Abstract
Background and objectives: ESRD has an adverse impact on patients who have had previous nonrenal solid-organ transplants (NRTxs; liver, heart, lung) and may be referred for a kidney transplant (KTx).
Design, setting, participants, & measurements: Using Scientific Registry of Transplant Recipients data for all KTx candidates who had NRTx and were listed between 1995 and 2008, incidence of NRTx listings were compared with trends in KTx without NRTX. The efficacy of kidney transplantation relative to dialysis was measured in time-dependent Cox models that incorporated candidates with the applicable previous organ transplant as a reference group.
Results: Overall, 4904 NRTx candidates were listed during the study period, growing from <1% of candidates before 1995 to 3.3% in 2008. A total of 38% of NRTx candidates were listed preemptively versus 21% of other candidates. NRTx candidates had dramatically shorter half-lives (≤ 4 years) after listing compared with previous KTx recipients (9.2 years). KTx demonstrated a survival advantage for each type of NRTx candidate relative to maintenance dialysis. Listing for expanded-criteria donor kidneys averaged 47% and did not differ significantly by previous transplant category.
Conclusions: KTx candidates who are placed on the waiting list after NRTx constitute a significant and more rapidly growing cohort compared with the general KTx candidate population. NRTx candidates are frequently listed preemptively but have rapid decline once placed on the waiting list. Targeted use of expanded-criteria donor and living-donor transplants in the NRTx population may be particularly important given their high mortality on the waiting list.
Renal failure may accompany or complicate transplantation of the liver, heart, lung, and small intestine (1). Ojo et al. (1), in their seminal study, reported a significant 5-year risk or development of chronic renal failure ranging from 7 to 21% by organ after transplantation of a nonrenal solid organ. That study also demonstrated the excess mortality with onset of renal failure and the survival benefit conferred by renal transplantation in this population.
Since the publication of the report by Ojo et al. (1), significant changes have been instituted in the organ allocation systems for lungs and livers in the United States that, in turn, have led to an increase in the numbers of these organs transplanted (2,3). Since early 2002, the Model for End Stage Liver Disease (MELD) has been used to allocate liver transplants in the United States with a sizable increase in liver transplants (2). The MELD includes serum creatinine and initiation of dialysis, its numerical value being higher with renal insufficiency. In May 2005, the new Lung Allocation Score (LAS) was introduced with emphasis on disease severity rather than waiting time as the primary determinant of eligibility for transplantation (4). Since the implementation of the LAS, a small increase in the number of lungs transplanted has occurred with unchanged in-hospital and 1-year mortality (3).
Given the foregoing and the increasing numbers of patients with nonrenal organ transplants (NRTxs), we decided to examine the trends in the kidney candidate population and use of kidney transplantation (KTx) in patients who have had a previous nonrenal organ transplant. Our principal aims were (1) to assess the incidence of KTx candidate placement on the waiting list after NRTx; (2) to evaluate the rate of preemptive listings, postlisting mortality, and donor quality among these candidates and recipients; and (3) to evaluate the efficacy of KTx for each type of NRTx candidate.
Materials and Methods
We examined data from the national Scientific Registry of Transplant Recipients for adult KTx candidates who were placed on the solitary KTx waiting list from 1995 through 2008 and had a previous heart, liver, or lung transplant. For patients with multiple listing dates during the study period, we included only the first listing date. We excluded patients who had previous pancreas or multiorgan transplants, because these populations were deemed a unique subset of the solid-organ transplant candidate pool. Demographic information and incidence rates of NRTx candidate listing were calculated and compared with trends in KTx patients without NRTx and those with previous KTxs alone. Kaplan-Meier plots and Cox proportional hazard models were used to assess risk factors for survival from the time of patients' placement on the waiting list. Covariates in the multivariable Cox models included age, percentage of panel-reactive antibodies (PRA %), race, body mass index, primary diagnosis, and presence and type of previous organ transplant. A multivariable logistic model was used to evaluate factors that were associated with preemptive listings using the same set of adjustment factors. The efficacy of KTx relative to patients who were listed as candidates was measured using time-dependent Cox models adjusted for age, race, and diabetes status, which incorporated the population of candidates with the applicable previous organ transplant as a reference group. For these models, follow-up time was censored on the date of living-donor transplantation. In addition, these models included only candidates who were listed after 1998, because candidates' initial dialysis date was unavailable in the United Network for Organ Sharing forms before this date. All analyses were conducted with SAS 9.2 (SAS Institute, Cary, NC).
Results
Demographics
During the study period, a total of 280,138 patients who were placed on the waiting list had no previous solid-organ transplants and 47,317 had received a previous KTx. A total of 4904 patients who were on the waiting list had received a previous NRTx, including 1607 with a previous heart transplant, 408 with a previous lung transplant, and 2889 with a previous liver transplant (Table 1). All candidate characteristics varied significantly across the various nonrenal organ transplant groups (P < 0.001). Black patients were represented more frequently in both primary and repeat listing for a KTx. Patients who were listed after a previous KTx were less likely to have private insurance as a primary payer and were more likely to be sensitized (PRA >30%). Those with a previous heart transplant were more likely to be older (>60 year) and were predominantly male. Among previous lung transplant recipients, women predominated. Previous lung transplant recipients were the least likely to be obese (Table 1).
Table 1.
Demographic characteristics of KTx candidates on the waiting list
| Demographic Characteristic (%) | No Previous Solid-Organ Transplant | Previous KTx | Previous Heart Transplant | Previous Lung Transplant | Previous Liver Transplant |
|---|---|---|---|---|---|
| N | 280,138 | 47,317 | 1607 | 408 | 2889 |
| Age ≥60 at listing | 25 | 10 | 46 | 30 | 31 |
| Female candidate | 40 | 42 | 20 | 53 | 34 |
| Black candidate | 29 | 27 | 15 | 4 | 10 |
| Obese candidate (BMI >30) | 33 | 21 | 23 | 8 | 23 |
| Diabetes as primary diagnosis | 17 | 10 | 3 | 1 | 5 |
| Private primary insurance | 45 | 35 | 39 | 48 | 47 |
| PRA ≥30% | 9 | 37 | 9 | 5 | 14 |
Characteristics all are statistically significantly different between study groups (P < 0.001). BMI, body mass index.
Growth in the Population of Candidates Listed after a Previous Renal or Nonrenal Solid-Organ Transplant
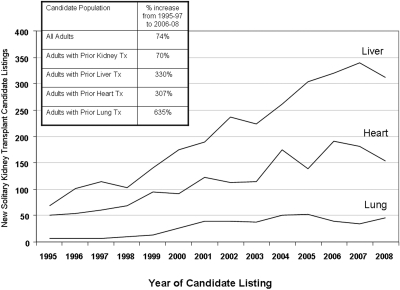
The proportions of KTx candidates who are on the waiting list and have had a previous renal or nonrenal solid-organ transplant have grown substantially over time (Figure 1). This growth has been particularly prominent in those who received previous liver (330%), heart (307%), and lung (635%) transplants. In contrast, the adult KTx primary listings grew by 74%, and kidney listings after a previous KTx grew by 70%.
Figure 1.
Growth in the population of KTx candidates with previous liver, heart, and lung transplants.
Preemptive Listing for Renal Transplantation after NRTx
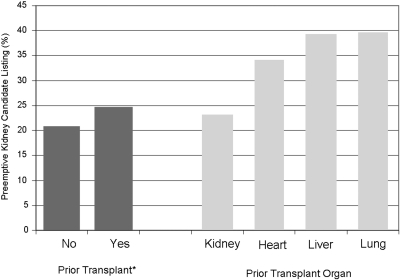
Predialysis listing was most frequent among candidates with previous liver and lung transplants and also more common among candidates with previous heart transplants as compared with candidates with previous KTx or no previous transplant (Figure 2). Overall, 38% of NRTx candidates were listed before dialysis initiation as opposed to 23% of candidates with previous KTx and 21% of candidates without previous organ transplantation (P < 0.001; Figure 2).
Figure 2.
Preemptive placement on the KTx waiting list by presence and type of previous organ transplant.
The results of the multivariate model examining factors that were associated with preemptive listing for KTx in the study population are depicted in Table 2. The likelihood for preemptive listing was higher in individuals who were aged 40 to 49 years and those who were aged >60 years relative to younger candidates who were aged 18 to 39 years. Patients who carried non-Medicare insurance were between two and four times more likely to be listed preemptively for a KTx compared with those with Medicare (private: adjusted odds ratio [aOR] 3.90 [95% confidence interval (CI) 3.81 to 3.97]; other: aOR 2.33 [95% CI 2.25 to 2.41]). Black and other nonwhite patients were associated with a lower likelihood of preemptive listing than white patients (black: aOR 0.43 [95% CI 0.42 to 0.44]; other: aOR 0.47 [95% CI 0.46 to 0.48]). On the basis of the same multivariate logistic regression model, relative to white patients, the adjusted odds of preemptive listings by other groups were as follows: American-Indian: aOR 0.44; Asian: aOR 0.62; Native Hawaiian or Other Pacific Islanders: aOR 0.44; Hispanic/Latino: aOR 0.41; and multiracial: aOR 0.54. Each of these categories was significantly different from white patients. Women were more likely to be listed preemptively than men (aOR 1.34; 95% CI 1.31 to 1.37). Higher PRAs were associated with a lower likelihood of preemptive listing. Preemptive listings were also increasingly more frequent in recent years. Preemptive listings for KTx occurred more frequently across all previous solid-organ transplant recipients including previous KTx recipients. There was also a statistically significantly greater likelihood for preemptive listing among previous heart and liver transplant recipients than candidates with a previous KTx. The median estimated GFR of patients who were listed preemptively were relatively similar by previous organ transplant (no previous KTx 15 ml/min per 1.73 m2; previous KTx 16 ml/min per 1.73 m2; previous liver transplant 17 ml/min per 1.73 m2; previous heart transplant 17 ml/min per 1.73 m2; and previous lung transplant 16 ml/min per 1.73 m2).
Table 2.
Likelihood for preemptive listing among newly listed KTx candidates
| Candidate Characteristic (Reference Group) | aOR | 95% CI |
|---|---|---|
| Age group (18 to 39) | ||
| 40 to 49 | 1.06 | 1.03 to 1.08 |
| 50 to 59 | 0.97 | 0.95 to 1.00 |
| ≥60 | 1.08 | 1.05 to 1.11 |
| Race/ethnicity (white) | ||
| black | 0.43 | 0.42 to 0.44 |
| other | 0.47 | 0.46 to 0.48 |
| Gender (male) | ||
| female | 1.34 | 1.31 to 1.37 |
| Year of listing (per year) | 1.06 | 1.06 to 1.06 |
| BMI group (19 to 24) | ||
| 13 to 18 | 0.80 | 0.75 to 0.85 |
| 25 to 29 | 1.18 | 1.15 to 1.21 |
| 30 to 34 | 1.17 | 1.14 to 1.20 |
| ≥35 | 1.14 | 1.10 to 1.18 |
| missing | 1.17 | 1.11 to 1.23 |
| Primary insurance (Medicare) | ||
| private | 3.90 | 3.81 to 3.97 |
| other | 2.33 | 2.25 to 2.41 |
| PRA % (0) | ||
| 1 to 9 | 0.88 | 0.85 to 0.90 |
| 10 to 29 | 0.82 | 0.79 to 0.86 |
| 30 to 79 | 0.60 | 0.58 to 0.63 |
| 80 to 100 | 0.40 | 0.38 to 0.42 |
| missing | 1.35 | 1.30 to 1.39 |
| Previous organ transplant (none) | ||
| kidney | 1.52 | 1.48 to 1.56 |
| livera | 2.06 | 1.89 to 2.24 |
| hearta | 1.87 | 1.67 to 2.10 |
| lung | 1.89 | 1.52 to 2.35 |
BMI, body mass index.
Statistically significantly greater likelihood for preemptive listing than candidates with a previous KTx.
Candidate Survival
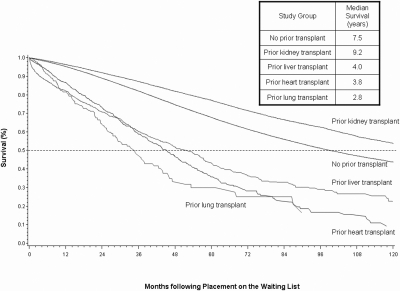
Candidate survival varied significantly between patients with previous organ transplants (Figure 3). The half-lives (median survival) on the waiting list were as follows: No previous transplant 7.5 years, previous KTx 9.2 years; previous liver transplant 6.0 years; previous heart transplant 3.8 years; and previous lung transplant: 2.8 years. On the basis of the multivariable Cox model, NRTx candidates all had a significantly increased hazard for death on the waiting list relative to candidates with no previous transplant (heart: adjusted hazard ratio [aHR] 1.85 [95% CI 1.65 to 2.08]; liver: aHR 1.82 [95% CI 1.67 to 1.99]; lung: AHR 2.79 [95% CI 2.25 to 3.46]). Despite improved unadjusted survival among candidates with a previous KTx as displayed in Figure 3, after adjustment in the Cox model, these candidates had slightly elevated hazard for death (aHR 1.07; 95% CI 1.03 to 1.11).
Figure 3.
Patient survival after placement on the waiting list by presence and type of previous transplant.
Donation Type by Presence and Type of Previous Organ Transplant
A total of 2480 recipients of NRTxs received a KTx within the study period. Overall, 24% of the patients who had received NRTxs and were receiving a KTx received it from a living donor, which was similar to the KTx population (23%). The proportion of living-donor transplants by presence and type of previous transplant were as follows: No previous transplant 30%; previous KTx 31%; previous liver transplant 18%; previous heart transplant 29%; and previous lung transplant 59%. Expanded-criteria donor (ECD) kidney listing (overall 47%) was relatively uniform across study groups (no previous KTx 47%; previous KTx 42%; previous heart transplant 52%; previous lung transplant 43%). In the multivariate model, none of the NRTx patients differed significantly with regard to the relative odds of being listed for an ECD KTx compared with those who did not receive a previous KTx (reference group; previous heart: aOR 1.94 [95% CI 0.83 to 1.06]; previous liver: aOR 1.06 [95% CI 0.97 to 1.16]; previous lung transplant: aOR 0.85 [95% CI 0.67 to 1.07]).
NRTx and non-NRTx recipients received 14 and 13% ECD KTxs, respectively (P = 0.08). the proportion of ECD transplant by presence and type of previous transplant were as follows: No previous transplant 18%; previous KTx 16%; previous liver transplant 14%; previous heart transplant 21%; and previous lung transplant 16%.
KTx was associated with a survival advantage for both renal transplant and NRTx candidates relative to maintenance dialysis. The relative benefit varied by NRTx organ as shown in Table 3. The magnitude of the survival benefit of transplantation was diminished among previous liver transplant recipients as compared with other nonrenal transplant and KTx recipients.
Table 3.
Survival advantage of KTx by previous transplant group
| Previous Transplant Groupa | HR for Mortality | 95% CI |
|---|---|---|
| None | 0.47 | 0.46 to 0.48 |
| Previous KTx | 0.44 | 0.42 to 0.47 |
| Previous liver transplant | 0.73 | 0.62 to 0.87 |
| Previous heart transplant | 0.42 | 0.33 to 0.54 |
| Previous lung transplant | 0.42 | 0.22 to 0.79 |
HR, hazard ratio.
Reference: Candidates on waiting list in appropriate previous transplant group.
Discussion
The number of candidates who are placed on the waiting list for KTx after a previous nonrenal transplant is growing significantly. NRTx candidates are listed before dialysis initiation more frequently than non-NRTx recipients and receive similar quality organs. Our data also point to a relative underuse of ECD KTx, and listing of NRTx candidates who have a poor prognosis on the waiting list particularly benefit from reduced waiting time.
NRTx candidates are also listed before dialysis initiation more frequently than those with KTx alone and receive organs of similar quality to those who receive KTx alone. It was shown previously that preemptive KTx confers a significant survival benefit over waiting for a KTx on dialysis (5,6); however, that preemptive renal transplantation is underused in the ESRD population at large but relatively more frequently used in the NRTx candidate pool raises serious concerns about the disparity in access in these two patient groups to optimal medical practices that could optimize survival. Although it is not possible to demonstrate that this is indeed the case, in our retrospective study, the more frequent predialysis listing of NRTx recipients for KTx likely reflects greater access of NRTx patients to close medical follow-up by transplant physicians. This finding further raises questions about the advantages to patients with physician advocacy on the one hand and the limitations that need to be in place to ensure fair and equitable access to optimal medical practices to all those in need on the other hand. It has been pointed out that it is important to consider the mortality risk of the thoracic transplant recipient before considering them for a subsequent KTx, the rationale being that kidneys should be offered to those who are likely to accrue the greatest mortality benefit (7). More recently, it was shown that preemptive transplants confer benefit to KTx candidates who were heart transplant recipients (8). Such a practice, in addition to careful patient selection, would certainly help to minimize waiting times given the poor survival of the NRTx recipient/KTx candidate on the waiting list (9,10).
Importantly, organ allocation systems for liver, lung, and heart transplants emphasize disease severity over wait time. In the case of KTx, disease severity is not accounted for in the deceased-donor allocation system. As such, an adult KTx candidate with poor expected survival on the waiting list could wait for years and could die on the waiting list before receiving a kidney offer (11). ECD kidneys may offer a survival benefit by helping to minimize waiting time, such as has been noted in older patients, patients with diabetes, and KTx candidates who reside in areas with long waiting times (12,13). Our results show, however, that despite a markedly higher mortality rate on the waiting list, NRTx recipients are not listed more frequently for ECD KTx, a strategy that could minimize deleterious effects of waiting time. This relative underuse of ECD KTx listing among NRTx recipients could thus represent a lost opportunity with regard to minimizing waiting time. It has also been shown that transplant center practices could have a significant impact on the effectiveness of ECD listing and use with a view to reducing waiting times (14). Given these considerations, although targeted listing for and transplantation of ECDs in NRTx recipients may be a viable option to reduce waiting time, as has been shown recently in the specific instance of candidates who received a previous heart transplant, the actual effectiveness of this strategy will likely be influenced by (1) the choice of the NRTx patient (and his or her physicians) to consider and/or accept ECD listing and (2) the transplant center's effectiveness in reducing waiting times by judicious use of ECD transplantation. In this regard, patients with NRTx could well benefit by being listed at a center that is known to have high transplant rates for those who are listed for ECD kidneys (8,14). It should be noted, however, that our findings derive from a retrospective analysis and only carefully designed prospective studies can validate some of our proposed measures to mitigate the deleterious effects of waiting time on NRTx recipient/KTx candidate survival.
In recent years there has also been significant growth in the number of KTxs performed simultaneously with the NRTx, particularly in liver transplantation (15). By design, MELD-based allocation for liver transplantation prioritizes liver transplantation in patients with pre–liver transplantation renal insufficiency (15,16). As such, the MELD-based liver allocation system could possibly select out patients who have pre–liver transplantation renal insufficiency and were destined for ESRD. A single-center study (17) noted no difference in the incidence of CKD in the pre- and post-MELD eras in liver transplant recipients; however, that study was conducted in a region with one of the lowest MELD scores in the country and/or inadequate statistical power. As noted in our study, that KTx listings after a previous liver transplant have significantly increased in the MELD era suggests that the incidence of chronic kidney disease (CKD) in patients who receive a liver transplant alone and progresses to ESRD may in fact be increasing (18).
Our analysis focuses on the NRTx recipient population as a whole, who are now candidates for KTxs. As shown in our analyses, these patients are a growing segment of the candidate pool for renal transplantation. These patients do have a substantial burden of mortality on the waiting list, and appropriate use of renal transplants in this population, whether living or deceased donor, raises several medical and ethical concerns. For example, it remains to be determined whether similar efforts by transplant centers are exercised in securing a live-donor kidney for an NRTx candidate as are usually used to secure a live donor for a kidney-only transplant. The higher frequency of preemptive listings of NRTx recipient/KTx candidates could reflect greater access to transplant care on the basis of past familiarity with the transplant process. The appropriateness of preemptive listing of an NRTx recipient who needs a transplant, however, is understandably justified on the basis of both excess mortality on the waiting list and the net benefit conferred by and expected of successful KTx. The actual number of years of additional life conferred by a renal transplant is greatest for those who receive a KTx alone as opposed to a KTx after NRTx, a phenomenon reflective of the lower overall survival in NRTx recipients compared overall with solitary KTxs. Our findings then prompt the question of maintaining equitable access to renal transplantation to the kidney-alone candidate who is on the waiting list and whose access to a kidney is primarily dictated by waiting list time.
In light of our discussion, we submit that the NRTx recipient has two chances to obtain a KTx, simultaneously with the NRTx or subsequently with the onset of renal failure after NRTx. Whereas the candidate for a KTx alone does not have the biological advantage of receiving the NRTx on the basis of disease severity and has to wait for a transplant, that latter process is associated with an annual mortality of at least 6%. In addition, recipients of simultaneous liver and kidney transplants receive organs from a greater number of standard-criteria donors compared with kidney-only candidates. Of greater concern is that preemptive renal transplants, widely known to confer a survival advantage to the patient with ESRD over placement on the waiting list and receiving dialysis, are offered less frequently than is in the case of the NRTx candidates. Furthermore, the overall patient and graft survival in the NRTx pool is significantly lower than that for those who receive KTx alone (Figure 3). The use of ECD kidneys may provide a means to minimize waiting times in such patients, as has been advocated for other patient groups with poor expected survival on the waiting list or long expected regional waiting times.
That kidney transplant listings are increasing in the NRTx population is perhaps an indication of (1) the growing burden of CKD in this population and (2) the overall growth in NRTx numbers. Given these complexities, the medical care of NRTx recipients may demand the structured involvement of physicians who are skilled in the organ-specific care of NRTx recipients as well as immunosuppression, care of CKD, and ESRD planning through all phases of transplantation.
Conclusions
The growing numbers of renal transplant listings after a previous NRTx and the disparate trends in renal transplantation in such candidates raise important questions about equitable access to transplantation in the KTx candidate pool. Judicious listing for and transplantation of ECD and living-donor kidneys in this population may help to shorten waiting times, because NRTx recipients/KTx candidates exhibit poor survival on the waiting list. Carefully designed prospective studies are needed to address practice and policy that will (1) mitigate the high mortality on the waiting list of NRTx recipients/KTx candidates and (2) ensure fairness and equitable allocation of resources to KTx candidates without previous NRTxs.
Disclosures
None.
Acknowledgments
The data reported here were supplied by the Arbor Research Collaborative for Health (Arbor Research) as the contractor for the Scientific Registry of Transplant Recipients. The study was approved by the Cleveland Clinic Institutional Review Board.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the Scientific Registry of Transplant Recipients or the US government.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM: Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 349: 931–940, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Kamath PS, Kim WR: The model for end-stage liver disease (MELD). Hepatology 45: 797–805, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kozower BD, Meyers BF, Smith MA, De Oliveira NC, Cassivi SD, Guthrie TJ, Wang H, Ryan BJ, Shen KR, Daniel TM, Jones DR: The impact of the lung allocation score on short-term transplantation outcomes: A multicenter study. J Thorac Cardiovasc Surg 135: 166–171, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, Grover FL: Development of the new lung allocation system in the united states. Am J Transplant 6: 1212–1227, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mange KC, Joffe MM, Feldman HI: Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 344: 726–731, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, Leichtman AB, Kaplan B: Effect of waiting time on renal transplant outcome. Kidney Int 58: 1311–1317, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Lonze BE, Warren DS, Stewart ZA, Dagher NN, Singer AL, Shah AS, Montgomery RA, Segev DL: Kidney transplantation in previous heart or lung recipients. Am J Transplant 9: 578–585, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Cassuto JR, Reese P, Bloom RD, Doyle A, Goral S, Naji A, Abt PL: Kidney transplantation in patients with a prior heart transplant. Transplantation 89: 427–433, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith CM, Brennan DC, Miller B, Wang C, Hmiel P, Shenoy S, Ramachandran V, Jendrisak MD, Ceriotti CS, Mohanakumar T, Lowell JA: Renal transplantation following previous heart, liver, and lung transplantation: An 8-year single-center experience. Surgery 130: 457–462, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Mason DP, Solovera-Rozas M, Feng J, Rajeswaran J, Thuita L, Murthy SC, Budev MM, Mehta AC, Haug M, 3rd, McNeill AM, Pettersson GB, Blackstone EH: Dialysis after lung transplantation: Prevalence, risk factors and outcome. J Heart Lung Transplant 26: 1155–1162, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU: Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol 4: 1239–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Schold JD, Meier-Kriesche HU: Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 1: 532–538, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Schold JD, Harman JS, Chumbler NR, Duncan RP, Meier-Kriesche HU: The pivotal impact of center characteristics on survival of candidates listed for deceased donor kidney transplantation. Med Care 47: 146–153, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N: Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the us: Where will MELD lead us? Am J Transplant 6: 2651–2659, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Locke JE, Warren DS, Singer AL, Segev DL, Simpkins CE, Maley WR, Montgomery RA, Danovitch G, Cameron AM: Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: Ineffective usage of renal allografts. Transplantation 85: 935–942, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Machicao VI, Srinivas TR, Hemming AW, Soldevila-Pico C, Firpi RJ, Reed AI, Morelli GJ, Nelson DR, Abdelmalek MF: Impact of implementation of the MELD scoring system on the prevalence and incidence of chronic renal disease following liver transplantation. Liver Transpl 12: 754–761, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Welch K, Eikstadt R, Marrero JA, Fontana RJ, Lok AS: Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl 15: 1142–1148, 2009 [DOI] [PubMed] [Google Scholar]