Abstract
Background and objectives: To determine, in a national cohort of incident hemodialysis patients, whether meeting a greater number of National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guideline goals at dialysis initiation was independently associated, in a graded manner, with lower first-year mortality rates.
Design, setting, participants, & measurements: Patients who initiated hemodialysis between June 1, 2005, and May 31, 2007, in the US were included in this retrospective cohort analysis. Guidelines examined were (1) use of arteriovenous fistula or graft at initiation; (2) hemoglobin ≥11 g/dl; and (3) albumin at goal. The primary predictor variable was number of guideline goals (zero, one, two, or three) met at dialysis initiation. Cox regression analysis was used to compare time to death, adjusting for baseline characteristics.
Results: At dialysis initiation, 59%, 31%, 9%, and 1.6% of patients met zero, one, two, or three guideline goals, respectively (total n = 192,307). After multivariate adjustment, mortality hazard ratios (95% confidence intervals) were 0.81 (0.80 to 0.83) for patients who met one, 0.53 (0.51 to 0.56) for patients who met two, and 0.34 (0.30 to 0.39) for patients who met three guideline goals, compared with patients who met none. Meeting each individual goal was also associated with lower mortality.
Conclusions: These findings suggest a graded association between meeting a greater number of evidence-based guideline goals at dialysis initiation and lower risk of death during the first year on dialysis.
The number of patients with end-stage renal disease (ESRD) requiring dialysis is increasing (1,2). Patients with ESRD have exceedingly high morbidity and mortality rates, particularly in the first year after dialysis initiation, when mortality exceeds 25% (1). To improve outcomes of patients with ESRD, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) developed evidence-based guidelines for the care of kidney disease patients. For patients with chronic kidney disease (CKD) approaching ESRD, the KDOQI guidelines recommend timely nephrology referral, nutritional consultation, fistula placement for dialysis access, and control of anemia, acidosis, and mineral and bone metabolism parameters (3). Evidence from prevalent dialysis patients (4) and from dialysis patients who survived on dialysis for at least 4 to 6 months (5,6) suggests that greater adherence to the KDOQI guidelines is associated with improved patient outcomes. Survival bias in these studies limits their generalizability to patients receiving renal replacement therapy who have survived beyond the initiation period. Whether guideline adherence at the time of dialysis initiation is associated with improved outcomes, particularly during the first year on dialysis, has not been examined.
We aimed to determine, in a large nationally representative cohort of incident hemodialysis patients, whether meeting a greater number of KDOQI guideline goals, specifically goals related to vascular access, anemia management, and serum albumin, at dialysis initiation is independently associated, in a graded manner, with survival during the first dialysis year.
Materials and Methods
Data Sources
This study used data from the United States Renal Data System (USRDS), a national data system that collects information on most new dialysis patients in the US from the Centers for Medicare & Medicaid Services (CMS). Information was obtained from the ESRD Medical Evidence Report (form CMS-2728), which is completed by providers for each new ESRD patient within 45 days of dialysis initiation. Data extracted included age, sex, race, primary cause of ESRD (diabetes, hypertension, glomerulonephritis, other), body mass index, comorbid conditions (heart failure, atherosclerotic heart disease, peripheral vascular disease, cerebrovascular disease, diabetes, hypertension, chronic obstructive pulmonary disease, cancer), smoking status, alcohol intake, drug use, inability to ambulate or transfer, need for help with activities of daily living, institutionalization status (nursing home residence), and insurance (Medicare, private insurance, Medicaid, Medicare and Medicaid, Veterans Administration, none, other). Pre-ESRD nephrology care was ascertained from question 18b on form CMS-2728: “Before ESRD was patient under care of a nephrologist?” If yes, and if the duration >12 months was chosen, the patient was defined as having received nephrology care for >12 months before ESRD. If yes, and if the duration 6 to 12 months or neither duration was chosen, the patient was defined as having received nephrology care for 0 to 12 months before ESRD. Similar assumptions were made to define duration of dietitian care based on question 18c: “Before ESRD was patient under care of kidney dietitian?”
Patients
We assembled a cohort of patients aged 20 years and older who initiated hemodialysis between June 1, 2005, and May 31, 2007, in the US. Patients who returned to hemodialysis from another renal replacement therapy modality, or who changed dialysis modality, underwent kidney transplant, or recovered kidney function within 60 days of their first ESRD service date were excluded from the analysis.
Meeting Guideline Goals
Data used to determine guideline adherence at dialysis initiation were obtained from form CMS-2728. Dialysis access information was obtained from question 18d: “What access was used on first outpatient dialysis? If not arteriovenous (AV) fistula: Is maturing AV fistula present? Is maturing AV graft present?” Hemoglobin and albumin values were obtained within 45 days before the first dialysis treatment.
The primary independent variable was number of guideline goals (zero, one, two, or three) met at dialysis initiation. The guideline goals examined in the analysis were: (1) use of AV fistula or graft at initiation; (2) hemoglobin ≥ 11 g/dl; and (3) serum albumin ≥ 4.0 g/dl when the method of measurement was bromcresol green or unspecified or ≥3.7g/dl when the method of measurement was bromcresol purple.
Follow-Up
Follow-up began on the first dialysis date and continued for up to 365 days. Follow-up was censored at modality change (to peritoneal dialysis or home dialysis), transplantation, or recovery of kidney function. Death information was obtained from the Death Notification (form CMS-2746); this information has been shown to be accurate in 99.5% of cases (7).
Statistical Analyses
Baseline characteristics were compared between groups defined by the number of guideline goals met at dialysis initiation using ANOVA for continuous variables and the Chi-square test for categorical variables. Logistic regression analyses were performed to identify independent associations between study variables and meeting individual guideline goals. Kaplan-Meier curves of cumulative survival for groups that met each guideline goal, and number of guideline goals met (zero to three), were plotted and compared using the log rank test. Cox regression analysis was used to compare survival times adjusting for baseline sociodemographic characteristics, smoking status, alcohol intake and drug use, functional status parameters, and comorbid conditions as collected on form CMS-2728. All analyses were performed using version 9.1 SAS software. Estimated P values are reported without adjustment for making multiple comparisons.
Results
Overall, 201,425 patients initiated hemodialysis between June 1, 2005, and May 31, 2007. Of these, 198,904 were aged 20 years or older at the time of initiation, resided in one of the 50 U.S. states, the District of Columbia, Puerto Rico, or the U.S. Territories, and had valid information on form CMS-2728. We excluded 6597 patients who changed dialysis modality, underwent kidney transplant, or recovered renal function during the first 60 days after dialysis initiation, leaving 192,307 patients in the analytical cohort.
Baseline Patient Characteristics
Of the 192,307 patients included in the cohort, 65% were white, 30% were African American, 44% were women, 46% had ESRD secondary to diabetes, and 25% had private insurance (Table 1). The cohort primarily consisted of elderly patients; mean age was 64 ± 15 years.
Table 1.
Baseline patient characteristics by number of KDOQI guideline goals achieved at hemodialysis initiation
| Baseline Characteristics | Total | Number of Guideline Goals Meta |
|||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| n | 192,307 | 113,605 | 58,949 | 16,641 | 3112 |
| Age (mean ± SD) | 64 ± 15 | 63 ± 15 | 65 ± 15 | 65 ± 14 | 65 ± 15 |
| Women, n (%) | 85,228 (44) | 52,509 (46) | 25,129 (43) | 6475 (39) | 1115 (36) |
| Race, n (%) | |||||
| White | 124,875 (65) | 71,965 (63) | 39,161 (66) | 11,459 (69) | 2290 (74) |
| African American | 57,293 (30) | 35,891 (32) | 16,528 (28) | 4231 (25) | 643 (21) |
| Asian | 7762 (4.0) | 4280 (3.7) | 2562 (4.4) | 762 (4.6) | 158 (5.3) |
| Native American | 2112 (1.2) | 1310 (1.2) | 613 (1.5) | 170 (1.3) | 19 (0.6) |
| Other | 265 (0.1) | 159 (0.1) | 85 (0.1) | 19 (0.1) | 2 (0.1) |
| Cause of ESRD, n (%) | |||||
| Diabetes | 89,179 (46) | 53,328 (47) | 27,575 (47) | 7269 (44) | 1007 (32) |
| Hypertension | 54,339 (28) | 30,967 (27) | 17,163 (29) | 5116 (31) | 1093 (35) |
| Glomerulonephritis | 12,203 (6.4) | 6618 (5.8) | 3881 (7) | 1396 (8.4) | 308 (10) |
| Other | 36,586 (19) | 22,692 (20) | 10,330 (18) | 2860 (17) | 704 (23) |
| Comorbid conditions, n (%) | |||||
| ASHD | 44,389 (23) | 25,680 (23) | 14,194 (24) | 3823 (23) | 692 (22) |
| CHF | 68,138 (35) | 42,340 (37) | 20,714 (35) | 4489 (27) | 595 (19) |
| PVD | 29,540 (15) | 17,779 (16) | 9097 (15) | 2289 (14) | 375 (12) |
| CVA/TIA | 19,861 (10) | 11,660 (10) | 6317 (11) | 1635 (10) | 249 (8) |
| Functional status | |||||
| ADL help, n (%) | 22,175 (12) | 14,638 (13) | 6325 (11) | 1096 (6.6) | 116 (3.7) |
| Institutionalized, n (%) | 15,655 (8) | 10,617 (9) | 4342 (7) | 637 (3.8) | 59 (1.9) |
| Employed, n (%) | 18,121 (9) | 9892 (9) | 5641 (10) | 2067 (12) | 521 (17) |
| BMI (mean ± SD) | 28.6 ± 7.7 | 28.6 ± 7.8 | 28.5 ± 7.6 | 28.7 ± 7.3 | 28.2 ± 6.6 |
| eGFR (mean ± SD) | 10.5 ± 4.9 | 10.4 ± 5.0 | 10.8 ± 4.8 | 10.6 ± 4.3 | 10.5 ± 3.9 |
| Erythropoietin use, n (%) | 56,744 (30) | 26,269 (23) | 20,654 (35) | 8078 (49) | 1743 (56) |
| Predialysis nephrology care, n (%) | |||||
| 0 to 12 months | 66,616 (35) | 35,609 (31) | 22,571 (38) | 7153 (43) | 1283 (41) |
| >12 months | 41,747 (22) | 17,939 (16) | 15,442 (26) | 6759 (41) | 1607 (52) |
| None | 61,542 (32) | 44,861 (40) | 14,831 (25) | 1730 (10) | 120 (3.9) |
| Missing | 22,402 (12) | 15,196 (13) | 6105 (10) | 999 (6.0) | 102 (3.3) |
| Predialysis dietitian care, n (%) | |||||
| 0 to 12 months | 13,327 (6.9) | 6059 (5.3) | 4827 (8.2) | 2026 (12) | 415 (13) |
| >12 months | 4913 (2.6) | 1764 (1.6) | 1842 (3.1) | 1029 (6.2) | 277 (8.9) |
| None | 138,200 (72) | 84,120 (74) | 41,385 (70) | 10,761 (65) | 1934 (62) |
| Missing | 35,867 (19) | 21,661 (19) | 10,895 (19) | 2825 (17) | 486 (16) |
| Hemoglobin (mean ± SD, g/dl) | 10.1 ± 1.7 | 9.2 ± 1.2 | 11.0 ± 1.7 | 11.7 ± 1.3 | 12.2 ± 0.9 |
| Albumin (mean ± SD, g/dl) | 3.1 ± 0.7 | 2.9 ± 0.6 | 3.2 ± 0.7 | 3.7 ± 0.6 | 4.2 ± 0.3 |
| Access at initiation, n (%) | |||||
| Arteriovenous fistula | 25,394 (13) | NA | 13,425 (23) | 9413 (57) | 2556 (82) |
| Arteriovenous graft | 8462 (4.4) | NA | 5199 (8.8) | 2707 (16) | 556 (18) |
| Catheter | 156,014 (81) | 111,902 (99) | 39,688 (67) | 4424 (27) | NA |
| Other | 2305 (1.2) | 1615 (1.4) | 598 (1.0) | 92 (0.6) | NA |
| Missing | 132 (0.1) | 88 (0.1) | 39 (0.1) | 5 (0) | NA |
| Maturing | 33,312 (17) | 22,190 (19) | 9486 (16) | 1599 (10) | 97 (3.1) |
| Arteriovenous graft maturing | 7747 (4.0) | 4673 (4.1) | 2385 (4.1) | 592 (3.6) | 37 (1.2) |
ADL, activities of daily living; ASHD, atherosclerotic heart disease; BMI, body mass index; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident/transient ischemic attack; eGFR, estimated GFR in ml/min per 1.73 m2; ESRD, end-stage renal disease; NA, not applicable; PVD, peripheral vascular disease.
All P 0.001 for comparisons between groups.
In the cohort, 113,605 patients (59%) met none of the guideline goals; 58,949 (31%) met one guideline goal; 16,641 (9%) met two guideline goals; and 3112 (1.6%) met all three guideline goals at hemodialysis initiation. Specifically, 18% of patients met the vascular access guideline goal, 26% the hemoglobin guideline goal, and 9% the albumin guideline goal.
Meeting Guideline Goals at Initiation by Pre-ESRD Nephrology Care
Among the included patients, 32% received no predialysis nephrology care, 35% received predialysis nephrology care for 12 months or less, and 22% received predialysis nephrology care for more than 12 months; predialysis nephrology care was not reported for 12%. Number of KDOQI guidelines goals met at dialysis initiation increased with presence and duration of predialysis nephrology care (Table 2). Of patients who received predialysis nephrology care for more than 12 months, 43% met zero, 37% met one, 16% met two, and 4% met all three goals at dialysis initiation. Predialysis dietitian care was related to nephrology care; 0.3% of patients without nephrology care received dietitian care, as did 16% of patients with nephrology care for 12 months or less and 18% of patients with nephrology care for more than 12 months (Table 2).
Table 2.
Meeting guideline goals at initiation by duration of nephrology follow-up
| Duration of Nephrology Follow-up |
||||
|---|---|---|---|---|
| None | 0 to 12 Months | >12 Months | Unknown/Missing | |
| Total (n) | 61,542 | 66,616 | 41,747 | 22,402 |
| Guideline goals met at initiation (%) | ||||
| 0 | 44,861 (73) | 35,609 (54) | 17,939 (43) | 15,196 (68) |
| 1 | 14,831 (24) | 22,571 (34) | 15,442 (37) | 6105 (27) |
| 2 | 1730 (2.8) | 7153 (11) | 6759 (16) | 999 (4.5) |
| 3 | 120 (0.2) | 1283 (1.9) | 1607 (3.9) | 102 (0.5) |
| Hemoglobin ≥ 11 g/dl | 12,395 (20) | 18,950 (29) | 13,510 (32) | 5194 (23) |
| Albumin at goal | 3650 (5.9) | 6838 (10) | 5723 (14) | 1451 (6.5) |
| Arteriovenous fistula or graft used at initiation | 2606 (4.2) | 14,938 (22) | 14,548 (35) | 1764 (7.9) |
| Arteriovenous fistula or graft maturing at initiation | 9579 (16) | 15,728 (24) | 9834 (24) | 2957 (13) |
| Dietitian care before dialysis | 174 (0.3) | 10,579 (16) | 7437 (18) | 50 (0.2) |
All P values for Chi-square test are < 0.0001.
Independent Correlates of Meeting Individual Guideline Goals
Female sex, presence of most comorbid conditions, and functional status limitations were associated with lower frequency of meeting individual guideline goals (Table 3). Conversely, employment at dialysis initiation was associated with higher frequency of meeting all of the individual goals. African American race was associated with higher likelihood of starting dialysis with an AV fistula or AV graft, but lower likelihood of meeting the hemoglobin and albumin guideline goals. Older age was associated with better dialysis access and meeting the hemoglobin guideline goal. Hypertension and glomerulonephritis as causes of ESRD were independently associated with greater likelihood of meeting the hemoglobin and albumin guideline goals at dialysis initiation, compared with diabetes as cause of ESRD. History of cerebrovascular accident was associated with a 9% increased likelihood of hemoglobin ≥ 11 g/dl. Predialysis nephrology care was independently associated, in a graded manner, with increased likelihood of meeting all of the individual guideline goals (Table 3).
Table 3.
Associations of individual guideline goals achieved at dialysis initiationa
| Variables | Arteriovenous Fistula or Arteriovenous Graft RR (95% CI)c | Hemoglobin ≥11 g/dl RR (95% CI)c | Albumin at GoalbRR (95% CI)c |
|---|---|---|---|
| Age (per 5 years) | 1.03 (1.02 to 1.03) | 1.03 (1.03 to 1.04) | 1.00 (0.99 to 1.00) |
| Sex | |||
| Men | Reference | Reference | Reference |
| Women | 0.77 (0.75 to 0.79) | 0.90 (0.88 to 0.92) | 0.87 (0.84 to 0.90) |
| Race | |||
| White | Reference | Reference | Reference |
| African American | 1.10 (1.07 to 1.14) | 0.76 (0.74 to 0.78) | 0.85 (0.82 to 0.88) |
| Asian | 1.17 (1.10 to 1.24) | 1.05 (0.99 to 1.10) | 1.00 (0.92 to 1.08) |
| Native American/other | 1.02 (0.91 to 1.14) | 0.95 (0.86 to 1.05) | 0.65 (0.55 to 0.78) |
| Cause of ESRD | |||
| Diabetes | Reference | Reference | Reference |
| Hypertension | 0.98 (0.94 to 1.02) | 1.08 (1.04 to 1.11) | 1.49 (1.41 to 1.56) |
| Glomerulonephritis | 1.00 (0.94 to 1.06) | 1.09 (1.04 to 1.15) | 1.25 (1.16 to 1.34) |
| Other | 0.81 (0.78 to 0.85) | 1.00 (0.97 to 1.04) | 1.37 (1.30 to 1.45) |
| Comorbid conditions | |||
| ASHD | 0.95 (0.92 to 0.98) | 1.02 (0.99 to 1.05) | 1.04 (1.00 to 1.09) |
| CHF | 0.70 (0.68 to 0.72) | 0.84 (0.82 to 0.86) | 0.73 (0.70 to 0.76) |
| PVD | 0.92 (0.89 to 0.96) | 0.92 (0.89 to 0.95) | 0.87 (0.83 to 0.92) |
| CVA/TIA | 1.04 (0.99 to 1.08) | 1.09 (1.05 to 1.13) | 0.97 (0.92 to 1.03) |
| Functional status | |||
| ADL help | 0.81 (0.77 to 0.86) | 0.95 (0.91 to 0.99) | 0.80 (0.75 to 0.87) |
| Institutionalized | 0.74 (0.70 to 0.79) | 0.97 (0.92 to 1.01) | 0.58 (0.53 to 0.64) |
| Employed | 1.09 (1.04 to 1.14) | 1.08 (1.04 to 1.13) | 1.33 (1.26 to 1.40) |
| Body mass index | 1.01 (1.01 to 1.01) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.01) |
| eGFR | 1.00 (1.00 to 1.00) | 1.04 (1.04 to 1.04) | 0.99 (0.99 to 1.00) |
| Erythropoietin use | 1.70 (1.65 to 1.75) | 1.10 (1.07 to 1.12) | 1.12 (1.08 to 1.16) |
| Nephrology care | |||
| 0 to 12 months | 4.64 (4.42 to 4.86) | 1.42 (1.38 to 1.46) | 1.74 (1.66 to 1.82) |
| >12 months | 7.97 (7.59 to 8.37) | 1.67 (1.61 to 1.73) | 2.29 (2.18 to 2.41) |
| None | Reference | Reference | Reference |
| Missing | 1.79 (1.67 to 1.92) | 1.11 (1.06 to 1.17) | 1.16 (1.07 to 1.25) |
RR, relative risk.
The logistic regression method was used to assess associations of meeting the individual guideline goals at dialysis initiation.
≥4.0 g/dl when the method of measurement was bromcresol green or unspecified or ≥3.7g/dl when the method of measurement was bromcresol purple.
Also adjusted for history of hypertension, diabetes, cancer, chronic obstructive pulmonary disease, inability to ambulate and transfer, smoking, alcohol use, drug use, dietitian follow-up, and insurance at dialysis initiation.
First Year Survival by Number of KDOQI Guideline Goals Met at Dialysis Initiation
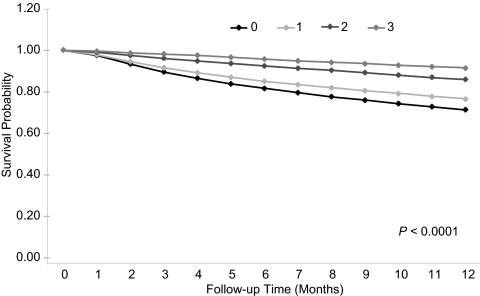
During follow-up, 47,809 patients (25%) died; 2957 (1.5%) were censored because of dialysis modality change, 3502 (1.8%) because of kidney transplant, and 4387 (2.3%) because of discontinuing dialysis due to restored kidney function. Unadjusted patient survival by number of KDOQI guideline goals met at dialysis initiation is presented in Figure 1. Survival at 1 year was 71%, 76%, 86%, and 91% when zero, one, two, and three guideline goals were met, respectively, (P < 0.001). A survival benefit was associated with meeting all individual guideline goals (Figures 2 through 4). Survival at 1 year was 81%, 77%, and 70% for patients who received predialysis nephrology care for more than 12 months, for 12 months or less, and not at all, respectively (P < 0.001).
Figure 1.
Survival during the first year of hemodialysis by a number of guideline goals achieved at dialysis initiation (unadjusted Kaplan-Meyer survival curves).
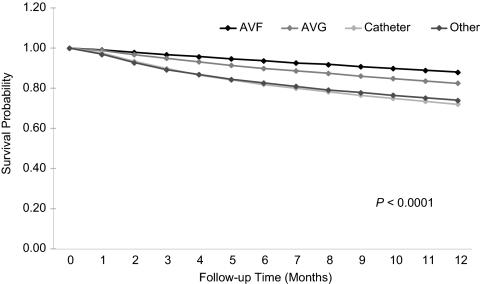
Figure 2.
Survival during the first year of hemodialysis by dialysis access at initiation (unadjusted Kaplan-Meyer survival curves). AVF, arteriovenous fistula; AVG, arteriovenous graft.
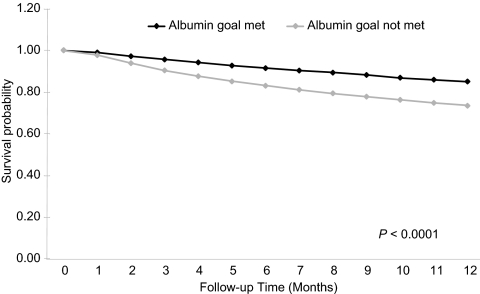
Figure 4.
Survival during the first year of hemodialysis by meeting or not meeting the KDOQI albumin guideline goal (≥4.0 g/dl when the method of measurement was bromcresol green or unspecified or ≥3.7 g/dl when the method of measurement was bromcresol purple) at initiation (unadjusted Kaplan-Meyer survival curves).
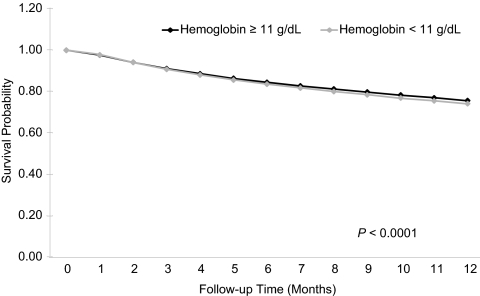
Figure 3.
Survival during the first year of hemodialysis by meeting or not meeting the KDOQI anemia guideline goal (hemoglobin ≥ 11 g/dl) at dialysis initiation (unadjusted Kaplan-Meyer survival curves).
In multivariable analysis, a greater number of guideline goals met at dialysis initiation was independently associated with lower mortality during the first year of dialysis. Compared with meeting none of the guideline goals, meeting one, two, or three goals was each independently associated with lower first-year mortality rates: Adjusted hazards ratios (95% confidence interval) for death were 0.81 (0.80 to 0.83) for one goal, 0.53 (0.51 to 0.56) for two goals, and 0.34 (0.30 to 0.39) for three goals met (Table 4). Meeting each of the individual guideline goals was also independently associated with survival benefit after adjusting for multiple covariates including meeting the other two goals (Table 4). Predialysis nephrology care was also independently associated, in a graded manner, with survival benefit after multivariable adjustments including meeting each of the individual guideline goals: Adjusted hazards ratios (95% confidence interval) for death were 0.82 (0.80 to 0.84) for nephrology care for 12 months or less and 0.73 (0.70 to 0.75) for nephrology care for more than 12 months compared with no nephrology care.
Table 4.
Hazard ratios for first-year mortality by number of KDOQI guideline goals met at dialysis initiation
| Guideline Goals at Initiation | HR (CI) Unadjusted | P | HR (CI) Adjusted | P |
|---|---|---|---|---|
| Number meta | ||||
| 0 | 1 | 1 | ||
| 1 | 0.79 (0.78 to 0.81) | <0.0001 | 0.81 (0.80 to 0.83) | <0.0001 |
| 2 | 0.45 (0.43 to 0.47) | <0.0001 | 0.53 (0.51 to 0.56) | <0.0001 |
| 3 | 0.26 (0.23 to 0.30) | <0.0001 | 0.34 (0.30 to 0.39) | <0.0001 |
| Arteriovenous fistula or arteriovenous graftb | 0.43 (0.42 to 0.45) | <0.0001 | 0.56 (0.54 to 0.58) | <0.0001 |
| Hemoglobin at goalb,c | 0.94 (0.92 to 0.96) | <0.0001 | 0.94 (0.92 to 0.96) | <0.0001 |
| Albumin at goalb,d | 0.52 (0.50 to 0.54) | <0.0001 | 0.67 (0.65 to 0.70) | <0.0001 |
Cox proportional hazards models adjusted for age, sex, race, body mass index, smoking, alcohol use, drug dependence, cause of end-stage renal disease, functional status (inability to ambulate or transfer, assistance with activities of daily living, institutionalization, employment at the time of end-stage renal disease onset), comorbid conditions (history of atherosclerotic heart disease, congestive heart failure, cerebrovascular accident, peripheral vascular disease, hypertension, diabetes, chronic obstructive pulmonary disease, cancer), and predialysis nephrology and dietitian care.
Cox proportional hazards models adjusted for the above variables with arteriovenous fistula or graft used at initiation, hemoglobin > 11 g/dl, and albumin at goal replacing the “number of guidelines achieved at initiation” variable.
Goal: Hemoglobin > 11 g/dl.
Goal: Albumin ≥ 4.0 g/dl when the method of measurement was bromcresol green or unspecified or ≥3.7 g/dl when the method of measurement was bromcresol purple.
Discussion
Meeting KDOQI guideline-recommended goals for vascular access, anemia, and serum albumin at dialysis initiation, individually and cumulatively, was independently associated with significantly lower mortality during the first year of dialysis.
Different patient characteristics were independently associated with meeting individual guideline goals at dialysis initiation. Despite regular access to medical care, institutionalized patients were less likely to meet the goals. This might be explained by a high burden of disease in this patient population. We also found racial and gender disparities. Women were less likely than men to meet all of the three guideline goals. Our study confirms findings by other investigators that women are underrepresented among hemodialysis patients with AV fistulas (8,9). This difference may be attributable to smaller vascular diameter and higher rates of early primary fistula failure among female patients (10). Albumin and hemoglobin are also reported to be lower in women at dialysis initiation (11). In the general population, hemoglobin is lower in women than in men (12); the etiology of lower albumin in women is less clear. African American patients were also less likely to meet hemoglobin and albumin goals. Other investigators have reported that African Americans start dialysis with lower levels of hemoglobin and albumin (13), and require higher doses of erythropoietin to maintain hemoglobin at goal (14). The lower hemoglobin at dialysis initiation has been attributed to lower predialysis use of erythropoietin (13) and higher prevalence of the thalassemia gene (15) in African Americans.
Racial and gender disparities in anemia and nutritional parameters are difficult to modify. The CMS ESRD quality improvement efforts for dialysis patients resulted in decreased disparity in adequacy of hemodialysis dose, but not in anemia and nutritional management (16). These disparities might be the result of biologic differences as well as differences in access to care, quality of care, patient knowledge, health beliefs, and clinician bias (17). Regardless of the cause, these findings indicate a need to target interventions aimed at improved preparation for renal replacement therapy toward women and African Americans with advanced CKD.
Ours is the first study to report that African Americans are more likely than whites to initiate hemodialysis with AV fistulas or grafts. Other studies did not report association of race/ethnicity with vascular access (8) or reported that African American dialysis patients were less likely to use a catheter and more likely to use an AV graft compared with white patients (18). One might speculate that African Americans are more likely to live in large urban centers with good access to vascular surgery services.
The low frequency of patients meeting KDOQI guideline goals at dialysis initiation suggests opportunity for improvement. In our cohort, 18% of patients started dialysis with AV fistulas or grafts, 26% with hemoglobin ≥ 11 g/dl, and 9.2% with albumin at goal. The proportions of patients who met guideline goals were much lower than reported for incident patients who survived on dialysis for at least 4 to 6 months (5,6). Among incident participants in the international Dialysis Outcomes and Practice Patterns Study, 29.7% started dialysis with AV fistulas or grafts, hemoglobin was ≥11 g/dl for 41%, and albumin was ≥3.5 g/dl for 60% (19). Because over 95% of incident hemodialysis patients who initiated dialysis between June 2005 and May 2007 in the US were included in our cohort, the numbers reported in this study accurately reflect the far less than optimal state of dialysis preparedness in the US.
Our findings are in agreement with findings from previous investigations that reported that achieving clinical performance targets for hemoglobin and serum albumin and using fistulas for vascular access were associated with reduced deaths among dialysis patients (4–6). While previous studies included prevalent dialysis patients or those who survived on dialysis for at least 4 to 6 months, our follow-up began at dialysis initiation, and our results suggest that meeting guideline goals at initiation is associated with greater survival during the first year of dialysis, when the risk of mortality is highest. Current evidence suggests that after dialysis initiation, the proportion of patients who meet guideline goals increases (6). Average time to target hemoglobin for dialysis patients is only 1.3 months (14). Therefore, values obtained after 4 to 6 months on dialysis are more likely to reflect a homogenous protocol-based approach to treatment practiced by dialysis facilities rather than more variable predialysis care. Patients receiving in-center hemodialysis receive regular medical care that provides most interventions. Before CKD patients begin dialysis, access to care and quality of care is widely variable. To date, a large effort has been made to improve the quality of care provided to patients on renal replacement therapy, with resultant improvement in prevalent patient outcomes (20). Unfortunately, there is much less emphasis on improving predialysis care. Findings from our study underscore the need to identify measures to decrease variability and improve quality of predialysis care.
We found a graded survival benefit associated with longer duration of predialysis nephrology care. While prior studies defined “early referral” as 3 to 6 months before dialysis initiation (21–25), we found that nephrology care for more than 12 months, compared with less than 12 months, is associated with greater likelihood of meeting evidence-based guideline goals and improved survival during the first year of dialysis. Optimal duration of predialysis nephrology care remains to be determined. Of particular concern, even among patients who received nephrology care for more than 12 months, KDOQI guideline adherence was alarmingly low: 43% met zero, 37% met one, 16% met two, and only 4% met all three KDOQI guideline goals at dialysis initiation. Our study reveals extensive opportunity for improvement in patient care provided by nephrologists. Further research is needed, focusing on defining barriers to adequate postreferral care and on developing interventions aimed at improving the quality of postreferral care. Potential interventions may include use of case managers and multidisciplinary clinics to improve attainment of clinical performance measures in patients with CKD who are likely to need dialysis.
This study was performed in a large national cohort of incident dialysis patients. The study period followed the widespread implementation of KDOQI guidelines and provides current information. Despite its strengths, this study has several limitations inherent in observational studies. Causality cannot be inferred. More severe illness, unidentified comorbidity, acuity, or noncompliance might explain some of the poor outcomes among patients who did not meet guideline goals at initiation. Despite adjustment for multiple covariates known to be associated with outcomes in dialysis patients, residual bias due to unmeasured confounders likely remains. The finding of an association between meeting the hemoglobin goal of ≥11 g/dl at dialysis initiation and better survival should be interpreted with caution. We chose targets recommended by the KDOQI guidelines at the time of care for the patient cohort to define guideline goal attainment. Several recent trials revealed no survival advantage and evidence of harm from efforts to achieve higher hemoglobin levels in patients with CKD (26–28). In our cohort, 7006 (12.3%) patients who were treated with erythropoietin before dialysis initiation had hemoglobin levels >12 g/dl. We used meeting the hemoglobin goal as the best surrogate for quality of predialysis medical care. In addition, our data were limited by a single measurement of laboratory values used to define guideline goal achievement, and methods for obtaining the values were not standardized. No data on control of bone metabolism parameters were available for analysis. Missing data were interpreted as not meeting the guideline goal. Furthermore, nephrology and dietitian care, as reported by patients' physicians on the Medical Evidence Report (form CMS-2728), was used in our models and in previous publications, but not validated (21).
In conclusion, this study suggests a graded association between meeting evidence-based guideline goals at initiation of dialysis and lower mortality during the first year of dialysis. We also report alarmingly poor guideline goal attainment at dialysis initiation in the US, even among patients seeing a nephrologist. Clinical practices associated with meeting evidence-based guideline goals before dialysis initiation should be identified and implemented to improve early patient outcomes on dialysis.
Disclosures
None.
Acknowledgments
This study was performed as a deliverable under contract no. HHSN267200715002C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD). The work was supported in part by a grant from the Minneapolis VA Center for Epidemiologic and Clinical Research (CECR) #04S-CRCOE-001 (Y.S.). The authors have no conflicts of interest with its subject matter. The authors wish to thank Beth Forrest, Shane Nygaard, and Nan Booth, MSW, MPH, of the United States Renal Data System, for regulatory assistance, manuscript preparation, and manuscript editing, respectively.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States, 2009 ed, Bethesda MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 2.Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ: Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 16: 3736–3741, 2005 [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation: NKF K/DOQI Guidelines: Clinical Practice Guidelines and Clinical Practice Recommendations. 2006Available at http://www.kidney.org/professionals/Kdoqi/guideline_upHD_PD_VA/va_guide1.htm Accessed May 12, 2010
- 4.Rocco MV, Frankenfield DL, Hopson SD, McClellan WM: Relationship between clinical performance measures and outcomes among patients receiving long-term hemodialysis. Ann Intern Med 145: 512–519, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Plantinga LC, Fink NE, Jaar BG, Sadler JH, Levin NW, Coresh J, Klag MJ, Powe NR: Attainment of clinical performance targets and improvement in clinical outcomes and resource use in hemodialysis care: A prospective cohort study. BMC Health Serv Res 7: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tentori F, Hunt WC, Rohrscheib M, Zhu M, Stidley CA, Servilla K, Miskulin D, Meyer KB, Bedrick EJ, Johnson HK, Zager PG: Which targets in clinical practice guidelines are associated with improved survival in a large dialysis organization? J Am Soc Nephrol 18: 2377–2384, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, Levey AS: Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded hemodialysis. Health Care Financing Administration. Am J Kidney Dis 39: 146–153, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Lee T, Barker J, Allon M: Associations with predialysis vascular access management. Am J Kidney Dis 43: 1008–1013, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, Canaud BJ, Pisoni RL: Vascular access use and outcomes: An international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 23: 3219–3226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus RJ, Marcus DA, Sureshkumar KK, Hussain SM, McGill RL: Gender differences in vascular access in hemodialysis patients in the United States: Developing strategies for improving access outcome. Gend Med 4: 193–204, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Obrador GT, Ruthazer R, Arora P, Kausz AT, Pereira BJ: Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol 10: 1793–1800, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Beutler E, Waalen J: The definition of anemia: What is the lower limit of normal of the blood hemoglobin concentration? Blood 107: 1747–1750, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward MM: Laboratory abnormalities at the onset of treatment of end-stage renal disease: Are there racial or socioeconomic disparities in care? Arch Intern Med 167: 1083–1091, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ishani A, Guo H, Arneson TJ, Gilbertson DT, Mau LW, Li S, Dunning S, Collins AJ: Possible effects of the new Medicare reimbursement policy on African Americans with ESRD. J Am Soc Nephrol 20: 1607–1613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beutler E, West C: Hematologic differences between African-Americans and whites: The roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood 106: 740–745, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehgal AR: Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA 289: 996–1000, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Fiscella K, Franks P, Gold MR, Clancy CM: Inequality in quality: Addressing socioeconomic, racial, and ethnic disparities in health care. JAMA 283: 2579–2584, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Reddan D, Klassen P, Frankenfield DL, Szczech L, Schwab S, Coladonato J, Rocco M, Lowrie EG, Owen WF, Jr: National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol 13: 2117–2124, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW: Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 20.McClellan WM, Frankenfield DL, Frederick PR, Helgerson SD, Wish JB, Sugarman JR: Improving the care of ESRD patients: A success story. Health Care Financ Rev 24: 89–100, 2003 [PMC free article] [PubMed] [Google Scholar]
- 21.McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV: Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol 20: 1078–1085, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora P, Obrador GT, Ruthazer R, Kausz AT, Meyer KB, Jenuleson CS, Pereira BJ: Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 10: 1281–1286, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kazmi WH, Obrador GT, Khan SS, Pereira BJ, Kausz AT: Late nephrology referral and mortality among patients with end-stage renal disease: A propensity score analysis. Nephrol Dial Transplant 19: 1808–1814, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Winkelmayer WC, Owen WF, Jr, Levin R, Avorn J: A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol 14: 486–492, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de ZD, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]