Abstract
Background and objectives: Acute kidney injury (AKI) is a frequent complication of cardiopulmonary bypass (CPB). Serum creatinine (SCr), the current standard, is an inadequate marker for AKI since a delay occurs before SCr rises. Biomarkers that are sensitive and rapidly measurable could allow early intervention and improve patient outcomes. We investigated the value of serum cystatin C as an early biomarker for AKI after pediatric CPB.
Design, setting, participants, & measurements: We analyzed data from 374 prospectively enrolled children undergoing CPB. Serum samples were obtained before and at 2, 12, and 24 hours after CPB. Cystatin C was quantified by nephelometry. The primary outcome was AKI, defined as a ≥50% increase in SCr. Secondary outcomes included severity and duration of AKI, hospital length of stay, and mortality. A multivariable stepwise logistic regression analysis was used to assess predictors of AKI.
Results: One hundred nineteen patients (32%) developed AKI using SCr criteria. Serum cystatin C concentrations were significantly increased in AKI patients at 12 hours after CPB (P < 0.0001) and remained elevated at 24 hours (P < 0.0001). Maximal sensitivity and specificity for prediction of AKI occurred at a 12-hour cystatin C cut-off of 1.16 mg/L. The 12-hour cystatin C strongly correlated with severity and duration of AKI as well as length of hospital stay. In multivariable analysis, 12-hour cystatin C remained a powerful independent predictor of AKI.
Conclusion: Serum cystatin C is an early predictive biomarker for AKI and its clinical outcomes after pediatric CPB.
Acute kidney injury (AKI) occurs commonly world-wide, affecting 2% to 5% of hospitalized patients and independently predicting mortality and morbidity (1). Once established, the treatment of AKI is largely supportive, at an annual cost surpassing $10 billion in the US alone (2). The diagnosis currently depends on detection of reduced kidney function by a rise in serum creatinine (SCr) concentration, which is a delayed and unreliable measure in the acute setting (3). Notably, experimental studies have identified interventions that may prevent or treat AKI if instituted early in the disease process, well before the SCr rises (4). The lack of early predictive biomarkers has impaired our ability to translate these promising findings to human AKI.
Cardiac surgery with cardiopulmonary bypass (CPB) is the most frequent major surgical procedure performed in hospitals worldwide, with well over a million operations undertaken each year. AKI is a common and serious complication encountered in 30% to 40% of adults and children after CPB (5,6). AKI requiring dialysis occurs in up to 5% of these cases, in whom the mortality rate approaches 80% (6). However, even minor degrees of postoperative AKI as manifest by only a 0.2 to 0.3 mg/dl rise in SCr from baseline and often thought to be clinically unimportant, portend a significant increase in short-term mortality in adults (7). AKI after cardiac surgery is also associated with a number of adverse outcomes, including prolonged intensive care and hospital stays, diminished quality of life, and increased long-term mortality (8). Infants and children with congenital heart disease may be especially vulnerable to developing AKI since many require multiple surgeries for stepwise repair of complex congenital anomalies. These patients represent an ideal group for the validation of AKI biomarkers since confounding co-morbid conditions, such as advanced age, pre-existing renal insufficiency, hypertension, atherosclerotic vascular disease, and diabetes are usually absent.
Serum cystatin C has been validated as a marker to estimate GFR in several patient populations, including kidney transplants (9) and critically ill patients (10), and more recently has shown promise as an early biomarker of AKI after adult cardiac surgery (11). Cystatin C is an endogenous cysteine proteinase inhibitor produced by nucleated cells at a constant rate. It is freely filtered at the glomerulus, reabsorbed and catabolized, but is not secreted by the tubules. Importantly, cystatin C is readily measurable using clinical laboratory platforms and does not increase with urinary tract infection or in chronic nonrenal disease, such as malignancy.
For this study, we sought to (1) determine the accuracy of early serum cystatin C measurements for the prediction of AKI after pediatric CPB; (2) determine the relationship between cystatin C measurements and renal outcomes (duration and severity of AKI); and (3) determine the relationship between cystatin C measurements and clinical outcomes (mortality and length of hospital stay).
Materials and Methods
Study Design
This study was approved by the Institutional Review Board of the Cincinnati Children's Hospital Medical Center. Written informed consent was obtained from the legal guardian of each patient before enrollment. All patients <18 years of age undergoing CPB at our center for surgical correction or palliation of congenital heart lesions between January 2004 and May 2007 were approached for study inclusion. Exclusion criteria included pre-existing renal insufficiency (based on age-adjusted normal ranges for SCr), diabetes mellitus, peripheral vascular disease, and the use of nephrotoxic agents up to 1 week before or during the study period. We utilized the Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1) consensus-based scoring system to categorize the complexity of surgery (12). This method of risk stratification is a widely accepted tool for the evaluation of differences in outcomes of surgery for congenital heart disease.
To obviate postoperative volume depletion and prerenal azotemia, subjects received at least 80% of their maintenance fluid requirements during the first postoperative 24 hours and 100% maintenance subsequently. All patients received intraoperative methylprednisolone during CPB. No other steroids were administered within the study period in any patient. Serum samples were obtained before and at various intervals after the initiation of CPB (2, 12, and 24 hours) and stored at −70°C. SCr was measured at baseline and was routinely monitored at least daily in the postoperative period.
The primary outcome variable was the development of AKI, defined as a 50% or greater increase in SCr from baseline within 48 hours of surgery. Secondary outcomes included severity of AKI based on the pediatric-modified RIFLE criteria (13), duration of AKI, hospital length of stay, and hospital mortality. The pediatric RIFLE classification stratifies kidney injury according to severity: Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and ESRD, using creatinine clearance or urine output criteria. The first three categories correspond to AKI and the latter two to chronic kidney disease. Because urine output may be affected by multiple intraoperative and postoperative variables such as the use of ultrafiltration or the administration of diuretics, we calculated scores using creatinine clearance criteria estimated by the newly modified Schwartz formula (14), with “Risk” corresponding to an estimated creatinine clearance (eCCl) decrease by 25% from preoperative eCCl baseline, “Injury” corresponding to an eCCl decrease by 50%, and “Failure” corresponding to an eCCl decrease by 75% or absolute value <35 ml/min per 1.73 m2. Duration of AKI was defined as the number of days SCr was elevated >50% above baseline. Candidate variables assessed for the prediction of AKI included age, sex, race, CPB duration, prior CPB, RACHS-1 score, postoperative inotrope score at 24 hours, and postoperative serum cystatin C concentrations. To provide a quantified index of the subject's postoperative hemodynamic status, we utilized the dosages of inotropic infusions to calculate an inotrope score at 24 hours after CPB, as described previously for similar patient cohorts (15).
Biomarker Measurements
All cystatin C measurements were made in duplicate, and the laboratory investigators were blinded to the sample sources and clinical outcomes until the end of the study. The concentration of serum cystatin C was measured by nephelometry using a standardized clinical laboratory platform (BN ProSpec II; Siemens Healthcare Diagnostics, Marburg, Germany), according to the manufacturer's recommendations. The inter- and intra-assay coefficient variations were <5% for batched samples analyzed on the same day, and <10% for samples measured 6 months apart. No change in biomarker concentration was noted following up to three freeze-thaw cycles.
Statistical Methods
Statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC). Descriptive analyses are reported as means ± SD for continuous variables and proportions for categorical variables. A two-sample t test was used to test for group differences in continuous variables and Chi-squared (χ2) or Fisher exact test was used for categorical variables, as indicated.
Pearson correlation coefficients were calculated between cystatin C concentration at each time-point and the following clinical parameters: Age, duration of CPB, RACHS-1 score, postoperative inotrope score, percent change in SCr, hospital length of stay after surgery, and duration of AKI.
Univariable and multivariable stepwise logistic regression analyses were undertaken to assess predictors of AKI. Potential independent predictor variables included age, sex, race, CPB duration, prior CPB, RACHS-1 score, postoperative inotrope score, and postoperative serum cystatin C concentration. Variables significant at P ≤ 0.10 in univariable analyses were included in a multivariable regression model and were retained in the final model after backward elimination if P < 0.05.
To calculate the sensitivity and specificity for 2-, 12-, and 24-hour serum cystatin C concentrations at varying cutoff values, conventional receiver-operating characteristic (ROC) curves were generated, and an area under the curve (AUC) was calculated to ascertain the quality of cystatin C as a biomarker. An AUC of 0.5 is no better than expected by chance, whereas a value of 1.0 signifies a perfect biomarker. This analysis was conducted in two phases. The cohort was randomly divided into two equal subcohorts with the same incidence of AKI. The primary analysis set (n = 188) was used to identify the optimal time-point and cystatin C threshold for predicting AKI. The optimal time-point was defined as the earliest time-point that provided significant discrimination between AKI and non-AKI, weighing the AUC and P value from the logistic regression model. Lastly, we identified the cystatin C values that provided 95% sensitivity, 95% specificity, and optimal sensitivity and specificity using the ROC curve at the best time-point. The threshold with optimal sensitivity and specificity was then applied to the second analysis set (n = 186) to determine its validity in an independent sample.
Results
Three hundred seventy-six children were enrolled. Two children were excluded from analysis because their baseline SCr level was not available. The primary outcome, AKI, defined as a 50% or greater increase in SCr from baseline, occurred in 119 of the 374 analyzed patients within 48 hours of surgery, yielding an incidence of 32%. SCr change was 108.8 ± 92.5% in patients who developed AKI and 9.2 ± 19.7% in those who did not (P < 0.0001). Demographic, clinical, and laboratory characteristics are shown in Table 1. Patients who developed AKI were significantly younger, had longer CPB times, higher 24-hour inotrope scores after CPB, and longer hospital length of stay. AKI patients also had higher mortality (P = 0.04), although the overall mortality was low in both groups. RACHS-1 scores were similar in AKI and non-AKI patients and notably, the non-AKI patients were more likely to have had prior CPB. Within the AKI group, 55 (46%) were in the “Risk” category, 41 (34%) were in the “Injury” category, and 23 (19%) were in the “Failure” category. In the AKI patients, AKI was diagnosed at a mean of 1.08 ± 0.31 days after CPB with peak SCr at a mean of 1.66 ± 1.17 days after CPB. The duration of AKI averaged 2.5 ± 1.1 days. No patients in the AKI group underwent dialysis or other renal replacement therapy during the study period.
Table 1.
Demographic, clinical, and laboratory characteristics
| AKI (n = 119) | Non-AKI (n = 255) | P | |
|---|---|---|---|
| Age (years ± SD) | 1.5 ± 2.9 | 4.4 ± 5.4 | <0.0001 |
| Bypass time (minutes ± SD) | 138.6 ± 69.8 | 105.4 ± 52.9 | <0.0001 |
| Baseline serum creatinine (mg/dl ± SD) | 0.35 ± 0.15 | 0.48 ± 0.19 | <0.0001 |
| Creatinine change (% ± SD) | 108.8 ± 92.5 | 9.2 ± 19.7 | <0.0001 |
| Baseline serum cystatin C (mg/L ± SD) | 0.81 ± 0.20 | 0.79 ± 0.17 | 0.16 |
| Baseline eCCL (ml/min per 1.73 m2) | 113.2 ± 52.3 | 78.9 ± 17.8 | 0.19 |
| Hospital stay (days ± SD) | 17.8 ± 31.0 | 9.6 ± 18.2 | 0.008 |
| Inotrope score (mean ± SD) | 5.1 ± 6.7 | 3.4 ± 6.1 | 0.02 |
| Sex (n [%] male) | 60 (50) | 140 (55) | 0.42 |
| Race (n [%] white) | 105 (89) | 221 (88) | 0.80 |
| Prior surgery (n [%]) | 39 (33) | 119 (47) | 0.01 |
| Death (n [%]) | 5 (4) | 2 (1) | 0.04a |
| RACHS score (n [%]) | |||
| 1 | 6 (5) | 30 (12) | |
| 2 | 62 (52) | 116 (45) | |
| 3 | 42 (35) | 93 (36) | 0.33a |
| 4 | 4 (3) | 9 (4) | |
| 5 | 2 (2) | 3 (1) | |
| 6 | 3 (3) | 4 (2) | |
| pRIFLE (n [%]) | |||
| R | 55 (46) | — | — |
| I | 41 (34) | — | — |
| F | 23 (19) | — | — |
| Duration of AKI (days ± SD) | 2.5 ± 1.1 | — | — |
Two sample t test was used to report P values for continuous variables. Chi-square test was used to report P values for categorical variables.
Fisher exact test was used for categorical variables with small cell counts.
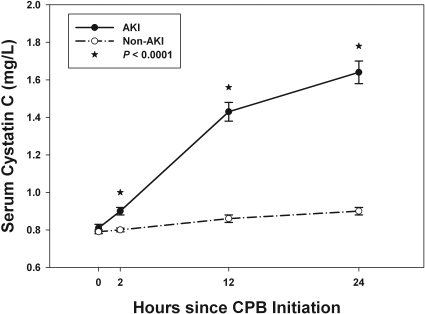
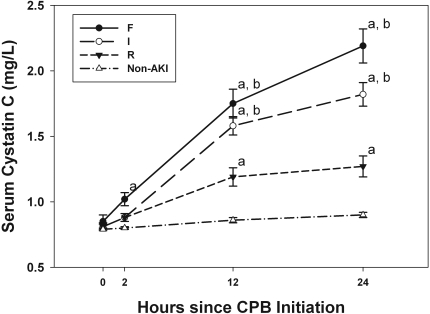
Serum cystatin C concentrations were similar in the AKI and non-AKI patients before surgery but began to increase in the AKI patients at 2 hours after onset of CPB and became significantly higher at 12 and 24 hours after CPB (Figure 1, P < 0.0001). As demonstrated in Figure 2, cystatin C concentrations significantly correlated with severity of AKI by RIFLE score with a stepwise increase in cystatin C with worsening disease severity. Significant differences exist between each of the RIFLE categories and the non-AKI patients at 12 and 24 hours after CPB (P < 0.0001 by paired comparison). Significant difference is also seen between the “R” patients and the higher severities (“I” and “F”) at 12 and 24 hours after CPB (P < 0.0001).
Figure 1.
Cystatin C concentrations for AKI and non-AKI patients using least square means ± SEM from generalized estimating equations. P < 0.0001 between groups.
Figure 2.
Cystatin C concentrations for AKI patients stratified by pRIFLE criteria and non-AKI patients. Error bars are least square means ± SEM. “a” represents significant difference (P < 0.0001) between AKI subgroup (“R,” “I,” or “F”) and non-AKI group at the same time-point. “b” represents significant difference (P < 0.0001) between AKI subgroup “I” or “F” and the subgroup “R” at the same time-point.
To examine the relationship of serum cystatin C concentrations with clinical characteristics and outcomes, we performed correlation analysis. Focusing on the 12- and 24-hour time-points, serum cystatin C concentration at 12 hours significantly correlated with younger age at surgery (r = −0.16, P = 0.002), greater SCr change (r = 0.55, P < 0.0001), longer hospital stay (r = 0.16, P = 0.002), longer CPB time (r = 0.28, P < 0.0001), higher RACHS-1 score (r = 0.13, P = 0.01), and longer duration of AKI among AKI patients (r = 0.32, P = 0.0006). The same relationships were observed at the 24-hour time-point. Additionally, the 24-hour cystatin C correlated with 24-hour postoperative inotrope score (r = 0.14, P = 0.007).
We then performed univariable and multivariable logistic regression analysis of the 12-hour cystatin C concentration as well as clinical factors for the prediction of AKI. Univariable logistic regression identified younger patient age (P < 0.001), longer CPB time (P < 0.0001), higher 24-hour postoperative inotrope score (P = 0.04), and longer hospital stay (P = 0.008) as significantly associated with higher odds of AKI. A history of prior CPB was associated with lower odds of AKI. (P = 0.012) A stepwise logistic regression analysis was used to determine the most parsimonious model among these significant variables. The final model revealed that younger age (P = 0.0001) and 12-hour cystatin C concentration (P < 0.0001) were the only significant independent predictors of AKI in our cohort. For every 0.25-year increase in age, the odds of AKI decreased by 4% (odds ratio [OR], 0.96; 95% confidence interval [CI]: 0.93 to 0.98). For every 0.1 mg/L increase in 12-hour serum cystatin C, the odds of AKI increased by 38% (OR, 1.38; 95% CI: 1.28 to 1.49).
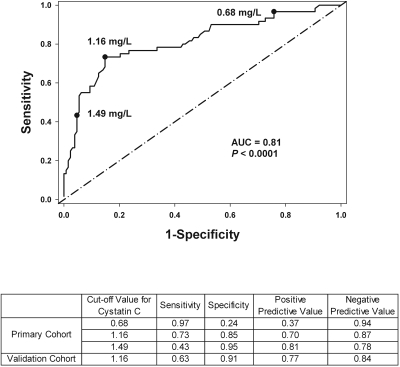
Lastly, to determine the ability of serum cystatin C to detect early AKI, ROC curves were generated for each time-point after CPB (2, 12, and 24 hours). AUC were 0.59 (95% CI: 0.50 to 0.69) for the 2-hour cystatin C (P = 0.02), 0.81 (95% CI: 0.74 to 0.88) for the 12-hour cystatin C (P < 0.0001), and 0.84 (95% CI: 0.78 to 0.91) for the 24-hour cystatin C (P < 0.0001). These values indicate that the 12- and 24-hour cystatin C are very good predictors for the early diagnosis of AKI. After weighing the AUC, timing of measurement, and P values from the predictive logistic model, we selected the 12-hour cystatin C as the “optimal” time-point. Analyzing the 12-hour ROC curve, optimal sensitivity and specificity occurred at a value of 1.16 mg/L (Figure 3). The derived sensitivities, specificities, and predictive values at different cutoff concentrations for cystatin C at 12 hours after CPB for the primary and the validation cohorts are shown. These results indicate that a 12-hour cystatin C concentration of ≥1.16 mg/L is a valid threshold for the detection of AKI.
Figure 3.
ROC curve analysis using the 12-h postoperative serum cystatin C concentration in the primary cohort. The values 0.68, 1.16, and 1.49 are the 12-hour cystatin C concentrations (mg/L) corresponding to 95% sensitivity, optimal sensitivity and specificity, and 95% specificity, respectively. The table lists the sensitivity, specificity, predictive values for the primary cohort at each concentration and for the validation cohort at the optimal concentration.
Discussion
Our results confirm that cystatin C performs very well as an early predictor for AKI following pediatric CPB. Notably, the diagnosis of AKI could be predicted with reasonable certainty within 12 hours of CPB. Cystatin C is recognized to be a superior marker to SCr in assessments of GFR in chronic kidney disease (16). The measurement of cystatin C is highly accurate and precise and importantly, a commercially available immunonephelometric assay provides rapid measurements with immediate results. Unlike other potential biomarkers for AKI, cystatin C is not affected by storage conditions or interfering substances and does not increase with urinary tract infection or other chronic nonkidney disease states, such as malignancy.
The diagnosis of AKI after an insult such as CPB is currently delayed and inaccurate. Unlike acute coronary syndrome, in which biomarkers such as cardiac troponins have revolutionized clinical care by establishing the early diagnosis, predicting severity, and guiding therapeutic maneuvers, similar biomarkers for AKI have been lacking. In this study, AKI was diagnosed by SCr criteria at a mean of 1.08 days after CPB. However, in almost 10% of our patients, the diagnosis was delayed until 48 hours after surgery. In contrast, a cystatin C concentration of 1.16 mg/L predicted AKI at 12 hours after CPB and with additional accuracy and clinical prognostic information. Thus, serum cystatin C offers a unique opportunity to dramatically impact the management of AKI by delivering diagnostic, severity, and prognostic information at an early time-point following a renal insult such as CPB.
We employed a temporally predictable human model of AKI to validate cystatin C as a biomarker. AKI occurs commonly after CPB and even minor degrees of postoperative AKI are associated with a significant increase in mortality in adult cohorts (7) and an increase in morbidity in pediatric patients (17). Infants and children with congenital heart diseases may be especially vulnerable to developing AKI. Indeed, the incidence of AKI in our study was 32%, similar to previous reports in pediatric cardiac surgery cohorts (5).
This study has several strengths. First, we utilized a prospective cohort design of subjects undergoing CPB and employed a rigorous protocol to prospectively collect specimens, followed by a blinded measurement of cystatin C. Second, our homogeneous cohort of pediatric subjects had normal baseline kidney function and was free of co-morbid conditions, such that the only obvious etiology for AKI would be the result of CPB. The study design also allowed for the precise determination of the temporal rise in cystatin C following CPB.
This study also has important limitations. First, it represents a single center study of pediatric patients with congenital heart disease and predominantly ischemic AKI. While this cohort was intentionally chosen to eliminate confounding variables and co-morbid conditions, it is acknowledged that AKI is often multifactorial, and our results will need to be validated in a larger randomized prospective trial, including adults undergoing CPB, in whom biomarker performance may differ (18–20). Second, this was a short-term study of post-CPB AKI. We did not follow patients to determine the decline in or normalization of cystatin C or the long-term impact of post-CPB AKI. This will be an important future study. Third, the definition of AKI was based on elevations in SCr, which raises the conundrum of using a flawed outcome variable to analyze the performance of novel biomarkers. This study may have yielded different results had there been a true “gold standard” for AKI. Instead, using a change in SCr potentially sets up the biomarkers for lack of accuracy due to either false positives (true tubular injury but no significant change in SCr) or false negatives (absence of true tubular injury, but elevations in SCr due to prerenal or other causes). Indeed, in this study, 30 of the 111 patients (27%) who had serum cystatin C concentrations above 1.16 mg/dl did not meet SCr criteria for AKI. There are two possible explanations for this: (1) that cystatin C concentration is a more sensitive marker of AKI and that SCr failed to rise in these children who were truly unrecognized AKI cases or (2) that cystatin C rose due to nonrenal causes, and these children truly did not have AKI. Given this, it has become increasingly evident that a single biomarker for AKI may not provide complete information and that simultaneous examination of other serum and urinary biomarkers may be ideal (21). Recent studies have uncovered other AKI biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) (5), IL-18 (22), liver-type fatty acid binding protein (23), and kidney injury molecule-1 (18) in similar clinical cohorts. However, all biomarkers have their individual strengths and weaknesses. Given the multifactorial etiologies of AKI (21), it is not surprising that any single biomarker is insufficient. Indeed, even in the cardiac surgical population, measurements of single biomarkers such as NGAL have yielded a wide range of AUC (0.59 to 0.99) for the prediction of AKI (18). It is anticipated that a panel of strategically selected candidates may prove optimal for early and rapid diagnosis of AKI and its clinical outcomes. The present study identifies cystatin C as an excellent candidate for inclusion in this panel. Such a tool would be indispensable for the timely institution of potentially effective therapies in early human AKI.
Disclosures
Dr. Devarajan is a co-inventor on patents related to NGAL as an AKI biomarker and is a consultant to Abbott Diagnostics and Biosite, Inc.
Acknowledgments
We are indebted to our additional study coordinators, Tracey VanVliet, R.N., Lauren Von Moll, R.N., and Julie Ciambarella, R.N., for their assistance and to our patients and their families for their participation.
Funding Sources: Supported by grants from the National Institutes of Health (NIH; RO1-HL08676, RO1-HL085757, RO1-DK069749) and a Translational Research Initiative Grant from Cincinnati Children's Hospital.
Presented at the 2009 American Heart Association Scientific Sessions; November 17, 2009; Orlando, Florida.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL: Mortality after acute renal failure: Models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Kellum JA, Ronco C: Defining acute renal failure: Physiological principles. Intensive Care Med 30: 33–37, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J: Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lok CE, Austin PC, Wang H, Tu JV: Impact of renal insufficiency on short- and long-term outcomes after cardiac surgery. Am Heart J 148: 430–438, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bokenkamp A, Ozden N, Dieterich C, Schumann G, Ehrich JH, Brodehl J: Cystatin C and creatinine after successful kidney transplantation in children. Clin Nephrol 52: 371–376, 1999 [PubMed] [Google Scholar]
- 10.Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, Philipp T, Kribben A: Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M: Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—a prospective cohort study. Crit Care Med 37: 553–560, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Jenkins KJ: Risk adjustment for congenital heart surgery: The RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 7: 180–184, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL: Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71: 1028–1035, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW, Wessel DL: Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92: 2226–2235, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, Hyder A, Alkandari O: A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int 76: 885–892, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV: Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73: 863–869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P: Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol 3: 665–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A: Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 54: 1012–1024, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Lameire N, Van Biesen W, Vanholder R: Acute kidney injury. Lancet 372: 1863–1865, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL: Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70: 199–203, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P: Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 73: 465–472, 2008 [DOI] [PubMed] [Google Scholar]