Abstract
Background and objectives: Clinical guidelines support vascular access surveillance to detect access dysfunction and alter the clinical course by radiologic or surgical intervention. The objective of this study was to explore the association between loss of primary functional patency within 6 months of first use and demographic and clinical characteristics of patients receiving chronic renal replacement therapy with arteriovenous fistulas.
Design, setting, participants, & measurements: This was a retrospective study of all chronic hemodialysis patients followed by the Southern Alberta Renal Program from January 1, 2005 to June 30, 2008. Demographic and clinical variables and initial intra-access blood flow (IABF) were compared between those with and without loss of primary functional patency. To determine the contribution of independent variables to the dependant variable of loss of primary functional patency, a multivariable analysis using logistic regression was performed.
Results: The incidence of primary failure was 10% (81 of 831). Multivariable analysis found that older age (>65 years, odds ratio [OR] 3.6, P < 0.001), history of diabetes (OR 2.3, P = 0.007), history of smoking (OR 4.3, P < 0.001), presence of forearm fistulas (OR 4.0, P < 0.001), and low initial IABF (<500 ml/min, OR 29, P < 0.001) were independently associated with loss of primary patency.
Conclusions: The set of patient risk factors identified in this study, particularly initial IABF, can be used to identify patients who are most at risk for developing vascular access failure and to guide a more directed approach for a vascular access screening protocol.
A significant challenge for physicians and other healthcare professionals who care for patients with chronic renal failure is the preservation of vascular access for hemodialysis. Vascular access failure, particularly loss of primary functional patency of a surgically created access, is a cause of considerable morbidity, discomfort, inconvenience, and cost. Approximately 20% to 30% of all hospital admissions for patients with ESRD are related to complications of vascular access (1–4). To decrease the incidence of loss of primary functional patency, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines recommend that all vascular accesses undergo regular surveillance by measurement of the intra-access blood flow (IABF) to identify hemodynamically significant dysfunction (5). Although following these guidelines leads to the identification of access dysfunction in arteriovenous fistulas (AVFs) and grafts, there remains considerable room for improvement. For example, identification of major risk factors for problems with access patency might allow redirection of surveillance effort away from patients with few risk factors toward those with more risk factors. The main objective of this study was to explore the association between loss of primary functional patency within 6 months of first use and demographic and clinical characteristics in patients with AVFs receiving chronic renal replacement therapy.
Materials and Methods
Subjects
This was a retrospective cohort of patients in southern Alberta, Canada, receiving chronic renal replacement therapy with an AVF created for dialysis. Patients were followed by the Southern Alberta Renal Program (SARP), which manages between 600 and 700 hemodialysis patients per year and has incorporated a multidisciplinary approach to access management since January 2002. A vascular access coordinator, nephrologist, interventional radiologist, and vascular access surgeon staff the program. This study included all chronic hemodialysis patients who met the following inclusion criteria: (1) were 18 years of age or older, (2) received hemodialysis at any of the SARP facilities between January 2005 and June 2008, (3) had a mature AVF created by a SARP-affiliated vascular access surgeon, and (4) had a successful first cannulation of the access with adequate dialysis achieved. Patients were excluded if (1) they were receiving peritoneal dialysis, (2) a central line was used as the method of vascular access, (3) the vascular access was in a location other than the upper extremity, (4) an intervention was performed to achieve maturation, or (5) first cannulation but adequate dialysis was not achieved.
The study was approved by the University of Calgary Conjoint Health Research Ethics Board. Because the study involved analysis of data already collected and the data had individual identifying information removed, the ethics board waived the need for individual patient consent.
Variables
The SARP and Southern Alberta Transplant Program (ALTRA) databases were reviewed to obtain demographic, clinical, and vascular access data. Demographic data included age at the time that the surgical procedure was performed, gender, and race. Clinical data included the etiology of renal insufficiency (e.g., diabetes, GN, ischemia/hypertension, interstitial nephritis, polycystic kidney disease, or unknown/other) and specific comorbidities such as diabetes, hypertension, peripheral vascular disease, history of smoking (currently smoking or self-classified as a regular smoker within the past 5 years) and use of medications (aspirin, anticoagulants, angiotensin-converting enzyme inhibitor). Vascular access data included the side of the surgery (right or left), anatomic location (forearm or upper arm fistula), and the surgeon of record. The surgeon of record was made anonymous before data extraction from the database.
The primary outcome of interest was loss of primary functional patency (6) defined as thrombosis of the graft (including abandonment of the access site) or the need for surgical or percutaneous endovascular intervention, whichever came first after initial cannulation and achievement of adequate dialysis and that occurred within 6 months after first use.
Successful Cannulation and Initial IABF Measurement
The decision for first use of a vascular access was determined by agreement between the vascular access nephrologist and vascular access coordinator using a standardized ultrasound assessment. The assessment determined a minimum graft diameter of 0.5 cm and a minimum depth below the skin of 0.5 cm. Successful cannulation was defined as affording an extracorporeal blood flow of at least 300 ml/min for at least 3 hours using arterial and venous needles placed in the vascular access. This variable was measured in days from surgery to the time of first cannulation. All patients had an initial IABF measurement performed within 2 weeks after initial cannulation and successful use of the access for hemodialysis. All vascular access blood flow measurements were performed in a standardized fashion using the ultrasound dilution technique (HD01 Monitor, Transonic Systems, Inc., Ithaca, NY). This technique has been extensively validated in vivo and in vitro (7). All access flow measurements were done within the first hour of the hemodialysis treatment because these results are reproducible and unaffected by changes in cardiac output associated with ultrafiltration. Blood flow measurements were not performed during periods of clinically significant hypotension (at the discretion of the dialysis unit nurses). All access blood flow measurements were performed in duplicate and subsequently averaged. If a measurement differed by >10%, a third measurement was taken and the average of the measurements was calculated.
Statistical Analyses
Demographic and clinical variables and initial IABF were compared between those with and without loss of primary functional patency using a two-sample t test for continuous variables and χ2 test for discrete variables. The relative risk (RR) of loss of primary functional patency was calculated. To determine the potential contribution of independent variables to the dependant variable of loss of primary functional patency, a multivariable analysis using logistic regression was performed. In our model, we considered all plausible two-way interactions. On the basis of previous research, a priori, age was stratified as ≤65 years and >65 years (8–10) and initial IABF was stratified as ≤500 ml/min and >500 ml/min for the RR and multivariate analyses. All statistical analyses were performed using Stata 9.0 (Stata Corp, College Station, TX).
Results
Patient Characteristics
During the 42-month study period from January 2005 to June 2008, a total of 831 AVF procedures were performed at the Foothills Hospital in Calgary. All patients included in the study were on renal replacement therapy at the time of the first access cannulation. Table 1 describes characteristics of the patient sample. Most patients had brachiocephalic fistulas in the upper arm. The usual practice is to attempt a forearm location first and then an upper arm location if the forearm was unsuccessful (surgical construction was not successful at the time of operation or first cannulation did not occur). No brachiobasilic fistulas were placed during the study time. The most common underlying disease causing renal insufficiency was diabetes, which was present in 44% of the patients. Other causes included hypertensive nephropathy (14%), GN (12%), interstitial nephritis (4%), and polycystic kidney disease (4%). In 22% of the patients, no definite etiological factor was identified (Table 1).
Table 1.
Baseline demographics and comorbid variables in patients with loss of primary functional patency (PFP) compared with patients without failure
| Variable | AVFs with PFP | AVFs with loss of PFP | All AVFs | P |
|---|---|---|---|---|
| Number of patients, n (%) | 750 (90) | 81 (10) | 831 (100) | |
| Age, mean ± SD | 67 ± 15 | 72 ± 11 | 68 ± 15 | 0.0098a |
| Male gender, n (%) | 464 (62) | 47 (58) | 511 (62) | 0.49b |
| Ethnicity, n (%) | 0.3b | |||
| Caucasian | 550 (73) | 53 (65) | 604 (72) | |
| other | 220 (27) | 28 (35) | 227 (28) | |
| Primary renal disease, n (%) | 0.4b | |||
| diabetes | 331 (44) | 32 (40) | 363 (44) | |
| GN | 91 (12) | 12 (15) | 103 (12) | |
| ischemic/hypertension | 109 (15) | 11 (14) | 120 (14) | |
| interstitial nephritis | 29 (4) | 1 (1) | 30 (4) | |
| polycystic kidney disease | 26 (3) | 6 (7) | 32 (4) | |
| unknown/other | 164 (22) | 19 (23) | 183 (22) | |
| Comorbidities, n (%) | ||||
| diabetes | 347 (46) | 57 (70) | 404 (49) | <0.001b |
| hypertension | 432 (58) | 64 (79) | 496 (60) | <0.001b |
| peripheral vascular disease | 230 (31) | 44 (54) | 274 (33) | <0.001b |
| smoking | 317 (42) | 61 (75) | 378 (45) | <0.001b |
| medications | 58 (8) | 7 (9) | 65 (8) | 0.77b |
| Surgeon, n (%) | 0.6b | |||
| A | 221 (30) | 29 (36) | 250 (30) | |
| B | 269 (36) | 29 (36) | 298 (36) | |
| C | 227 (30) | 21 (26) | 248 (30) | |
| D | 33 (4) | 2 (3) | 35 (4) | |
| Location, n (%) | 0.48b | |||
| right | 203 (27) | 19 (24) | 222 (27) | |
| left | 547 (73) | 62 (76) | 609 (73) | |
| Anatomical location, n (%) | <0.001b | |||
| forearm AVF | 207 (28) | 48 (59) | 255 (31) | |
| upper arm AVF | 543 (72) | 33 (41) | 576 (69) | |
| Initial IABF | ||||
| mean ± SD ml/min | 992 ± 510 | 421 ± 138 | 936 ± 515 | <0.001a |
P values for continuous variables are based on two sample t test.
P values for categorical variables are based on χ2 test of two samples.
Primary Functional Patency Failure of the AVF
There was a mean (±SD) duration of 70 ± 10 days between access creation and first cannulation. From the IABF monitoring, 210 patients were sent for angiography. Loss of primary functional patency of the AVF occurred in 81 (10%) of total cases. The median time from first cannulation to loss of primary functional patency was 48 days (interquartile range 12 to 95). Sixty-six of these patients (81%) had thrombosis as a cause. Of the 81 patients, 75 had a percutaneous endovascular intervention, of which only 9 were successful. Four patients had surgery, of which all were successful. In two patients, a decision was made to abandon the access without intervention. Therefore, 13 of 81 interventions returned or maintained functional patency of the access.
Baseline demographics and comorbid variables of patients who developed loss of the primary functional patency of the AVF were compared with patients without AVF failure in Table 1. Patients with primary functional patency failure were significantly more likely to have a current comorbid diagnosis of diabetes, hypertension, peripheral vascular disease, or smoking and were more likely to have a forearm fistula. Additionally, patients with primary functional patency failure were significantly older. Most importantly, the initial IABF was lower in those who subsequently developed primary functional patency failure. No differences in gender, ethnicity, underlying primary kidney disease, use of medications, surgeons, or location of the access were demonstrated between those with and without primary functional patency failure (Table 1).
RR for Primary Functional Patency Failure
RR calculations of the patient characteristic variables showed that patients with initial IABF <500 ml/min were 16 times more likely to experience primary functional patency failure; smokers were 3.7 times more likely; those with forearm fistulas were 3.3 times more likely; and those older than 65 and those with a history of diabetes, hypertension, or peripheral vascular disease were 2.4 to 2.5 times more likely to experience primary functional patency failure from any cause as those without these characteristics (Table 2). The RRs based on gender, ethnicity, medications, or AVF location were NS (data not shown).
Table 2.
RR of loss of primary functional patency (PFP) from any cause in patients with AVFs
| Variable | Number with Characteristic, % (ratio) | Incidence of PFP Loss among Patients with Characteristic, % (ratio) | Incidence of PFP Loss among Patients without Characteristic, % (ratio) | RR (95% CI) |
|---|---|---|---|---|
| Age ≥65 years | 64 (537/831) | 12 (66/537)) | 5 (15/294) | 2.4 (1.4 to 4.1)a |
| Diabetes | 49 (404/831) | 14 (57/404) | 5 (24/427) | 2.5 (1.6 to 4.0)a |
| Hypertension | 60 (496/831) | 13 (64/496) | 5 (17/335) | 2.5 (1.5 to 4.3)a |
| Peripheral vascular disease | 33 (274/831) | 16 (44/274) | 7 (37/557) | 2.4 (1.6 to 3.6)a |
| Smoking | 46 (378/831) | 16 (61/378) | 4 (20/453) | 3.7 (2.2 to 5.9)a |
| Anatomical location (forearm) | 31 (255/831) | 19 (48/255) | 6 (33/576) | 3.3 (2.2 to 5.0)a |
| Initial IABF (<500 ml/min) | 14 (116/831) | 51 (59/116) | 3 (22/715) | 16 (11 to 26)a |
P < 0.001; variables with no significant P value are not shown in table.
Multivariable Modeling
A stepwise logistic modeling procedure was used. The only variables chosen for inclusion in the multivariable logistic regression analysis were those found in the univariate analysis to be significantly correlated with loss of primary functional patency. Variables that met this criterion were age (≥65 years); presence of diabetes, hypertension, and peripheral vascular disease; history of smoking; having a forearm AVF; and having an initial IABF <500 ml/min. Gender was NS in this analysis (P = 0.5); however, in other research, being female has been associated with a higher incidence of loss of primary functional patency, hypothesized to be due to smaller vascular structures in women (11). Therefore, gender was included in the multiple regression models despite its lack of statistical significance in the univariate analysis. A stratified analysis using the Mantel–Haenszel test did not indicate that any variable acted as a confounder or effect modifier of the association between loss of primary functional patency and initial IABF, the variable identified as having the highest RR.
At the multivariable modeling stage of the analysis, assessment for interaction was done. All possible pairwise interactions were tested and none were found to be statistically significant. Higher order (three-way) interaction terms (e.g., gender by age by diabetes mellitus) were not considered.
Final Model
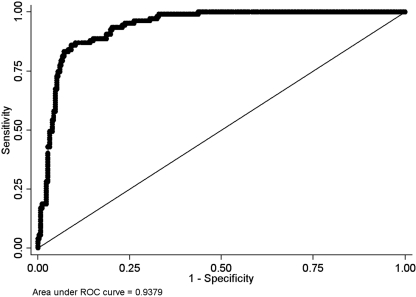
The analysis yielded a model that included age, history of diabetes, history of smoking, anatomical location, and initial IABF dichotomized at 500 ml/min. Hypertension and peripheral vascular disease, although significant with regard to RR, were NS in this analysis; gender was also NS. Assessment of the model fit using the Hosmer–Lemeshow goodness of fit test yielded high P values (c = 14.80, P = 0.19). This model was found to have excellent discrimination as determined by the area under the receiver operator characteristics curve of 0.93 (Figure 1). The final regression model demonstrates that all selected variables remain statistically significant (Table 3). Significant odds of experiencing vascular access failure occurred in those patients with initial IABF of <500 ml/min (odds ratio [OR] = 29 and 95% confidence interval [CI] 15 to 52), adjusting for all other covariates. A history of smoking, being older than 65, having a forearm fistula, and a history of diabetes produced significant ORs between 4.3 and 2.3 (Table 3), adjusting for all of the other covariates. In our cohort, the baseline probability of access failure was 0.2% (IABF > 500 ml/min, younger than 65 years of age, nonsmoker, no diabetes, and with upper arm fistula).
Figure 1.
Area under the receiver operating characteristic (AUROC) curve. Association of loss of primary patency and a model including age, history of diabetes, history of smoking, anatomical location of procedure, and initial IABF dichotomized at 500 ml/min. The AUROC curve for our model is 0.9336. The AUROC curve for a perfect diagnostic test would be 1 and a model with no predictive value would be ≤0.5 (solid diagonal line).
Table 3.
Final multiple logistic regression model: Retained dichotomized variables for loss of primary functional patency in patients with AVFs
| Variablea | Coefficient | OR | 95% Confidence Interval | P |
|---|---|---|---|---|
| IABF_500b | 3.4 | 29 | 15 to 52 | <0.001 |
| Age_65c | 1.3 | 3.6 | 1.7 to 7.7 | 0.001 |
| Diabetes | 0.9 | 2.3 | 1.3 to 4.5 | 0.007 |
| Smoking | 1.4 | 4.3 | 2.2 to 8.3 | <0.001 |
| Anatomical location (forearm) | 1.4 | 4.0 | 2.1 to 7.4 | <0.001 |
Adjusted for age, diabetes, history of smoking, and access type.
Initial IABF dichotomized at 500 ml/min.
Age dichotomized at 65 years.
Discussion
Vascular access failure remains a frequent cause of costly morbidity in patients receiving chronic hemodialysis. Investigation of determinants of vascular access failure in AVF patients will have direct implications for patient care. First, it will allow us to potentially identify subgroups of patients at higher risk of vascular access failure and to consider modifying surveillance of fistulas accordingly. Given that there is a subgroup of patients at high risk, efforts need to be directed toward finding ways for early prediction and prevention of vascular access failure in these patients. Second, increased surveillance of high-risk patients may identify patients that should undergo early fistula rescue therapy with balloon angioplasty to prevent total vascular access failure. Third, a modification of surveillance systems according to risk group may result in cost savings for the healthcare system.
This study retrospectively analyzed 831 patients with functional AVFs, which had successfully matured and were used for dialysis, to determine risk factors for subsequent loss of primary functional patency within 6 months after first use. Because the main objective of the study was to investigate fistulas that failed after maturation and to explore reasons associated with these failures, patients that required intervention (surgical or percutaneous endovascular) to achieve maturation were specifically excluded. The mean duration of 70 days between access creation and first use suggests that subsequent problems with loss of primary functional patency were not as a result of low flow from early use of a nonmature access. In contrast to a previously reported primary failure rate of 33% (12), our failure rate was only 10%, similar to that reported by Korten et al. (13).
The results demonstrated that the initial IABF was a powerful predictor of vascular access failure. Even without any additional risk factors, patients with an initial IABF of <500 ml/min had a high RR (RR = 16) of subsequent vascular access failure. Therefore, these results support that these patients should be routinely monitored. Additionally, the study identified other risk factors for vascular access failure in patients with AVFs: age ≥65 years, history of smoking or diabetes, and AVF located in the forearm. It is important to note that our study had a relatively short follow-up of 6 months. Given the strong association of initial IABF with loss of primary functional patency at 6 months, it is reasonable to assume, but cannot be concluded from our study, that this is likely to be a risk factor beyond 6 months. Further research is needed to confirm this hypothesis.
In contrast to previous studies (8,14–16), in which no significant difference was noticed between patient age and risk of loss of primary patency, our results indicated that age >65 years was a risk factor for the vascular access failure. Our results are similar to those of Windus et al. (16) and Huijbregts et al. (17) and suggest that patients who start hemodialysis therapy after 65 years of age need special increased surveillance to maintain their vascular access patency.
Smoking history has been examined in a few studies of vascular access morbidity with inconsistent results (15,18,19). Previous peripheral vascular damage in former and active smokers may lead to acute access thrombosis, which may partially explain the inconsistency in results. In this study, smoking resulted in an OR of 4.3, again identifying smokers as a group of patients who should have routine vascular access monitoring. Similarly, our study confirmed previous findings that diabetic patients are at risk for increased primary failure rates (12) and reduced primary patency rates (20).
In our study, forearm fistulas were at greater risk of loss of primary functional patency compared with those located in the upper arm. The smaller size of the vessels at this location may play a role in the difference noted; Poiseuille's law (Q − r4) physiologically supports the presence of lower IABF in the forearm location. In upper arm fistulas, the diameter of the vessels is expected to be bigger than in the forearm, and more significant roles for systemic aspects such as cardiac output and blood pressure can be expected (13,21).
An association of female sex with a greater risk for access-related failure has been reported in two large studies (1,22), although neither study investigated the results by access type. In contrast, our study did not show a significant association between sex and the risk of AVF failure, even after adjusting for age, presence of diabetes mellitus, hypertension, history of peripheral vascular disease, smoking history, and type of procedure. These results are similar to those of another study that found no significant differences in access survival between 80 men and 43 women on hemodialysis therapy with a first AVF (23). It has been suggested that differences in vessel diameter may account for the increased access-related morbidity experienced by women (1). Consistent with this notion, it was found that arterial size was a significant predictor of subsequent AVF survival (17), although gender was not (18), and that vessel size predicted fistula failure in the first 3 months after surgery (24). Our study did not have data on baseline vessel diameter or size.
In conclusion, current guidelines aimed to prevent vascular access failure are resource-intensive and are based on ongoing surveillance of all patients with evidence of vascular access dysfunction who receive early intervention. The results of our study offer novel findings and a more directed approach to surveillance techniques. Specifically, we have identified a set of patient risk factors (age), clinical risk factors (diabetes, smoking history), and vascular access characteristics (anatomical location, low initial IABF) that identify patients who are most at risk for developing vascular access failure. Of these, low initial IABF is the most significant. These risk factors can be used to guide a more directed approach for a vascular access screening protocol. Further work is required to confirm these initial findings and to translate this work into meaningful clinical practice.
Disclosures
None.
Acknowledgments
We thank Dr. Ann Kyle for editorial assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System: 2001 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2001 [Google Scholar]
- 3.The economic cost of ESRD, vascular access procedures, and Medicare spending for alternative modalities of treatment. U.S. Renal Data System. Am J Kidney Dis 30: S160–S177, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Lazarus JM, Huang WH, Lew NL, Lowrie EG: Contribution of vascular access-related disease to morbidity of hemodialysis. In: Vascular Access for Hemodialysis III, edited by Henry ML, Ferguson RM.Chicago, Gore and Associates and Precept Press, 1993; pp 23–42 [Google Scholar]
- 5.NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis 30: S150–S191, 1997 [PubMed] [Google Scholar]
- 6.Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M, Jr, Miller A, Scher L, Trerotola S, Gregory RT, Rutherford RB, Kent KC: Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 35: 603–610, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Krivitski NM: Access flow measurement during surveillance and percutaneous transluminal angioplasty intervention. Semin Dial 16: 304–308, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Miller PE, Carlton D, Deierhoi MH, Redden DT, Allon M: Natural history of arteriovenous grafts in hemodialysis patients. Am J Kidney Dis 36: 68–74, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Lin SL, Huang CH, Chen HS, Hsu WA, Yen CJ, Yen TS: Effects of age and diabetes on blood flow rate and primary outcome of newly created hemodialysis arteriovenous fistulas. Am J Nephrol 18: 96–100, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Ridao-Cano N, Polo JR, Polo J, Perez-Garcia R, Sanchez M, Gomez-Campdera FJ: Vascular access for dialysis in the elderly. Blood Purif 20: 563–568, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Huijbregts HJ, Bots ML, Moll FL, Blankestijn PJ: Hospital specific aspects predominantly determine primary failure of hemodialysis arteriovenous fistulas. J Vasc Surg 45: 962–967, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Korten E, Toonder IM, Schrama YC, Hop WC, van der Ham AC, Wittens CH: Dialysis fistulae patency and preoperative diameter ultrasound measurements. Eur J Vasc Endovasc Surg 33: 467–471, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hodges TC, Fillinger MF, Zwolak RM, Walsh DB, Bech F, Cronenwett JL: Longitudinal comparison of dialysis access methods: Risk factors for failure. J Vasc Surg 26: 1009–1019, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Churchill DN, Taylor DW, Cook RJ, LaPlante P, Barre P, Cartier P, Fay WP, Goldstein MB, Jindal K, Mandin H, et al. : Canadian Hemodialysis Morbidity Study. Am J Kidney Dis 19: 214–234, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Windus DW, Jendrisak MD, Delmez JA: Prosthetic fistula survival and complications in hemodialysis patients: Effects of diabetes and age. Am J Kidney Dis 19: 448–452, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Blankestijn PJ: Access blood flow and the risk of complications in mature forearm and upper arm arteriovenous fistulas. Blood Purif 27: 212–219, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Reilly DT, Wood RF, Bell PR: Prospective study of dialysis fistulas: Problem patients and their treatment. Br J Surg 69: 549–553, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Wetzig GA, Gough IR, Furnival CM: One hundred cases of arteriovenous fistula for haemodialysis access: The effect of cigarette smoking on patency. Aust N Z J Surg 55: 551–554, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ: Hemodialysis arteriovenous fistula patency revisited: Results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 3: 714–719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandeya S, Lindsay RM: The relationship between cardiac output and access flow during hemodialysis. ASAIO J 45: 135–138, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Ifudu O, Mayers JD, Cohen LS, Paul H, Brezsnyak WF, Avram MM, Herman AI, Friedman EA: Correlates of vascular access and nonvascular access-related hospitalizations in hemodialysis patients. Am J Nephrol 16: 118–123, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Prischl FC, Kirchgatterer A, Brandstatter E, Wallner M, Baldinger C, Roithinger FX, Kramar R: Parameters of prognostic relevance to the patency of vascular access in hemodialysis patients. J Am Soc Nephrol 6: 1613–1618, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A: Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg 12: 207–213, 1996 [DOI] [PubMed] [Google Scholar]