Abstract
Background and objectives: Although lesions of renal osteodystrophy have traditionally been defined by bone turnover, alterations in skeletal mineralization and volume are also prevalent and may contribute to significant morbidity in patients with chronic kidney disease (CKD). The study presented here was undertaken to compare the traditional spectrum of renal osteodystrophy defined by bone turnover to a new classification system that includes T (turnover), M (mineralization), and V (volume) and to determine the value of biochemical parameters as predictors of specific TMV lesions.
Design, setting, participants, & measurements: Pediatric patients (n = 161) treated with peritoneal dialysis were enrolled into the study.
Results: Increased bone turnover and abnormal mineralization were prevalent (57% and 48%, respectively); bone volume was normal or increased in all subjects. Predictive algorithms for different skeletal diagnoses were established by Classification and regression tree analysis. Serum parathyroid hormone (PTH) less than 400 pg/ml in combination with alkaline phosphatase values less than 400 IU/L provided the highest correct prediction rate for patients with both normal bone turnover and normal mineralization. Levels of PTH were higher and serum calcium levels were lower in patients with defective mineralization, irrespective of bone turnover.
Conclusions: Although no single biochemical marker is able to provide a complete assessment of renal osteodystrophy, a combination of serum calcium, alkaline phosphatase, and PTH levels may lead to a more precise noninvasive assessment of turnover and mineralization abnormalities in this population.
Renal osteodystrophy, a disorder of bone remodeling, is a common complication of chronic kidney disease (CKD). Traditionally, renal osteodystrophy has been diagnosed by bone histomorphometry and classified according to lesions of bone turnover ranging from high rates of bone formation (mild lesions of secondary hyperparathyroidism and osteitis fibrosa) to low turnover (adynamic bone) (1,2). However, alterations in skeletal mineralization and bone volume also occur in a substantial proportion of pediatric patients with CKD (2,3) and may contribute to the increased fracture rates, boney deformities, poor linear growth, and chronic bone pain that persist despite adequate control of secondary hyperparathyroidism (4).
Underscoring the potential of parameters other than bone turnover to bone disease in CKD, a new definition for renal osteodystrophy, emphasizing the assessment of three key histologic descriptors—bone turnover (T), mineralization (M), and volume (V) (TMV system)—has been recommended in the interpretation of bone biopsies in all patients with CKD (5). Although several traditional biomarkers, including serum calcium, phosphorus, parathyroid hormone (PTH), and alkaline phosphatase, are routinely measured in patients with CKD, their ability to assess skeletal parameters other than bone turnover have not been evaluated. Currently, bone biopsy is the only available method for assessing all three recommended areas of bone histology (5,6), but this invasive procedure is not routinely used in any clinical setting and very few laboratories are equipped to perform bone histomorphometric analysis. Thus, the study presented here was undertaken to characterize the spectrum of renal osteodystrophy on the basis of the TMV classification system (5), to compare this classification system to the traditional spectrum of renal osteodystrophy as defined by bone turnover, and to determine the value of biochemical parameters as predictors of specific TMV diagnoses in pediatric dialysis patients.
Materials and Methods
Bone histomorphometric and biochemical data from 161 sequential pediatric patients treated with continuous cycling peritoneal dialysis who had undergone bone biopsy at our institution between the years of 1990 and 2005 were reviewed. All of the subjects were part of various clinical investigations to characterize the spectrum of renal osteodystrophy in pediatric dialysis patients (3,7–11); only baseline biopsies were used for the current analysis. All patients received calcium-based phosphate binders at the time of the bone biopsy, and in those treated with daily oral calcitriol, such therapy was held for 4 weeks before bone biopsy. Exclusion criteria were parathyroidectomy within the previous 12 months, the presence of aluminum staining on bone biopsy, treatment with immunosuppressive agents, or recombinant human growth hormone therapy within the 6 months before the bone biopsy.
Patients were admitted to the University of California–Los Angeles (UCLA) General Clinical Research Center, and full thickness bone biopsies were obtained from the anterior iliac crest (2 cm below the anterior superior iliac spine) using a modified Bordier trephine needle under general anesthesia. Double tetracycline labeling (10 mg/kg per day for 2 days, followed by a 10-day tetracycline-free period, a subsequent 2 days of relabeling, and a final 2- to 4-day tetracycline-free period) was performed in all patients before bone biopsy. Biopsy specimens were 0.5 cm in diameter by 1 to 2 cm in length. Specimens were fixed in 10% phosphate-buffered formalin for 48 hours, then dehydrated in alcohol, cleared with xylene, and embedded in methylmethacrylate. Static histomorphometric parameters were evaluated in lamellar portions of undecalcified 5-μm sections treated with Goldner stain; tetracycline labeling was assessed in unstained 10-μm sections of lamellar bone, as previously reported (12).
Primary bone histomorphometric parameters were assessed in trabecular bone under 200× magnification. Bone histology was reported using terminology established by the Nomenclature Committee of the American Society of Bone and Mineral Research (13) and the TMV classification for renal osteodystrophy (5). Mineralized bone was defined by green-staining areas; red-staining seams at least 1.5 μm in width were included in measurements of unmineralized osteoid. Derived indices were calculated by formulas displayed in the appendix. Normal values for all histomorphometric parameters were previously obtained from double-tetracycline-labeled iliac crest bone biopsy specimens from 31 pediatric patients with normal kidney function (mean age 12.4 ± 8.9 years, 71% male, 48% Caucasian, and 26% Hispanic) who were undergoing elective orthopedic surgery (14). Bone specimen acquisition, fixation, staining, and measurement for these normal volunteers were performed as described above for study subjects. At the time of bone biopsy, serum biochemical variables including calcium, phosphorus, alkaline phosphatase, and PTH were obtained.
Bone turnover was determined by bone formation rate/bone surface (BFR/BS) after tetracycline labeling and was classified as low, normal, or high on the basis of normal values previously established at our institution (12,14). Biochemical values were evaluated for each bone turnover subgroup. Subsequently, all specimens were reclassified, considering parameters of mineralization and volume in addition to bone turnover (5). Mineralization was assessed by a combination of osteoid accumulation and osteoid mineralization rate. Patients with abnormalities in osteoid volume (OV/BV) and osteoid maturation time (OMT) were deemed to have abnormal skeletal mineralization. To validate the choice of these two parameters in representing the mineralization status of each patient, skeletal mineralization was subsequently re-evaluated using osteoid thickness (O.Th) and mineralization lag time (MLT) as defining parameters. Bone volume was defined by the parameter of bone volume/tissue volume (BV/TV). Biochemical parameters were then re-evaluated according to each new classification subtype.
Serum calcium, phosphorus, and alkaline phosphatase levels were determined using a Technicon Autoanalyzer II. Serum PTH concentrations were determined by first-generation immunometric assay (Nichols, San Juan Capistrano, CA) (15). The UCLA Human Subjects Protection Committee approved the study protocols, and informed consent was obtained from each patient and from parents or guardians, where appropriate.
Statistical Methods
Results were expressed as mean ± SEM. Before analysis, logarithmic transformation was applied to variables with skewed distributions (PTH, alkaline phosphatase, BFR, OMT, and MLT). Differences in mean values of biochemical variables between TMV groups were assessed by t tests, Mann–Whitney test, or ANOVA. Spearman correlation coefficients were used to determine relationships between bone and biochemical parameters. A P value less than 0.05 indicated statistical significance.
Classification and regression tree (CART) analysis (S-Plus statistical software) was performed to establish predictive algorithms for different lesions of renal osteodystrophy. In brief, binary trees were used to establish biochemical cutoff values for the diagnosis of normal versus abnormal bone histology. Cutoff values were not determined a priori by the investigators but were established from the data set such that each subsequent subset yielded a higher correct prediction rate for the bone parameter of interest. The biochemical parameter with greatest discriminatory capability was chosen as the first branch point; parameters with lower predictive capacity determined subsequent branchings. χ2 test was used to compare the significance of the values in each split. Patients with low bone turnover were excluded from CART analysis because of the small sample size.
Results
The patient characteristics are depicted in Table 1. Patients were 14.1 ± 1.2 (0.7 to 20) years of age and had been treated with peritoneal dialysis for 13 ± 3 months at the time of the bone biopsy. There was no difference in age, time on dialysis, or history of previous renal transplantation between the different subtypes of renal osteodystrophy.
Table 1.
Patient characteristics
| n | % | |
|---|---|---|
| Age | ||
| 0 to 5 years | 21 | 13 |
| 6 to 12 years | 46 | 29 |
| 13 to 20 years | 94 | 58 |
| Gender (male/female) | 81/80 | |
| Race | ||
| Hispanic | 102 | 63 |
| Caucasian | 36 | 23 |
| African American | 13 | 8 |
| Asian | 10 | 6 |
| Primary kidney disease | ||
| dysplasia/obstructive/reflux | 52 | 32 |
| glomerulonephritides | 62 | 38 |
| unknown | 34 | 22 |
| others | 13 | 8 |
| Dialysis duration | ||
| <12 months | 107 | 66 |
| 13 to 24 months | 26 | 17 |
| 25 to 60 months | 21 | 13 |
| >60 months | 7 | 4 |
When patients were classified according to traditional bone histomorphometric criteria (low, normal, or high bone turnover), high turnover bone disease was observed in 57%, normal bone turnover in 39%, and low bone turnover in 4% of subjects. Table 2 displays the biochemical characteristics corresponding to bone turnover. Although there were no differences in serum calcium levels between groups, serum PTH and alkaline phosphatase values were higher in patients with high bone turnover, the predominant bone lesion.
Table 2.
Bone histomorphometry according to bone turnover with corresponding biochemical values (n = 161)
| Turnover (BFR/BS) | Serum Calcium (mg/dl) | Serum Phosphorus (mg/dl) | Alkaline Phosphatase (IU/L) | PTH (pg/ml) |
|---|---|---|---|---|
| Low (n = 7) | 9.1 ± 0.6 | 8.2 ± 0.6a | 212 ± 40 | 163 ± 48 |
| Normal (n = 62) | 9.3 ± 0.1 | 6.0 ± 0.2 | 214 ± 18 | 356 ± 36 |
| High (n = 92) | 9.0 ± 0.1 | 6.4 ± 0.1 | 436 ± 28a | 781 ± 49a |
P < 0.01 from other two groups.
Subsequently, patients were reclassified according to the TMV classifications system. Because bone volume was normal (73%) or increased (27%) in all individuals and because no biochemical parameters correlated with indices of bone volume, subjects were stratified as to bone formation rate (low, normal, or high) and mineralization status (normal versus abnormal) alone. Abnormal mineralization (concurrent increase in osteoid volume and OMT) was present in 48% of all patients, including 58% of subjects with high bone turnover, 38% with normal bone turnover, and 29% with low bone turnover. Table 3 describes the biochemical variables according to the TMV classification.
Table 3.
Bone histomorphometry according to turnover and mineralization with corresponding biochemical values (n = 161)
| Turnover (BFR/BS) | Mineralization (OV/BV + OMT) | Serum Calcium (mg/dl) | Serum Phosphorus (mg/dl) | Alkaline Phosphatase (IU/L) | PTH (pg/ml) |
|---|---|---|---|---|---|
| Low (n = 7) | Normal (n = 5) | 9.6 ± 0.4 | 8.2 ± 0.6 | 197 ± 26 | 116 ± 15 |
| Abnormal (n = 2) | 8.1 ± 2.0 | 8.2 ± 2.2 | 250 ± 160 | 282 ± 162 | |
| Normal (n = 62) | Normal (n = 39) | 9.6 ± 0.1 | 6.0 ± 0.2 | 198 ± 16 | 286 ± 38 |
| Abnormal (n = 23) | 8.9 ± 0.2a | 5.9 ± 0.3 | 243 ± 41 | 477 ± 68a | |
| High (n = 92) | Normal (n = 39) | 9.2 ± 0.2 | 6.2 ± 0.2 | 340 ± 31 | 587 ± 58 |
| Abnormal (n = 53) | 8.8 ± 0.1 | 6.5 ± 0.2 | 506 ± 39 | 924 ± 67a |
P < 0.01 from subjects with normal mineralization.
Although serum PTH and alkaline phosphatase levels were both correlated directly with bone turnover and osteoid volume (PTH: r = 0.61, P < 0.01 [OV/BV] and r = 0.58, P < 0.01 [OMT]; alkaline phosphatase: r = 0.51, P < 0.01 [OV/BV] and r = 0.51, P < 0.01 [OMT]), serum calcium concentrations were inversely related to mineralization (osteoid volume: r = −0.35, P < 0.01 and OMT: r = −0.34, P < 0.01) but did not relate to bone turnover. Overall, for any given level of bone turnover, levels of PTH were higher and serum calcium values were lower in patients with a concomitant mineralization defect (Table 3). Indeed, patients with abnormal amounts of osteoid accumulation and prolonged OMT, despite normal bone turnover, had lower serum calcium levels and higher serum PTH levels (P < 0.01, Table 3). Similar relationships between PTH, alkaline phosphatase, and calcium were obtained when mineralization status was defined by alterations in O.Th and MLT. Bone volume did not correlate with any biochemical parameters.
CART
To establish algorithms that might simultaneously differentiate between patients with various combined alterations in bone turnover and skeletal mineralization, CART analysis was performed on the biochemical values of 154 patients with normal or high bone turnover. The limited number of patients with adynamic bone disease (n = 7) made CART analysis impossible for this patient subset; thus, these subjects were excluded from CART analysis.
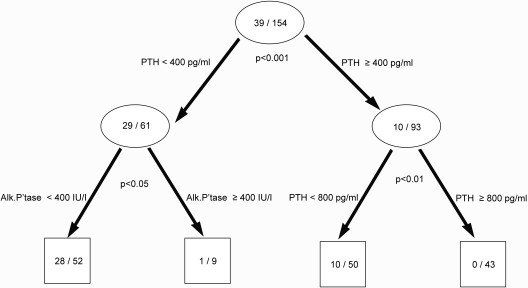
CART 1 (Figure 1).
Figure 1.
Prediction tree for patients with both normal bone turnover and mineralization. Numbers in the circles and the boxes define the number of the patients with both normal bone formation rate and normal O.Th over total number of patients in the cohort (excluding those with adynamic bone). The P value under each circle represents the significance of the split on the basis of the χ2 test.
To characterize patients with normal turnover and mineralization (n = 39) from all other patients, PTH was identified as the first discriminator and alkaline phosphatase as the second. Cutoff levels of PTH less than 400 pg/ml and alkaline phosphatase less than 400 IU/L identified 28 of 39 patients (72%) with both normal turnover and mineralization (sensitivity: 72%) whereas excluding 79% with abnormalities in turnover and/or mineralization (specificity: 79%). The overall correct prediction rate was 77%. A similar correct prediction rate was obtained when using O.Th and MLT as a measure of mineralization.
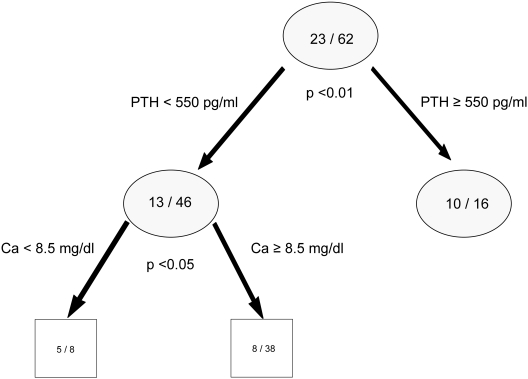
CART 2 (Figure 2).
Figure 2.
Prediction tree for the patients with normal bone turnover and defective mineralization. Numbers in the circles and the boxes define the number of the patients with normal bone formation rate and high O.Th over total number of patients with normal bone formation rate. The P value under each circle represents the significance of the split on the basis of the χ2 test.
To characterize patients with normal bone turnover and defective mineralization, 62 of 154 patients were identified with normal turnover. Twenty-three of these had increased osteoid volume in addition to prolonged OMT. Serum PTH was selected as the first discriminator and calcium as the second level predictor. In this subset of patients, abnormal mineralization was associated with serum PTH values greater than 550 pg/ml or serum calcium levels of less than 8.5 mg/dl. The sensitivity, specificity, and correct prediction rate of these parameters for increased excess osteoid volume and prolonged OMT in the context of normal turnover were 65%, 77%, and 73%, respectively.
Discussion
The study presented here compared the traditional system for the classification of renal osteodystrophy (based on bone turnover) to the recently recommended TMV classification system in pediatric patients treated with maintenance dialysis. Although lesions of low bone volume were not identified in the current cohort, the new classification system identified a high prevalence of mineralization defects, which has been previously underappreciated. Indeed, 57% of this population undergoing bone biopsy between 1990 and 2005 displayed high bone turnover, and defective mineralization was also present in almost half of those studied, irrespective of bone turnover. Serum PTH and alkaline phosphatase values were directly related to bone formation rate, whereas defective mineralization was associated with higher serum PTH and lower serum calcium levels.
The study presented here demonstrates the benefit of applying the new TMV classification system in shedding light on the large prevalence of skeletal mineralization defects in pediatric dialysis patients. Although reports from prior decades demonstrated a prevalence of the mixed lesion of renal osteodystrophy in adult and pediatric dialysis patients (1,14), these lesions were defined primarily by excess osteoid accumulation, which was attributed to aluminum intoxication and/or to the stimulatory effects of PTH on osteoblast matrix secretion. However, in these findings, the mineralization defect was defined as a combination of osteoid accumulation and delayed osteoid maturation—a parameter that would not be expected to be altered by excess PTH alone. Using these criteria, the data presented here suggest that defective mineralization continues to be a challenge, even in the postaluminum era, in the pediatric dialysis population. Indeed, although alterations in turnover and mineralization are present in many patients, a significant percentage of the population displayed abnormalities in mineralization despite normal bone turnover.
The etiology and specific clinical consequences of defective mineralization in pediatric dialysis patients are unknown—indeed, this problem is widespread despite treatment with calcium-based phosphate binders, active vitamin D sterols, and normal to increased phosphate levels (i.e., sufficient substrate to properly mineralize bone). Interestingly, bone pain, growth failure, and skeletal deformities that accompany childhood renal osteodystrophy are similar to the phenotypic expression of vitamin-D-deficient and vitamin-D-resistant rickets in pediatric patients with normal kidney function (16,17), suggesting that factors such as 25-hydroxy-vitamin D (25(OH)-vitamin D) deficiency and elevated fibroblast growth factor (FGF)-23 levels may influence mineralization in the uremic milieu. Although 25(OH)-vitamin D and FGF-23 values were not measured in the current cohort of patients, recent studies have demonstrated a high prevalence of 25(OH)-vitamin D deficiency in adults and children with CKD (18,19), and Langman et al. have previously demonstrated that 25(OH)-vitamin D therapy is effective in treating this mineralization defect in children with moderate (stage 3) CKD (20). Moreover, FGF-23 levels, which are markedly elevated in pediatric and adult patients treated with maintenance dialysis (21), have been shown to relate to parameters of skeletal mineralization in this population (12).
The prevalence of adynamic bone in the cohort presented here was only 4%, consistent with the relatively low prevalence of this lesion in pediatric patients over the years (22). Indeed, adynamic bone in children develops primarily after therapy with intermittent, high-dose calcitriol and calcium-based binder therapy (23) and none of the subjects in the cohort presented here had been exposed to such intensive active vitamin D sterol therapy before the bone biopsy. These findings are in sharp contrast to the abundance of low bone turnover commonly observed in the adult population because of aging and diabetes (24,25). The complete absence of low bone volume in pediatric patients also differs from the osteoporotic lesions described in older individuals (5,26).
The study presented here also emphasized the importance of the use of multiple biomarkers in the noninvasive diagnosis of pediatric renal osteodystrophy. Currently, serum PTH levels are commonly used as biomarkers for bone turnover; however, PTH levels, in addition to bone turnover, are also associated with osteoid accumulation, thus making the simultaneous noninvasive differentiation between altered turnover and mineralization difficult. However, the use of multiple biochemical markers in CART analysis allows for the discrimination between different histologic subtypes. The use of CART also identified the relationship between serum calcium concentrations and defective mineralization. Indeed, low serum calcium levels and high serum PTH values were the strongest predictors of mineralization defect in patients with bone turnover within the normal range, a finding that should be taken into consideration in view of the availability of therapeutic agents, such as calcium-free phosphate binders (10,27) and calcimimetic agents (28), which maintain serum calcium levels within the lower normal range. None of the patients included in this analysis received any of these agents before bone biopsy.
Through use of the TMV classification system, the study presented here identified a large prevalence of mineralization defects in pediatric dialysis patients that was previously underrecognized. These data suggest that a combination of serum PTH and alkaline phosphatase levels may be useful in discriminating patients with normal turnover and defective mineralization from those with normal mineralization, whereas a combination of serum PTH and calcium levels may be used to further identify individuals with an increased likelihood of poor skeletal mineralization. Although no single biochemical marker is able to provide a complete assessment of renal osteodystrophy, a combination of biochemical parameters may allow for improved assessment of turnover and mineralization abnormalities in pediatric patients treated with maintenance dialysis. The effect of these target ranges will need to be confirmed by prospective randomized studies.
Disclosures
Dr. Salusky receives Honoraria from Genzyme, Amgen, and Johnson & Johnson.
Acknowledgments
The authors thank Dr. William Goodman for his contribution in the interpretation of the bone biopsies. This work was supported in part by U.S. Public Health Services grants DK-35423, DK-67563, and RR-00865 and funds from the Casey Lee Ball Foundation. The Technological and Scientific Research Council of Turkey (TUBITAK) supported S.A.B. during her research fellowship at UCLA. S.A.B. and K.W.P. contributed equally to the preparation of this manuscript.
Appendix
Appendix.
Formulas used to calculate derived bone histomorphometric indices from primary measurements
| Abbreviation | Parameter | Unit | Formula |
|---|---|---|---|
| Structural | |||
| BV/TV | Bone volume/tissue volume | % | Bone area/tissue area × 100 |
| Static formation | |||
| OV/BV | Osteoid volume/bone volume | % | Osteoid area/bone area × 100 |
| O.Th | Osteoid thickness | μm | (Osteoid area/osteoid perimeter) × 2/1.2 |
| Dynamic formation | |||
| MS/BS | Mineralizing surface/bone surface | % | (Double-label perimeter + 1/2 single-label perimeter)/bone perimeter × 100 |
| MAR | Mineral apposition rate | μm/d | (Distance between labels/interlabel period)/1.2 |
| MLT | Mineralization lag time | days | O.Th/AjAr |
| OMT | Osteoid maturation time | days | O.Th/MAR |
| BFR/BS | Bone formation rate/bone surface | μm3/μm2 per year | MAR × MS/BS × 3.65 |
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Value of the New Bone Classification System for Pediatric Patients with Chronic Kidney Disease,” on pages 1730–1731.
References
- 1.Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, Saiphoo C, Fenton SS, Segre GV: The spectrum of bone disease in end-stage renal failure—an evolving disorder. Kidney Int 43: 436–442, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Salusky IB, Brill J, Oppenheim W, Goodman WG: Features of renal osteodystrophy in pediatric patients receiving regular peritoneal dialysis. Semin Nephrol 9: 37–42, 1989 [PubMed] [Google Scholar]
- 3.Salusky IB, Ramirez JA, Oppenheim W, Gales B, Segre GV, Goodman WG: Biochemical markers of renal osteodystrophy in pediatric patients undergoing CAPD/CCPD. Kidney Int 45: 253–258, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Groothoff JW, Offringa M, Van Eck-Smit BL, Gruppen MP, Van De Kar NJ, Wolff ED, Lilien MR, Davin JC, Heymans HS, Dekker FW: Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int 63: 266–275, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 6.K/DOQI clinical practice guidelines for bone metabolism and disease in children with chronic kidney disease. Am J Kidney Dis 46: S1–S121 2005 [PubMed] [Google Scholar]
- 7.Salusky IB, Kuizon BD, Belin TR, Ramirez JA, Gales B, Segre GV, Goodman WG: Intermittent calcitriol therapy in secondary hyperparathyroidism: A comparison between oral and intraperitoneal administration. Kidney Int 54: 907–914, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Kuizon BD, Goodman WG, Juppner H, Boechat I, Nelson P, Gales B, Salusky IB: Diminished linear growth during intermittent calcitriol therapy in children undergoing CCPD. Kidney Int 53: 205–211, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Salusky IB, Goodman WG, Kuizon BD, Lavigne JR, Zahranik RJ, Gales B, Wang HJ, Elashoff RM, Juppner H: Similar predictive value of bone turnover using first- and second-generation immunometric PTH assays in pediatric patients treated with peritoneal dialysis. Kidney Int 63: 1801–1808, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, Elashoff RM, Juppner H: Sevelamer controls parathyroid hormone-induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. J Am Soc Nephrol 16: 2501–2508, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Wesseling-Perry K, Harkins GC, Wang HJ, Sahney S, Gales B, Elashoff RM, Juppner H, Salusky IB: Response of different PTH assays to therapy with sevelamer or CaCO3 and active vitamin D sterols. Pediatr Nephrol 24: 1355–1361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB: Relationship between plasma FGF-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab 94: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR: Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2: 595–610, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, Goodman WG: Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int 53: 1358–1364, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nozawa-Ung K, Kim LY, Keutmann HT, Wang CA, Potts JT, Jr, Segre GV: Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 33: 1364–1367, 1987 [PubMed] [Google Scholar]
- 16.Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. ADHR Consortium. Nat Genet 26: 345–348, 2000 [DOI] [PubMed] [Google Scholar]
- 17.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium Nat Genet 11: 130–136, 1995 [DOI] [PubMed] [Google Scholar]
- 18.DeVille J, Thorp ML, Tobin L, Gray E, Johnson ES, Smith DH: Effect of ergocalciferol supplementation on serum parathyroid hormone and serum 25-hydroxyvitamin D in chronic kidney disease. Nephrology (Carlton) 11: 555–559, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Zisman AL, Hristova M, Ho LT, Sprague SM: Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27: 36–43, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Langman CB, Mazur AT, Baron R, Norman ME: 25-hydroxyvitamin D3 (calcifediol) therapy of juvenile renal osteodystrophy: Beneficial effect on linear growth velocity. J Pediatr 100: 815–820, 1982 [DOI] [PubMed] [Google Scholar]
- 21.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Salusky IB, Coburn JW, Brill J, Foley J, Slatopolsky E, Fine RN, Goodman WG: Bone disease in pediatric patients undergoing dialysis with CAPD or CCPD. Kidney Int 33: 975–982, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Kuizon BD, Salusky IB: Intermittent calcitriol therapy and growth in children with chronic renal failure. Miner Electrolyte Metab 24: 290–295, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH: Improved assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int 60: 1460–1468, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Malluche HH, Siami GA, Swanepoel C, Wang GH, Mawad H, Confer S, Smith M, Pratt RD, Monier-Faugere MC: Improvements in renal osteodystrophy in patients treated with lanthanum carbonate for two years. Clin Nephrol 70: 284–295, 2008 [PubMed] [Google Scholar]
- 26.Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM, Dias CJ, Malluche HH: Low bone volume—a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol 4: 450–455, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Martin KJ, Juppner H, Sherrard DJ, Goodman WG, Kaplan MR, Nassar G, Campbell P, Curzi M, Charytan C, McCary LC, Guo MD, Turner SA, Bushinsky DA: First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int 68: 1236–1243, 2005 [DOI] [PubMed] [Google Scholar]