHARF) scoring system for the prognosis of acute renal failure: an independent multicenter, prospective study. Nephrol Dial Transplant 19: 2282–2288, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lins R, Elseviers M, Van der Niepen P, Hoste E, Malbrain ML, Damas P, Devriendt J; SHARF Investigators: Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: Results of a randomized clinical trial. Nephrol Dial Transplant 24: 512–518, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Snow KK, Kosinski M, Gandek B: MOS SF-36 Health Survey Manual and Interpretation Guide, Boston, MA, Health Institute, 1993 [Google Scholar]
- 21.Ware JE, Kosinski M, Keller SD: SF-36 Physical and Mental Health Summary Scales: A User's Manual, Boston, MA, The Health Institute: New England Medical Center, 1994 [Google Scholar]
- 22.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M; Alberta Kidney Disease Network: Renal replacement therapy in patients with acute renal failure: A systematic review. JAMA 299: 793–805, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Lins RL, Elseviers MM, Daelemans R: Severity scoring and mortality 1 year after acute renal failure. Nephrol Dial Transplant 21: 1066–1068, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Åhlström A, Tallgren M, Peltonen S, Räsänen P, Pettilä V: Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31: 1222–1228, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Spiegel DM, Ullian ME, Zerbe GO, Berl T: Determinants of survival and recovery in acute renal failure patients dialyzed in intensive care units. Am J Nephrol 11: 44–47, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Consentino F, Chaff C, Piedmonte M: Risk factors influencing survival in ICU acute renal failure. Nephrol Dial Transplant 9: 179–182, 1994 [PubMed] [Google Scholar]
- 29.Bhandari S, Turney JH: Survivors of ARF who do not recover renal function. QJM 89: 415–421, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 31.van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT; NECOSAD Study Group: Netherlands Co-operative Study on the Adequacy of Dialysis-2: How to adjust for comorbidity in survival studies in ESRD patients: A comparison of different indices. Am J Kidney Dis 40: 82–89, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML: A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 108: 609–613, 2000 [DOI] [PubMed] [Google Scholar]
- 33.van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT; NECOSAD Study Group: Adjustment for comorbidity in studies on health status in ESRD patients: Which comorbidity index to use? J Am Soc Nephrol 14: 478–485, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Eddleston JM, White P, Guthrie E: Survival, morbidity, and quality of life after discharge from intensive care. Crit Care Med 28: 2293–2299, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Chrispin PS, Scotton H, Rogers J, Lloyd D, Ridley SA: Short form 36 in the intensive care unit: Assessment of acceptability, reliability and validity of the questionnaire. Anaesthesia 52: 15–23, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Meyer KB, Espindle DM, DeGiacomo JM, Jenuleson CS, Kurtin PS, Davies AR: Monitoring dialysis patients' health status. Am J Kidney Dis 24: 267–279, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Bullinger M, Kaasa S, Leplege A, Prieto L, Sullivan M: Cross-validation of the item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA project. International Quality of Life Assessment. J Clin Epidemiol 51: 1171–1178, 1998 [DOI] [PubMed] [Google Scholar]