Abstract
Background and objectives: The optimal time of dialysis initiation is unclear. The goal of this analysis was to compare survival outcomes in patients with early and late start dialysis as measured by kidney function at dialysis initiation.
Design, setting, participants, & measurements: We performed a retrospective analysis of patients entering the U.S. Renal Data System database from January 1, 1995 to September 30, 2006. Patients were classified into groups by estimated GFR (eGFR) at dialysis initiation.
Results: In this total incident population (n = 896,546), 99,231 patients had an early dialysis start (eGFR >15 ml/min per 1.73 m2) and 113,510 had a late start (eGFR ≤5 ml/min per 1.73 m2). The following variables were significantly (P < 0.001) associated with an early start: white race, male gender, greater comorbidity index, presence of diabetes, and peritoneal dialysis. Compared with the reference group with an eGFR of >5 to 10 ml/min per 1.73 m2 at dialysis start, a Cox model adjusted for potential confounding variables showed an incremental increase in mortality associated with earlier dialysis start. The group with the earliest start had increased risk of mortality, wheras late start was associated with reduced risk of mortality. Subgroup analyses showed similar results. The limitations of the study are retrospective study design, potential unaccounted confounding, and potential selection and lead-time biases.
Conclusions: Late initiation of dialysis is associated with a reduced risk of mortality, arguing against aggressive early dialysis initiation based primarily on eGFR alone.
Despite the widespread use of chronic dialysis, there remains a lack of consensus about the optimal time at which renal replacement therapy should be initiated. Recommendations from the National Kidney Foundation-Dialysis Outcomes Quality Initiative (NKF-DOQI) are generally used as a guideline, although they have been predominantly opinion-based (1). Initial NKF-DOQI guidelines suggested beginning dialysis at a GFR of ∼10.5 ml/min per 1.73 m2, equivalent to a creatinine clearance of 9 to 13 ml/min (2). Updated NKF-DOQI guidelines in 2006 emphasized the need for a risk-benefit analysis when patients reach stage 5 chronic kidney disease or even earlier in certain circumstances (3). Although these guidelines suggest using clinical judgment, in practice, renal function at the time of dialysis initiation has been increasing over time (4). Early dialysis was believed to decrease mortality, hospitalization, and costs of treatment (5). However, early initiation creates lifestyle hardships, can be a limiting factor for employment and travel, and impacts the quality of life of patients and their families (6). Furthermore, multiple studies from the United States and Europe reported a lower level of renal function at dialysis initiation than recommended by the NKF-DOQI guidelines (7,8).
Because randomized prospective controlled trials addressing this important practical point are lacking, the goal of this project was to study the mortality associated with early compared with late dialysis initiation based on retrospective data from the U.S. Renal Data System (USRDS).
Materials and Methods
Study Population
Institutional review board approval was requested, and this study was determined to have an exempt status as a retrospective analysis of existing de-identified data. The data collected by the USRDS between January 1, 1995 and September 30, 2006 in the PATIENTS, MEDEVID, and RXHIST60 files were used. Adult patients (≥18 years old) on hemodialysis and peritoneal dialysis were included in the study. Patients were excluded from the primary analysis if they had subsequent renal transplantation, recovered renal function, or had values outside of the plausible ranges for height (120 to 200 cm), weight (23 to 180 kg), or body mass index (10 to 60 kg/m2). Independent variable values that were unlikely to be valid (e.g., age >100 years old) were eliminated, and records missing at least one value for any of the continuous covariates were excluded from analyses. In later additional analyses, we included patients who had received a renal transplant or the small subgroup of subjects who recovered renal function based on RXHIST60 file data.
Primary Variable of Interest and Outcome
The primary variable of interest was the level of renal function assessed by estimated GFR (eGFR, based on the Modification of Diet in Renal Disease formula) at the time of dialysis initiation, which is reported directly in the USRDS dataset. The following categories of eGFR at the time of dialysis initiation were chosen: >15, >10 to 15, >5 to 10, and ≤5 ml/min per 1.73 m2. We classified “early” and “late” dialysis initiation based solely on the level of renal function, where initiation at higher eGFR was considered “early start” and at lower eGFR was considered “late start.”
The outcome variable was patient survival from the time of dialysis initiation (variable FIRST_SE) to patient's death (variable DIED) or censor at September 30, 2006, which was the end of the available dataset. The causes of death recorded in the USRDS database were grouped into cardiovascular, infection, malignancy, and other causes.
Multivariate Models and Covariates
The Cox models were adjusted for covariates believed to be potential confounders that could be related to both the outcome and the primary variable of interest. All multivariate models were adjusted for the following variables at ESRD onset: age, height, weight, race, gender, diabetic status, comorbidity index (described below), duration of predialysis nephrology care, type of dialysis, type of vascular access, and cause of ESRD. The comorbidity index for each subject was calculated based on the Charlson index (9), with a method using only the terms available in the dataset (10): age, history of myocardial infarction, congestive heart failure, ischemic heart disease, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, cancer, and diabetes. Both the Charlson index and history of diabetes were included separately in the Cox models because diabetic status is a significant predictor of outcomes, and its effects cannot be completely explained by the Charlson index. The lack of colinearity between diabetic status and the Charlson index was confirmed before analysis.
Testing Proportionality Assumptions and Addressing Lack of Proportionality
In a separate analysis, we addressed the issue of proportionality of the hazard functions. The straightforward Cox model is based on the assumption that hazard functions for the groups compared, and covariates in the analysis are proportional across the time period studied. A major concern is that this assumption may not be valid. If either shows evidence of nonproportionality, more sophisticated analysis may be appropriate (incorporating time-varying interaction terms for covariates and/or describing the hazard function in piecewise manner, with separate analyses for early and late follow-up). We adopted both these approaches. First, we searched for variables that had significant time-varying terms, suggesting nonproportionality. This assessment was not computationally feasible for the entire massive dataset but was applied to two randomly sampled subsets of 50,000 observations. Nonproportionality was found in several variables including the primary variables of interest, which is not surprising given the high statistical power of these large datasets. To address nonproportionality for these variables, we included time-interaction terms in the models. To address nonproportionality attributed to the primary variable of interest, we analyzed short- and long-term outcomes separately. For example, using 18 months of follow-up as a cut-point between short and long term, we calculated the hazard ratio (HR) for the first 18 months and the HR for the follow-up period after 18 months. If a patient had an event in the first 18 months, they were excluded from the long-term (late) period. The procedure was repeated using 36- and 60-month cut-points to confirm that the results were not sensitive to the selection of any specific definition of long- and short-term follow-up.
Statistical Analyses
Logistic regression was used to evaluate factors associated with early and late dialysis start, and Kaplan-Meier graphs and Cox regression models were used in survival analysis. Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC). The population was stratified into subgroups by age, diabetic status, race, dialysis type, and level of comorbidity.
Results
We identified 1,070,228 subjects in the USRDS database with records in the Medical Evidence file from January 1, 1995 to September 30, 2006. After transplant recipients and other defined exclusions were applied, the number of subjects remaining was 896,546.
Baseline Characteristics of the Study Population
The baseline characteristics of the entire study population are presented in Table 1. This consisted of 896,546 patients with a mean age of 64.7 ± 14.5 years and included 46.9% females, 29.8% African Americans, and 64.2% whites. In addition, 46.7% of the recipients had ESRD caused by diabetes, and 28.4% had ESRD caused by hypertension. The Charlson comorbidity index was on average 7.0 ± 2.3. The group of patients with early start (eGFR > 15 ml/min per 1.73 m2) was different from the group with late start (eGFR ≤ 5 ml/min per 1.73 m2), in that those who started dialysis at higher eGFR (early start group) were of older age and higher comorbidity index and had a greater proportion of white patients, men, and patients with diabetes (Table 1).
Table 1.
Baseline characteristics of the study population (n = 896,546)
| Entire Study Population (n = 896,546)a | eGFR ≤ 5 ml/min per 1.73 m2 (n = 113,510) | eGFR >5 to 10 ml/min per 1.73 m2 (n = 463,277) | eGFR >10 to 15 ml/min per 1.73 m2 (n = 220,528) | eGFR > 15 ml/min per 1.73 m2 (n = 99,231) | |
|---|---|---|---|---|---|
| Age (years) | 64.7 (14.5) | 59.3 (15.8) | 64.2 (14.3) | 66.9 (13.8) | 68.4 (13.6) |
| Sex: male (%) | 53.1 | 44.1 | 51.7 | 57.4 | 60.8 |
| Height (cm) | 167.5 (11.0) | 166.6 (11.0) | 167.5 (11) | 167.9 (11.1) | 168 (11.2) |
| Weight (kg) | 75.2 (21.4) | 73.6 (21.4) | 75.3 (21.4) | 75.7 (21.4) | 75.5 (21.5) |
| Body mass index (kg/m2) | 26.8 (7.3) | 26.5 (7.2) | 26.8 (7.3) | 26.9 (7.3) | 26.7 (7.3) |
| Race (%) | |||||
| White | 64.2 | 55.4 | 63.6 | 67.4 | 70 |
| African American | 29.8 | 35.4 | 30.1 | 28 | 26.2 |
| Native American | 1.2 | 1.6 | 1.3 | 1.0 | 0.8 |
| Asian | 3.4 | 5.4 | 3.6 | 2.7 | 2.2 |
| Other | 1.3 | 2.3 | 1.4 | 0.9 | 0.8 |
| Cause of ESRD (%) | |||||
| Diabetes | 46.7 | 30 | 46.1 | 53.5 | 53.4 |
| Hypertension | 28.4 | 32.1 | 28.6 | 27 | 26.9 |
| Glomerulonephritis | 7.3 | 12.5 | 7.8 | 5.1 | 3.8 |
| Cystic kidney disease | 1.5 | 2.4 | 1.8 | 1.0 | 0.5 |
| Other urologic | 2.5 | 4.0 | 2.4 | 2.1 | 2.5 |
| Other cause | 9.7 | 12.9 | 9.8 | 8.1 | 9.2 |
| Unknown cause | 3.8 | 6.1 | 3.6 | 3.2 | 3.8 |
| History of diabetes (%) | 56.2 | 37.1 | 55.1 | 64.1 | 66.2 |
| Charlson comorbidity index | 7.0 (2.3) | 5.9 (2.4) | 6.9 (2.2) | 7.5 (2.1) | 7.8 (2.1) |
| Duration of nephrology care prior to dialysis | |||||
| <6 months | 17.4 | 17.2 | 17.1 | 17.1 | 18.7 |
| 6 to 12 months | 43.7 | 44.1 | 43.0 | 43.4 | 46.4 |
| >12 months | 38.9 | 38.8 | 39.9 | 39.5 | 35.0 |
| Employment status summary (%) | |||||
| Unemployed | 19.5 | 26.9 | 19.7 | 16.9 | 15.9 |
| Full-time employee | 5.8 | 9.4 | 6.5 | 4.1 | 2.6 |
| Part-time employee | 1.5 | 2.1 | 1.6 | 1.2 | 0.8 |
| Homemaker | 5.2 | 6.5 | 5.4 | 4.7 | 4.2 |
| Retired by age | 42.1 | 30.1 | 40.7 | 47.1 | 51.0 |
| Retired because of disability | 19.1 | 15.9 | 19.1 | 20.3 | 20.3 |
| Dialysis modality (%) | |||||
| Hemodialysis | 92.8 | 94.1 | 92.4 | 92.6 | 93.5 |
| Peritoneal | 7.2 | 5.9 | 7.6 | 7.4 | 6.5 |
| Access used on first outpatient dialysis (%)b | |||||
| Fistula | 12.6 | 8.4 | 13.6 | 14.2 | 9.5 |
| Graft | 4.7 | 3.0 | 4.7 | 5.4 | 4.2 |
| Catheter | 80.3 | 86.2 | 79.4 | 78.2 | 83.7 |
| Other | 2.4 | 2.5 | 2.3 | 2.3 | 2.6 |
| Serum creatinine (mg/dl) | 7.2 (3.5) | 13.7 (4.2) | 7.6 (1.7) | 4.9 (0.9) | 3.4 (0.7) |
| Blood urea nitrogen (mg/dl) | 87.8 (33.6) | 113.9 (37.2) | 88.3 (30.1) | 78.6 (30.8) | 72.7 (32.1) |
| HbA1c (%)c | 6.7 (1.6) | 6.3 (1.3) | 6.6 (1.6) | 6.9 (1.7) | 6.9 (1.7) |
| Hemoglobin (g/dl) | 9.8 (1.7) | 9.1 (1.9) | 9.8 (1.7) | 10.1 (1.6) | 10.3 (1.6) |
| Hematocrit (%) | 29.4 (5.4) | 27.1 (5.8) | 29.3 (5.3) | 30.4 (5.1) | 31.1 (5.2) |
Baseline characteristics for continuous variables are presented as mean (SD) and for categorical variables as percent of total. The P value by ANOVA (for continuous variables) or χ2 (for categorical variables) was <0.001 for all categories tested.
The categories of dialysis access are based on responses to question 18.d of CMS form 2728 filled by dialysis units. There is potential inconsistency, specifically if a PD catheter is included into the “Catheter” category vs. “Other” category.
Only reported on data form used after June 1, 2005; n = 21,458.
Predictors of Late and Early Dialysis Start
Using logistic regression, we identified factors independently associated with late (eGFR ≤ 5 ml/min per 1.73 m2) and early (eGFR > 15 ml/min per 1.73 m2) dialysis start (Table 2). Non-white race, female gender, lower comorbidity index, absence of diabetes, arteriovenous fistula or graft (compared with dialysis catheter), and hemodialysis had significant associations (P < 0.001) with a later dialysis start. White race, male gender, greater comorbidity index, presence of diabetes, and peritoneal dialysis were significantly (P < 0.001) associated with an early start.
Table 2.
Factors predicting late start and early start of dialysis in multivariate model
| Late Start (eGFR ≤15 ml/min) |
Early Start (eGFR >15 ml/min) |
|||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Weight at ESRD onset (per 5 kg) | 0.99 (0.98 to 0.99) | <0.001 | 1.00 (1.00 to 1.00) | 0.81 |
| Height at ESRD onset (per 10 cm) | 1.03 (1.02 to 1.04) | <0.001 | 0.95 (0.94 to 0.95) | <0.001 |
| Race: African American | 1.11 (1.09 to 1.12) | <0.001 | 0.96 (0.95 to 0.98) | <0.001 |
| Race: Asian | 1.91 (1.85 to 1.97) | <0.001 | 0.58 (0.56 to 0.61) | <0.001 |
| Race: Native American | 1.60 (1.51 to 1.68) | <0.001 | 0.64 (0.60 to 0.69) | <0.001 |
| Race: Other | 1.86 (1.77 to 1.95) | <0.001 | 0.62 (0.57 to 0.66) | <0.001 |
| Race: white | Reference | Reference | ||
| Sex: female (compared with male) | 1.73 (1.70 to 1.76) | <0.001 | 0.62 (0.61 to 0.64) | <0.001 |
| Diabetes (compared with no diabetes) | 0.57 (0.55 to 0.58) | <0.001 | 1.59 (1.56 to 1.63) | <0.001 |
| Charlson comorbidity index | 0.89 (0.89 to 0.90) | <0.001 | 1.11 (1.10 to 1.11) | <0.001 |
| Nephrology care for <6 months before dialysis | 0.53 (0.50 to 0.57) | <0.001 | 1.06 (1.52 to 1.68) | <0.001 |
| Nephrology care 6 to 12 months before dialysis | 0.56 (0.54 to 0.59) | <0.001 | 1.55 (1.50 to 1.60) | <0.001 |
| No nephrology care before dialysis | 0.93 (0.90 to 0.96) | <0.001 | 1.65 (1.61 to 1.70) | <0.001 |
| Nephrology care for >12 months before dialysis | Reference | Reference | ||
| Dialysis type: hemo- (versus peritoneal) | 1.51 (1.47 to 1.55) | <0.001 | 0.98 (0.96 to 1.01) | 0.23 |
| Vascular access: Fistula | 0.54 (0.50 to 0.58) | <0.001 | 0.88 (0.83 to 0.92) | <0.001 |
| Vascular access: graft | 0.51 (0.45 to 0.57) | <0.001 | 1.06 (0.98 to 1.14) | 0.18 |
| Vascular access: catheter | Reference | Reference | ||
| ESRD cause: hypertension | 1.09 (1.06 to 1.12) | <0.001 | 1.19 (1.16 to 1.21) | <0.001 |
| ESRD cause: glomerulonephritis | 1.41 (1.37 to 1.46) | <0.001 | 0.77 (0.74 to 0.80) | <0.001 |
| ESRD cause: cystic disease | 1.29 (1.23 to 1.36) | <0.001 | 0.53 (0.48 to 0.58) | <0.001 |
| ESRD cause: other | 1.27 (1.23 to 1.31) | <0.001 | 1.3 (1.27 to 1.34) | <0.001 |
| ESRD cause: diabetes | Reference | Reference | ||
Survival Analyses
An unadjusted Kaplan-Meier analysis was suggestive of incrementally increased survival of patients initiating dialysis later (Figure 1). In the Cox model adjusted for the potential confounding variables listed above, the time of dialysis initiation remained significantly associated with mortality risk (Table 3). Because of missing values for some of the continuous independent variables, the number of subjects for this analysis was reduced to 895,293.
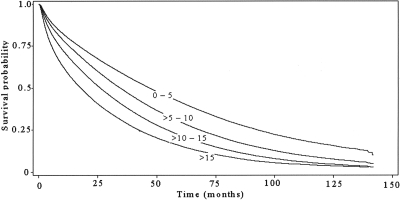
Figure 1.
Kaplan-Meier survival curves (survival versus time after dialysis initiation) for categories of patients divided by the residual renal function (eGFR, ml/min per 1.73 m2) at the initiation of dialysis.
Table 3.
Effect of timing of dialysis start on patient survival using Cox models in the entire study population and patient subgroupsa
| HR (95% CI) | P | |
|---|---|---|
| Entire study population (n = 895,293)b | ||
| eGFR >15 ml/min per 1.73 m2 | 1.44 (1.43 to 1.45) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.15 (1.15 to 1.16) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.88 (0.87 to 0.88) | <0.001 |
| Age categoriesb | ||
| Patients younger than 75 yr (n = 651,304) | ||
| eGFR >15 ml/min per 1.73 m2 | 1.48 (1.46 to 1.49) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.17 (1.16 to 1.17) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.86 (0.85 to 0.86) | <0.001 |
| Patients 75 or older (n = 243,989) | ||
| eGFR >15 ml/min per 1.73 m2 | 1.35 (1.33 to 1.37) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.11 (1.10 to 1.26) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.96 (0.94 to 0.97) | <0.001 |
| Dialysis modalityb | ||
| Patients treated with peritoneal dialysis (n = 63,691) | ||
| eGFR >15 ml/min per 1.73 m2 | 1.42 (1.37 to 1.47) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.10 (1.07 to 1.12) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.96 (0.93 to 0.99) | 0.02 |
| Patients treated with hemodialysis (n = 801,685) | ||
| eGFR >15 ml/min per 1.73 m2 | 1.49 (1.47 to 1.50) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.17 (1.16 to 1.17) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.87 (0.86 to 0.87) | <0.001 |
| Comorbidity categoriesb | ||
| Charlson comorbidity index <6 (n = 204,208) | ||
| eGFR >15 ml/min per 1.73 m2 | 1.46 (1.42 to 1.5) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.18 (1.16 to 1.21) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.84 (0.83 to 0.85) | <0.001 |
| Charlson comorbidity index 6 to 8 (n = 468,446) | ||
| eGFR >15 ml/min per 1.73 m2 | 1.46 (1.44 to 1.48) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.15 (1.14 to 1.16) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.90 (0.89 to 0.91) | <0.001 |
| Charlson comorbidity index >8 (n = 222,639) | ||
| eGFR >15 ml/min per 1.73 m2 | 1.37 (1.35 to 1.39) | <0.001 |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.13 (1.11 to 1.14) | <0.001 |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |
| eGFR ≤5 ml/min per 1.73 m2 | 0.94 (0.92 to 0.95) | <0.001 |
Only primary variables of interest are presented in the table. All models were adjusted for the following covariates: age at ESRD onset, height and weight at the time of ESRD onset, race, sex, diabetic status, Charlson comorbidity index, duration of pre-dialysis nephrology care, type of dialysis, type of vascular access, and cause of ESRD.
The number of subjects may be less than the entire study population because of missing variables.
In particular, compared with the group of patients who started dialysis at an eGFR of >5 to 10 ml/min per 1.73 m2, those that started dialysis at an eGFR of ≤5 ml/min per 1.73 m2 had a reduced risk of mortality (HR, 0.88; 95% confidence interval [CI], 0.84 to 0.92; P < 0.001), whereas there was an incremental increase in mortality risk associated with starting dialysis at an eGFR >10 to 15 ml/min per 1.73 m2 (HR, 1.15; 95% CI, 1.15 to 1.16; P < 0.001) or an eGFR >15 ml/min per 1.73 m2 (HR, 1.44; 95% CI, 1.43 to 1.45; P < 0.001).
To address potential residual confounding by age, race, diabetic status, dialysis modality, and comorbidity level, we performed analyses in subgroups stratified by these factors. In all subgroups, whether grouped by age (<75 or ≥75 years of age); dialysis modality; or comorbidity levels (Charlson index <6, 6 to 8, and >8 based on 25th and 75th percentiles), there was still a consistent and statistically significant trend toward greater mortality with an earlier start that was similar to the primary analysis (Table 3). The subgroups stratified by the presence or absence of diabetes and by racial categories also showed the same relationship. For example, patients with diabetes had an HR of 1.43 (95% CI, 1.41 to 1.45) and 0.89 (95% CI, 0.88 to 0.90) in the highest and lowest eGFR categories, respectively, whereas those without diabetes had HRs of 1.45 (95% CI, 1.44 to 1.47) and 0.88 (95% CI, 0.87 to 0.89).
In addition to our primary analysis, we also tested using alternate exclusion criteria. First, an analysis that included patients who recovered renal function based on USRDS RXHIST60 file data (0.9% of the study population) generated essentially the same results. Second, an analysis including patients who received a renal transplant after having been on dialysis for at least 60 days (total n = 979,686) also found similar results. In this second case, compared with the group of patients who started dialysis at an eGFR of >5 to 10 ml/min per 1.73 m2, those that started dialysis at an eGFR ≤5 ml/min per 1.73 m2 had reduced risk of mortality (HR, 0.87; 95% CI, 0.86 to 0.88; P < 0.001), whereas there was an increase in risk associated with starting dialysis at an eGFR >10 to 15 ml/min per 1.73 m2 (HR, 1.17; 95% CI, 1.16 to 1.18; P < 0.001) and at an eGFR >15 ml/min per 1.73 m2 (HR, 1.46; 95% CI, 1.45 to 1.48; P < 0.001) The results of subgroup analyses using these alternate exclusion criteria also remained essentially the same as those described above except that peritoneal dialysis patients in the earliest-start group did not have improved mortality compared with the reference group (HR, 0.98; 95% CI, 0.95 to 1.02).
Hemoglobin and albumin were not included in the primary model because approximately one third of the subjects were missing values for one or both. We repeated a separate analysis including hemoglobin and albumin as additional covariates using only those records with complete data. Both hemoglobin (HR, 0.995 per g/dl; 95% CI, 0.993 to 0.996; P < 0.001) and albumin (HR, 0.741 per g/dl; 95% CI, 0.737 to 0.744; P < 0.001) showed an association with mortality. However, this did not affect the observed association between the timing of dialysis start and mortality.
As the practice of nephrology might have changed over the 12 years of the study period, we divided the study period into six sequential pairs of years. Repeat analyses limited to each of these six cohorts still showed the same associations as in the primary analysis.
To address the issue of nonproportionality mentioned earlier, we analyzed short-term and long-term outcomes separately. There were differences in the magnitude of the association between eGFR and mortality between short-term and long-term follow-up, suggesting nonproportionality. However, overall, the findings were consistent with the conclusions of the primary analysis and not affected by the choice of definition (Table 4).
Table 4.
Separate analyses of early and late outcomea
| 18-Month Cut-Point |
36-Month Cut-Point |
60-Month Cut-Point |
||||
|---|---|---|---|---|---|---|
| Short-Term | Long-Term | Short-Term | Long-Term | Short-Term | Long-Term | |
| eGFR >15 ml/min per 1.73 m2 | 1.62 (P < 0.001) | 1.24 (P < 0.001) | 1.54 (P < 0.001) | 1.19 (P < 0.001) | 1.49 (P < 0.001) | 0.91 (P = 0.360) |
| eGFR >10 to 15 ml/min per 1.73 m2 | 1.20 (P < 0.001) | 1.08 (P < 0.001) | 1.18 (P < 0.001) | 1.03 (P = 0.373) | 1.14 (P < 0.001) | 1.07 (P = 0.23) |
| eGFR >5 to 10 ml/min per 1.73 m2 | Reference | |||||
| eGFR ≤5 ml/min per 1.73 m2 | 0.89 (P < 0.001) | 0.84 (P < 0.001) | 0.86 (P < 0.001) | 0.82 (P < 0.001) | 0.84 (P < 0.001) | 0.89 (P = 0.010) |
In this analysis, as a means of assessing nonproportionality, separate Cox models were constructed to evaluate for short-term and long-term outcomes. Numbers are hazard ratios. Three different definitions were used (18, 36, and 60 months) to assess the effect of the specific definition of short- vs. long-term outcome.
Discussion
The optimal timing of initiation of chronic dialysis remains elusive. There is a trend in the nephrology community toward earlier initiation of dialysis (11). Unfortunately, prospective data that could guide practice are lacking. The first multicenter, randomized controlled trial (Initiating Dialysis Early And Late, or IDEAL) was undertaken in Australia and New Zealand (12), but final results have not yet been reported.
In this project, we used a large dataset to retrospectively assess the association of dialysis timing with mortality. We found a dose-dependent increase in mortality associated with earlier dialysis initiation. After correcting for other factors, compared with those with an eGFR of >5 to 10 ml/min per 1.73 m2 at dialysis start, patients who initiated dialysis with a higher eGFR experienced a 44% greater mortality risk, whereas those who initiated dialysis at the lowest eGFR (≤5 ml/min per 1.73 m2) had a 12% lower risk of death. Similar results were shown in subpopulations grouped by similar age category, comorbidity score, diabetic status, or dialysis modality; in each group, mortality risk associated with earlier dialysis initiation changed in a dose-dependent fashion.
These results are in disagreement with some of the prior retrospective studies suggesting improved patient survival associated with earlier initiation of chronic dialysis (13–15). However, lead time bias—where mortality seems improved simply because of an earlier start point used to calculate survival—may have given an advantage in these reports to patients who began treatment earlier (8,16). Although our study also was subject to lead time bias, because we actually found a disadvantage associated with an earlier start, this type of bias could not explain our results. Our findings are consistent with other studies. Traynor et al. (17) calculated survival after reaching an estimated fixed level of kidney function and found that patients starting dialysis later had improved survival. Beddhu et al. (18), using propensity scores in a multivariate model in Dialysis Morbidity and Mortality Study Wave 2 patients, also showed an advantage of later start, albeit it was of lesser magnitude (HR, 1.14 for each 5 ml/min per 1.73 m2).
Selection bias might be a more serious issue. Specifically, those with an early dialysis start might have different reasons for dialysis initiation, e.g., acute illness or difficulty with volume control, whereas younger, healthier patients might tend to be started on dialysis later. Indeed, baseline characteristics of the study population (Table 1) showed that younger and healthier patients are more represented in the group with later start; however, multivariate analysis should address this to a degree. Wilson et al. (6) reported that a difference in survival between late and early start populations could be explained by taking into account covariates such as age, sex, employment status, vascular disease, and hypertension. On the other hand, Kazmi et al. (19), in a study of a smaller cohort of USRDS patients, found that a greater eGFR at initiation of dialysis was associated with a greater risk for death, which could not be completely explained by differences in comorbidity level. Similarly, in our study, we found that even when comorbidity and other covariates were taken into account, the association between early start and mortality remained robust.
In the overall context of existing literature, our finding of a lack of benefit of an early start even after adjustment for covariates conflicts with some reports (13–15) but supports the findings presented by other authors (17,18,19). Our findings are based on the largest and the most recent dataset and show an effect size and significance level greater than generally previously reported. We showed this association in multiple subgroups and showed dose-dependency in the incremental increase in risk of death with greater eGFR at dialysis start.
This retrospective study cannot establish causation, only association, and needs to be interpreted with caution. However, it is interesting to speculate about hazards of dialysis that could explain the advantage of the later start group even after adjustment for their lesser comorbidities and other covariates. These include clinical or subclinical bloodstream and peritoneal infections, heightened inflammation, exposure to antibiotic-resistant organisms or to bacterial fragments, hemodynamic effects including possible accelerated loss of remaining renal function (20), dialysis access complications, protein or blood loss, exposure to heparin, and the higher doses of erythropoietin required because of its reduced potency when given intravenously (21). Early start could cause an increased cumulative exposure to these hazards that may in the end be detrimental. There is also the potential negative impact of initiating dialysis on patients' quality of life, employment, and mood. Specifically, depression in dialysis patients can lead to a cascading effect toward a higher risk of mortality with lower medication adherence (22) and dialysis withdrawal (23). However, these considerations regarding mechanisms are purely speculative and should not be considered a part of the study results.
Aside from lead time bias and selection bias, our study has other limitations. Statistical techniques, including subgroup analysis, reduce but cannot provide a complete guarantee against residual confounding. Second, there is possible confounding by unassessed factors. For example, the reason for dialysis initiation was not available and may well have been different between the groups. Likewise, the rate of disease progression was not evaluated and might confound the results if patients with rapidly progressive disease were both initiated earlier and had poorer survival. Finally, survival bias is a potential issue, because only people who survived to the time of initiation of dialysis were analyzed, eliminating those who could have started dialysis early but instead died before initiation.
Despite these limitations, we believe there are potential practical implications. The main justification for initiating dialysis before a patient has symptoms is the expectation that it will confer a short- or long-term benefit in morbidity or mortality. Our results, although limited by being retrospective, do not suggest such a benefit for mortality and therefore argue against early dialysis start based simply on the degree of kidney function. These results should not be interpreted in a way that would delay dialysis for symptomatic patients or where there is hyperkalemia, poor volume control, or another clinical indication.
Conclusions
In conclusion, in the absence of a randomized trial, this retrospective analysis of the USRDS data suggests that late initiation of dialysis is associated with a reduced risk of mortality; a policy of early dialysis initiation based only on eGFR without clinical indications cannot be recommended.
Disclosures
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the U.S. government.
Acknowledgments
The data reported here have been supplied by the USRDS.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Obrador GT, Arora P, Kausz AT, Ruthazer R, Pereira BJG, Levey AS: Level of renal function at the initiation of dialysis in the U.S. end-stage renal disease population. Kidney Int 56: 2227–2235, 1999 [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation: NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 30: S67–S136, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Hemodialysis Adequacy 2006 Work Group: Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48Suppl 1: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Rosansky SJ, Clark WF, Eggers P, Glassock RJ: Initiation of dialysis at higher GFRs: Is the apparent rising tide of early dialysis harmful or helpful? Kidney Int 76: 257–261, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Hakim R, Lazarus J: Initiation of dialysis. J Am Soc Nephrol 6: 1319–1328, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Wilson B, Harwood L, Locking-Cusolito H, Chen SJ, Heidenheim P, Craik D, Clark WF: Optimal timing of initiation of chronic hemodialysis? Hemodial Int 11: 263–269, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Korevaar JC, Jansen MAM, Dekker FW, Jager KJ, Boeschoten EW, Krediet RT, Bossuyt PMM: When to initiate dialysis: Effect of proposed US guidelines on survival. Lancet 358: 1046, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Korevaar J, van Manen J, Boeschoten E, Dekker F, Krediet RNECOSAD Study Group: When to start dialysis treatment: Where do we stand? Perit Dial Int 25: S69–S72, 2005 [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Lin SJ, Koford JK, Baird BC, Habib AN, Reznik I, Chelamcharla M, Shihab FS, Goldfarb-Rumyantzev AS: The association between length of post-kidney transplant hospitalization and long-term graft and recipient survival. Clin Transplant 20: 245–252, 2006 [DOI] [PubMed] [Google Scholar]
- 11.U.S. Renal Data System: USRDS 2005 Annual Data Report, Bethesda, MD, National Institutes of Health, 2005 [Google Scholar]
- 12.Cooper B, Branley P, Bulfone L, Collins J, Craig J, Dempster J, Fraenkel M, Harris A, Harris D, Johnson D, Kesselhut J, Luxton G, Pilmore A, Pollock C, Tiller DIDEAL Study Steering Committee: The Initiating Dialysis Early and Late (IDEAL) study: Study rationale and design. Perit Dial Int 24: 176–181, 2004 [PubMed] [Google Scholar]
- 13.Bonomini V, Albertazzi A, Vangelista A, Bortolotti GC, Stefoni S, Scolari MP: Residual renal function and effective rehabilitation in chronic dialysis. Nephron 16: 89–99, 1976 [DOI] [PubMed] [Google Scholar]
- 14.Bonomini V, Feletti C, Scolari MP, Stefoni S: Benefits of early initiation of dialysis. Kidney Int Suppl 17: S57–S59, 1985 [PubMed] [Google Scholar]
- 15.Tattersall J, Greenwood R, Farrington K: Urea kinetics and when to commence dialysis. Am J Nephrol 15: 283–289, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Winchester JF, Harbord N, Audia P, Dubrow A, Gruber S, Feinfeld D, Amerling R: The 2006 K/DOQI guidelines for peritoneal dialysis adequacy are not adequate. Blood Purif 25: 103–105, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG: Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol 13: 2125–2132, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK: Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol 14: 2305–2312, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, Kausz AT: Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis 46: 887–896, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Shemin D, Bostom AG, Laliberty PD, Workin LD: Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis 38: 85–90, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Moist LM, Muirhead N, Wazny LD, Gallo KL, Heidenheim AP, House AA: Erythropoietin dose requirements when converting from subcutaneous to intravenous administration among patients on hemodialysis. Ann Pharmacother 40: 198–203, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL: Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int 75: 1223–1229, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Hackett AS, Watnick SG: Withdrawal from dialysis in end-stage renal disease: Medical, social, and psychological issues. Semin Dial 20: 86–90, 2007 [DOI] [PubMed] [Google Scholar]