Abstract
Background and objectives: Population-based studies have reported outcomes and risk factors for patients with chronic kidney disease (CKD), defined primarily by decreased estimated GFR (eGFR). They are characterized by old age, low proteinuria level, and stage 3 CKD. However, many patients referred to nephrologists are younger and have overt proteinuria and advanced CKD. This study evaluated the association between outcomes and those factors among referred CKD patients.
Design, setting, participants, & measurements: We retrospectively reviewed 461 referred patients with stage 3 to 5 CKD from January 2003 to December 2007. Key outcomes were death and ESRD. Patients were followed from the time of first serum creatinine measurement to December 2009.
Results: The median age of subjects was 67.0 years, and median follow-up was 3.2 years. Overt proteinuria was present in 57.0% of subjects. For stage 3, 4, and 5 CKD, cumulative mortality and probability of ESRD at 3 years was 9.5 and 6.5%, 11.2 and 27.8%, and 16.5 and 79.1%, respectively. Using proportional-hazards regression models, age was a determinant for death, whereas overt proteinuria was strongly associated with ESRD. Among stage 3 CKD patients older than 65 years without overt proteinuria, the incidence of death before renal replacement therapy (RRT) was 2.8/100 patient-years and none had ESRD. In patients with advanced CKD and overt proteinuria, the incidence of ESRD was substantially higher than that of death before RRT.
Conclusions: Stratification by age, proteinuria level, and CKD stage could predict the competing outcomes of death before RRT and ESRD among CKD patients.
The diagnostic classification of chronic kidney disease (CKD) (1) is useful to understand kidney function and risk of complications. Previous studies have demonstrated that CKD increases the risks of cardiovascular morbidity and mortality (2–13). The prevalence of CKD has been shown to be high in population studies (10% to 13%) (14–16), and for CKD patients in the general population, the risk of death, particularly due to cardiovascular disease (CVD), is much higher than that of ESRD requiring renal replacement therapy (RRT) (12–13). Population-based studies have also reported that CKD patients have a mean age of about 70 years (15–18), and most of them had little proteinuria/albuminuria and stage 3 CKD (15–16), primarily defined by decreased estimated GFR (eGFR).
However, many patients referred to nephrologists are relatively younger than those in population-based studies, and often have overt proteinuria and advanced CKD (19–20). A small but significant number of those patients should be carefully evaluated and intensively managed by nephrologists. It is important to clarify the prognoses and risk factors for these patients. Japan is an appropriate country for a survival analysis of CKD patients who have undergone nephrology care as it has a generous social-welfare system, kidney screening program, and public medical care insurance; thus almost all patients can receive sufficient care, including RRT (21).
In the present study, we investigated outcomes among referred patients with stage 3 to 5 CKD without previous RRT and evaluated the association between outcomes and clinically relevant risk factors, including age and proteinuria. We also assessed whether CKD patients experienced ESRD or died before RRT, and compared the incidence of these outcomes after stratifying patients by CKD stage, age, and proteinuria level. Unlike previous studies on the prognosis of CKD patients, we followed up patients even after they started chronic RRT.
Subjects and Methods
Study Population
This study was designed as a survival analysis of a historical cohort of consecutive adult patients (aged 20 years or older) with stage 3 to 5 CKD who were referred to the Department of Nephrology in Rinku General Medical Center from January 2003 to December 2007. No hospitals in Izumisano city and neighboring four municipalities have the department of nephrology, except our medical center. All subjects had one or more outpatient determinations of serum creatinine levels, and none had previously received RRT. Patients were followed from the time of first serum creatinine measurement. We used the new Japanese equation for GFR estimation [estimated GFR (eGFR) (ml/min per 1.73 m2) = 194 × Serum creatinine−1.094 × Age−0.287 × 0.739 (if female)] (22).
Entry criteria included eGFR <60 ml/min per 1.73 m2, which presumed the presence of CKD based on a clinical history of deterioration. We excluded patients with malignancy at the first consultation, those who had already received RRT, those who refused RRT, and those who received immunosuppressive agents for renal disease. Patients were referred to family doctors if they were clinically stable and determined to be at low risk, to dialysis centers if maintenance hemodialysis were started, or to kidney transplantation centers if they were eligible for a transplant. They were followed up as long as possible through collaboration between referred institutions by letters. Patient follow-up ended on December 31, 2009 or on the day when they underwent renal transplantation. Patients were considered lost to follow-up if no contact was documented for more than 6 months. This was a retrospective observational study approved by the Ethics Committee of Rinku General Medical Center.
Baseline Demographics and Comorbidities
Baseline clinical and laboratory variables included age, sex, diabetes (The International Classification of Diseases, Tenth Revision [ICD-10] codes E10–E14), hypertension (ICD-10 codes I10–I15), prior CVD, smoking history, eGFR, overt proteinuria, and hemoglobin, at referral. CVD was defined as a combination of a cardiac event and stroke (ICD-10 codes I60–I67). A cardiac event was defined as the combination of sudden death (ICD-10 codes I46.1, I46.9), ischemic heart disease (ICD-10 codes I20–I25), and heart failure (ICD-10 code I50). Overt proteinuria was defined as urinary protein-creatinine ratio ≥1.0 g protein/g creatinine, or urine dipstick ≥2+ if urinary protein-creatinine ratio was unavailable. We also extracted use of renin-angiotensin system (RAS) inhibitors at baseline or within 3 months after referral. Information on smoking habits was obtained through a standard questionnaire.
Outcomes
The primary endpoints were all-cause death and ESRD requiring chronic RRT. Causes of death were extracted from medical records, and chronic RRT was defined as use of RRT more than 2 months. The secondary outcome was hospitalization for cardiac events and stroke. We recorded only the first event of each endpoint. For survival analysis, we used death during the entire follow-up period as an outcome, regardless of RRT use. We also calculated the incidence of the competing outcomes of death before RRT and ESRD among stratified patients. These outcomes were ascertained by hospital medical records and questionnaire surveys.
Statistical Analysis
Descriptive statistics are presented as medians with interquartile ranges. Continuous variables were compared using the Kruskal-Wallis test, and post hoc analysis was performed by Mann-Whitney U tests. Categorical variables were compared using the chi-square test. Patients lost to follow-up were regarded as censored at the date of the last documented contact in the following survival analyses.
Three-year cumulative mortality was estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazard regression analysis of death was conducted for those aged 46 to 85 years to evaluate the effects of several clinical variables and cardiovascular risk factors at baseline.
Contrary to all-cause mortality, cumulative probability of ESRD calculated using Kaplan-Meier method and associations between baseline variables and ESRD evaluated with Cox model were obviously biased, since patients who died before developing ESRD requiring RRT had subsequently no probability of progression to ESRD, indicating that ESRD and all-cause mortality were competing risk events. Consequently, cumulative probability of ESRD was estimated using the competing risk method and compared using the Gray test in the presence of death as a competing risk (23). The substantially higher cumulative probability of ESRD among patients with stage 5 CKD showed an initial steep rise and then followed by a near-plateau, violating the proportional hazard assumption, which was tested by a time-covariate interaction term. Thus, univariate and multivariate competing risk regression of ESRD was conducted for patients aged 46 to 85 years with stage 3 and 4 CKD to evaluate the association between baseline characteristics and ESRD, using Fine and Gray model (24).
In multivariate model, the association between age and overt proteinuria with each primary outcome was adjusted using different models with forced entry. Effect modifications among age, overt proteinuria, and eGFR were assessed incorporating their simple interaction terms into multivariate models. To avoid multicollinearity, correlation analyses were performed among variables in each model based on phi coefficient for two nominal variables, correlation ratio (eta) for one nominal and one continuous variable, and Spearman's correlation coefficient for two continuous variables. The validity of the proportional-hazards assumption was tested by adding a time-dependent interaction variable for each of the predictors in the models. The assumption of linearity was checked in the model for the continuous variable by cubic splines (25). Finally, the outcomes of death before RRT and ESRD were re-analyzed stratifying all patients by age, overt proteinuria, and CKD stages.
A P value <0.05 for two-sided tests was considered significant, and confidence intervals (CI) reported are 95% intervals. P values were adjusted by the method of Holm in multiple testing (26). Missing data in smoking history and proteinuria were both 1.7%, which were imputed using multiple imputation method with five data sets (27). All analyses were conducted with the use of STATA/SE 11.0 for Windows (STATA Corp. LP, College Station, TX).
Results
Between January 2003 and December 2007, 461 patients (62% men) with a median age of 67 years (range, 57 to 76 years) met the inclusion criteria. Of these, 189 patients (41.0%) with stage 3 CKD, 144 (31.2%) with stage 4, and 128 (27.8%) with stage 5 had median observational periods of 2.9 years (range 2.0 to 4.5), 3.4 years (2.3 to 4.7), and 3.2 years (1.9 to 4.4), respectively. During follow-up, 12 (4.0%) of 303 patients were lost at our institution. We referred 158 patients to other medical institutions, and 22 patients (13.9%) of those were lost.
Baseline characteristics of the population stratified by CKD stages are shown in Table 1. More than two-thirds of patients with CKD stage 3 and 4 were male, while 55.5% of those with stage 5 CKD were female. Patients with stage 4 and 5 CKD had a higher prevalence of hypertension and prior CVD compared with patients with stage 3 CKD. As CKD stage progressed, the hemoglobin level decreased and the prevalence of overt proteinuria increased. RAS inhibitors were used in approximately 60% of patients with stage 3 and 4 CKD, and in 47.7% of those with stage 5 CKD.
Table 1.
Demographic and clinical data for 461 patients at each stage of CKD
| Variable | Stage 3(n = 189; 41.0%) | Stage 4(n = 144; 31.2%) | Stage 5(n = 128; 27.8%) | P |
|---|---|---|---|---|
| Age (years) | 65 (56 to 74) | 69 (60 to 76) | 67 (58 to 77) | 0.056 |
| Male (%) | 69.3 | 67.4 | 44.5a,b | <0.001 |
| eGFR (ml/min per 1.73 m2) | 43.6 (36.1 to 51.7) | 22.0 (17.9 to 26.1) | 9.7 (7.2 to 12.2) | N/A |
| Overt proteinuria (%)c | 37.6 | 62.7a | 80.3a,b | <0.001 |
| Hemoglobin (g/dl) | 12.9 (11.5 to 14.3) | 10.9 (9.8 to 12.1)a | 9.6 (8.8 to 10.4)a,b | <0.001 |
| Diabetes (%) | 31.8 | 41.7 | 39.1 | 0.148 |
| Hypertension (%) | 76.7 | 92.4a | 95.3a | <0.001 |
| Prior CVD (%) | 20.1 | 31.3 | 26.6 | 0.064 |
| Smoking history (%)c | 45.7 | 48.9 | 39.7 | 0.308 |
| RAS inhibitor (%) | 59.3 | 63.9 | 47.7b | 0.021 |
Continuous and categorical values are presented as median (interquartile range) and percentage of total, respectively. P values were adjusted by the method of Holm in multiple testing. Pt, patients; RAS, renin-angiotensin system; N/A, not applicable.
Significant difference versus stage 3 CKD.
Significant difference versus stage 4 CKD.
n = 453 due to missing data.
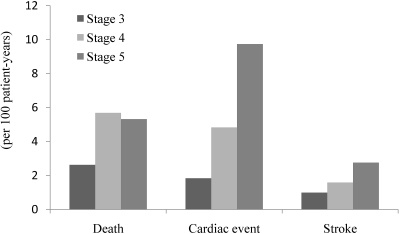
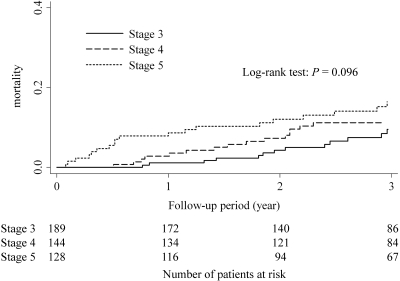
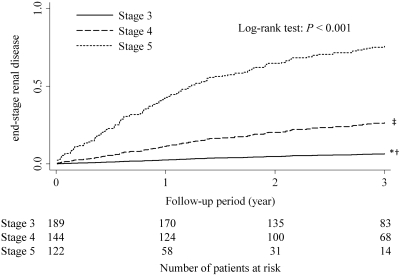
During the follow-up period, 168 patients experienced ESRD, including 12 patients (7.1%) with stage 3 CKD, 53 (31.5%) with stage 4 CKD, and 103 (61.3%) with stage 5 CKD. The incidence of death, cardiac events, and stroke (per 100 patient-years) increased as CKD stage progressed (Figure 1). The 3-year cumulative mortality for each CKD stage is shown in Figure 2. At 3 years after referral, the estimated mortality rates were 9.5% for those with stage 3 CKD, 11.2% for stage 4 CKD, and 16.5% for stage 5 CKD. There was no statistically significant differences in mortality among the 3 CKD groups (P = 0.096 by the Log-rank test). The 3-year cumulative probability of ESRD for each CKD stage is shown in Figure 3. At 3 years, the estimated rates of ESRD were 6.5% for stage 3 CKD, 27.8% for stage 4 CKD, and 79.1% for stage 5 CKD (Holm-adjusted P < 0.001).
Figure 1.
Incidence of death from any cause, cardiac events, and stroke during all study periods.
Figure 2.
Cumulative mortality among the three CKD groups.
Figure 3.
Cumulative probability of ESRD among the three CKD groups. *adjusted P < 0.001 versus stage 4 CKD; †adjusted P < 0.001 versus stage 5 CKD; ‡adjusted P < 0.0001 versus stage 5 CKD. P values were adjusted by the method of Holm.
Univariate regression analyses showed that older age, lower hemoglobin level, and prior CVD were significantly associated with death, whereas male gender, overt proteinuria, lower eGFR, lower hemoglobin level, diabetes, hypertension, and smoking history were significantly associated with ESRD (Table 2). Hazard ratios (with 95% CIs) for all-cause mortality and subhazard ratios (with 95% CIs) for ESRD among age and overt proteinuria are given for three different statistical models that include important clinical variables and cardiovascular risk factors (Table 3). After adjustment with respective models, age remained a significant risk factor for death whereas overt proteinuria was a strong predictor of ESRD. Age was also negatively associated with ESRD in Model 3.
Table 2.
Univariate Cox regression analysis of death and ESRD
| Variable | Death |
ESRDa |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | SHR | 95% CI | P | |
| Age (per 10 years) | 1.98 | (1.45 to 2.69) | <0.001 | 0.78 | (0.59 to 1.03) | 0.078 |
| Male | 0.95 | (0.57 to 1.61) | 0.861 | 1.86 | (1.01 to 3.45) | 0.048 |
| eGFR (per 10 ml/min per 1.73 m2) | 0.85 | (0.70 to 1.02) | 0.079 | 0.31 | (0.21 to 0.46) | <0.001 |
| Overt proteinuria | 1.22 | (0.71 to 2.08) | 0.471 | 9.10 | (4.20 to 19.7) | <0.001 |
| Hemoglobin (g/dl) | 0.79 | (0.69 to 0.89) | <0.001 | 0.74 | (0.66 to 0.84) | <0.001 |
| Diabetes | 0.80 | (0.46 to 1.37) | 0.407 | 2.06 | (1.24 to 3.43) | 0.005 |
| Hypertension | 2.22 | (0.69 to 7.09) | 0.180 | 10.7 | (1.52 to 76.0) | 0.017 |
| Prior CVD | 2.30 | (1.35 to 3.93) | 0.002 | 1.26 | (0.72 to 2.21) | 0.424 |
| Smoking history | 1.48 | (0.88 to 2.49) | 0.144 | 2.04 | (1.22 to 3.41) | 0.006 |
| RAS inhibitors | 0.66 | (0.39 to 1.10) | 0.110 | 0.78 | (0.47 to 1.29) | 0.326 |
Patients with stage 5 CKD were excluded.
Table 3.
Association of age and overt proteinuria with death and ESRD
| Variable | Adjustment | Death |
ESRDa |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | SHR | 95% CI | P | ||
| Age (per 10 years) | Model 1a | 2.05 | (1.49 to 2.82) | <0.001 | 0.87 | (0.64 to 1.18) | 0.364 |
| Model 2a | 1.87 | (1.35 to 2.60) | <0.001 | 0.81 | (0.60 to 1.10) | 0.170 | |
| Model 3a | 1.70 | (1.20 to 2.41) | 0.003 | 0.67 | (0.49 to 0.92) | 0.013 | |
| Overt proteinuria | Model 1a | 1.38 | (0.78 to 2.45) | 0.270 | 6.03 | (2.73 to 13.3) | <0.001 |
| Model 2a | 1.39 | (0.78 to 2.47) | 0.265 | 5.55 | (2.56 to 12.0) | <0.001 | |
| Model 3a | 1.13 | (0.62 to 2.05) | 0.697 | 4.97 | (2.23 to 11.1) | <0.001 | |
Model 1a: Overt proteinuria, sex, and estimated glomerular filtration ratio.
Model 1b: Age, sex, and estimated glomerular filtration ratio.
Model 2a/b: Model 1a/b + prior cardiovascular disease, diabetes and hypertension.
Model 3a/b: Model 2a/b + smoking history, hemoglobin and renin-angiotensin inhibitors.
Patients with stage 5 CKD were excluded.
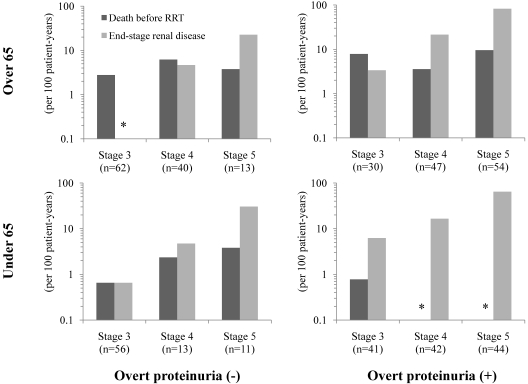
We then stratified all patients by CKD stage, age (65 years), and the presence or absence of overt proteinuria level. The incidence of death before RRT and ESRD among each group was shown in Figure 4 with logarithmic scale. Among stage 3 CKD patients older than 65 years without overt proteinuria, that is, the representative population of general CKD patients, the incidence of death before RRT was 2.8 in 100 patient-years and none of them experienced ESRD. For patients with more advanced CKD and overt proteinuria, the incidence of ESRD was substantially higher than that of death before RRT.
Figure 4.
Incidence of death before renal replacement therapy and end-stage renal disease stratified by age, overt proteinuria, and CKD stages. *No incidence.
Discussion
The present study describes how the characteristics of CKD patients referred to nephrologists in Japan differ from those in the general population. Previous population-based studies in the United States have shown that the prevalence of patients with stage 3 CKD and stage 4 to 5 CKD was about 7.7% and 0.35% (15), with a mean age of 66.5 to 71.6 and 70.1 to 73.6 years, respectively (11,13). Among those with stage 3 and 4 CKD, the prevalence of macroalbuminuria was 6.1% and 42.4%, respectively (15). Similar characteristics were also described in Japanese general CKD patients. The prevalence of stage 3 CKD and stage 4 to 5 CKD in a general Japanese population is about 10.4% and 0.2%, respectively (16). The prevalence of the elderly (older than 70 years) and positive proteinuria is 57.7% and 7.7% in stage 3 CKD, and 70.1% and 52.9% in stage 4 to 5 CKD, respectively (16). In contrast to these population-based studies, our patients were younger and were more likely to have advanced CKD and overt proteinuria. Thus the outcomes in the present study of referred patients differed from outcomes in population-based studies in terms of higher ESRD and lower mortality.
This discrepancy of outcomes was elucidated by proteinuria and age, both of which have attracted attention as predictors among CKD patients (28–30). As shown in the present study, proteinuria and age contributed differently to CKD outcomes. Proteinuria and albuminuria are accepted markers of kidney damage, and are associated with lower eGFR and an increased risk of subsequent ESRD (31–39). On the other hand, age is inversely associated with ESRD and positively associated with death (7,40–43). The stratification of heterogeneous CKD patients by those factors and CKD stage could be a simple strategy for the selection of patients who should be intensively treated by nephrologists. Whether this strategy could be applied to a more general population or not needs to be studied in future.
The incidence of outcomes among our older patients with stage 3 CKD without proteinuria were similar to those in a population-based study (13), and the incidence of ESRD was substantially higher than that of death before RRT among those with more advanced CKD and overt proteinuria. There are a few reports that describe the outcomes in non-general CKD patients, all of whom had stage 4 CKD (43–44). However, results from these reports are not consistent. One cohort study from the United Kingdom showed that crude mortality and the percentage of ESRD at 5 years were 47.5% and 24.7%, respectively (43), which is consistent with the population-based study (13). On the other hand, a study from Canada indicated that 7% of patients died, and 25% started RRT during the first 2 years of follow-up (44), which is similar to our results. In the UK cohort study, the median age (71.4 years) and the prevalence of proteinuria (62.1%) were similar to those in the population-based studies (13,43,45). On the other hand, patients in our study and the Canadian study were younger and showed better survival (median age of 69 years and 6.6% estimated mortality at 2 years; mean age of 67.3 years and 6.6% mortality at 2 years, respectively) (44), resulting in a higher incidence of ESRD because these outcomes are competing risk events. Although the prevalence of overt proteinuria in our study was much higher than other studies, indicating that they had more progressive CKD, the incidence of ESRD was similar to Canadian study, probably because of higher rate of RAS inhibitor use in our study than the Canadian study (63.9% versus 48.1%). Thus, the outcomes among advanced CKD patients could be predicted based on age and overt proteinuria, in addition to eGFR at baseline.
Several limitations of our study should be noted. First, although most baseline characteristics were obtained, this was a retrospective observational study and we lost 7.4% of patients to follow-up. Among 22 losses at stage 3 CKD, median age was 63 years (range, 49 to 69) and four patients (18%) had overt proteinuria. Among 11 losses at stage 4 CKD, median age was 76 years (range, 53 to 78) and six patients (55%) had overt proteinuria. There were no statistical differences in age and proteinuria between lost patients and those not lost (P = 0.091 and P = 0.072 in stage 3, and P = 0.85 and P = 0.49 in stage 4, respectively). Only one of 128 patients at stage 5 CKD was lost. Therefore, these lost patients were not supposed to have significant influence on our results. Second, because of the small study size, it is likely that our study had insufficient power to draw any conclusions about the prognosis and risk factors. Several variables that are supposed to be established risk factors of death (sex, diabetes, hypertension, and smoking history) were not statistically identified in our study. However, considering the prevalence of stage 4 to 5 CKD in Japanese general adult population (0.2%) (16) and the population of Izumisano city and neighboring four municipalities (78,291 and total of 162,143 in the 2005 national population census (46), respectively), our patients with stage 4 to 5 CKD in this study were supposed to account for majority of those in this area, although this study was a dynamic population cohort. We also investigated the prevalence of those who initiated RRT at our institution among four major dialysis centers in this area during this study period, and found it to be 68.7%. Third, we did not evaluate primary kidney disease. It is possible that the lack of primary kidney disease as a confounder made the impact of age and overt proteinuria on ESRD stronger than expected in our analysis. However, it is quite difficult to include precise diagnoses as a variable in analyses because clinical diagnosis is often ambiguous and incorrect, and renal biopsy is rarely performed for advanced CKD patients. Moreover, sometimes there are coexisting kidney diseases, such as nephrosclerosis and diabetic nephropathy. On the other hand, our stratification strategy with age, proteinuria, and CKD stage is simple and practical. Fourth, the study population consisted of only Japanese subjects without malignancy. Previous studies have also described longer survival and lower CVD event rates in Japanese high-risk population than those in other countries (47). These factors might contribute to the better survival in our study.
Conclusion
In the present study, we showed different backgrounds and outcomes in CKD patients referred to nephrologists compared with patients included in population-based studies. Advanced CKD and overt proteinuria were common among referred patients. Proteinuria and age contributed differently to CKD outcomes. Stratification of heterogeneous CKD patients by those factors and CKD stage could be a simple strategy for predicting the competing outcomes and for the selection of patients who should be intensively treated by nephrologists.
Disclosures
None.
Acknowledgments
We would like to thank the physicians at all 84 institutions for their cooperation with our questionnaire survey and management of these patients. Special thanks are extended to the Izumisano-Sennan Medical Association.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J: The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140: 167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Kaysen GA, Eiserich JP: The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol 15: 538–548, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA: Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: The heart and soul study. J Am Soc Nephrol 14: 3233–3238, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Drey N, Roderick P, Mullee M, Rogerson M: A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Shlipak MG, Stehman-Breen C, Vittinghoff E, Lin F, Varosy PD, Wenger NK, Furberg CD: Creatinine levels and cardiovascular events in women with heart disease: Do small changes matter? Am J Kidney Dis 43: 37–44, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Roderick PJ, Atkins RJ, Smeeth L, Mylne A, Nitsch DDM, Hubbard RB, Bulpitt C, Fletcher AE: CKD and mortality risk in older people: A community-based population study in the United Kingdom. Am J Kidney Dis 53: 950–960, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Patel UD, Young EW, Ojo AO, Hayward RA: CKD progression and mortality among older patients with diabetes. Am J Kidney Dis 46: 406–414, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan HA, Lydersen S, Holmen J: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275–2284, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S: Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 13: 631–632, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Okamura T, Hayakawa T, Kadowaki T, Kita Y, Ohnishi H, Saitoh S, Sakata K, Okayama A, Ueshima H: Chronic kidney disease is a risk factor for cardiovascular death in a community-based population in Japan: NIPPON DATA90. Circ J 70: 954–959, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 19.John R, Webb M, Young A, Stevens PE: Unreferred chronic kidney disease: A longitudinal study. Am J Kidney Dis 43: 825–835, 2004 [DOI] [PubMed] [Google Scholar]
- 20.St Peter WL, Schoolwerth AC, McGowan T, McClellan WM: Chronic kidney disease: Issues and establishing programs and clinics for improved patient outcomes. Am J Kidney Dis 41: 903–924, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Imai E, Yamagata K, Iseki K, Iso H, Horio M, Mkino H, Hishida A, Matsuo S: Kidney disease screening program in Japan: History, outcome, and perspectives. Clin J Am Soc Nephrol 2: 1360–1366, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Gray R: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals Statistics: 1141–1154, 1988 [Google Scholar]
- 24.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Statistical Assn 94: 496–497, 1999 [Google Scholar]
- 25.Royston P, Sauerbrei W, Freiburg G: Multivariable modeling with cubic regression splines: A principled approach. Stata J 7: 45, 2007 [Google Scholar]
- Holm A: Construction of efficient nuclear event analysis programs using a simple dedicated language. IEEE Transactions Nucl Sci 26: 4569–4571, 1979 [Google Scholar]
- 27.Royston P: Multiple imputation of missing values. Stata J 4: 227–241, 2004 [Google Scholar]
- 28.Agarwal R, Bunaye Z, Bekele DM, Light RP: Competing risk factor analysis of end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 28: 569–575, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, Kusek JW, Nayfield SG, Schmader K, Tian Y, Ashworth JR, Clayton CP, Parker RP, Tarver ED, Woolard NF, High KP: Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 20: 1199–1209, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Eckardt KU, Berns JS, Rocco MV, Kasiske BL: Definition and classification of CKD: The debate should be about patient prognosis–a position statement from KDOQI and KDIGO. Am J Kidney Dis 53: 915–920, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, Collins AJ, Kusek JW, Levey AS, Greene T: Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 73: 1310–1315, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR: Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 20: 1069–1077, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T: Proteinuria as a surrogate outcome in CKD: Report of a scientific workshop sponsored by the national kidney foundation and the us food and drug administration. Am J Kidney Dis 54: 205–226, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Makino H, Hishida A, Matsuo S: Slower decline of glomerular filtration rate in the Japanese general population: A longitudinal 10-year follow-up study. Hypertens Res 31: 433–441, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J: Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taal MW, Brenner BM: Renal risk scores: Progress and prospects. Kidney Int 73: 1216–1219, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Iseki K, Ikemiya Y, Iseki C, Takishita S: Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63: 1468–1474, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Iseki K, Kinjo K, Iseki C, Takishita S: Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis 44: 806–814, 2004 [PubMed] [Google Scholar]
- 39.Ishani A, Grandits GA, Grimm RH, Svendsen KH, Collins AJ, Prineas RJ, Neaton JD: Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 17: 1444–1452, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Evans M, Fryzek JP, Elinder CG, Cohen SS, McLaughlin JK, Nyren O, Fored CM: The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis 46: 863–870, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Johnson ES, Thorp ML, Yang X, Charansonney OL, Smith DH: Predicting renal replacement therapy and mortality in CKD. Am J Kidney Dis 50: 559–565, 2007 [DOI] [PubMed] [Google Scholar]
- 42.O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS: Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18: 2758–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Conway B, Webster A, Ramsay G, Morgan N, Neary J, Whitworth C, Harty J: Predicting mortality and uptake of renal replacement therapy in patients with stage 4 chronic kidney disease. Nephrol Dialysis Transplant 24: 1930–1937, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Levin A, Djurdjev O, Beaulieu M, Er L: Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 52: 661–671, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG: Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: The Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 113: 2713–2723, 2006 [DOI] [PubMed] [Google Scholar]
- 46.The number of households and population of municipalities in Osaka prefecture, The 2005 Japanese National Population Census. 2005. Available at http://www.pref.osaka.jp/toukei2/kokucho05/chouchou/xlslist.html Accessed December 10, 2009
- 47.Steg PG, Bhatt DL, Wilson PW, D'Agostino R, Sr, Ohman EM, Rother J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S: One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 297: 1197–1206, 2007 [DOI] [PubMed] [Google Scholar]