Abstract
Background and objectives: ESRD is associated with systemic oxidative stress, an important nontraditional risk factor for the development of cardiovascular disease. Since interventions aimed at reducing oxidative stress may be beneficial, we examined the pharmacokinetics and pharmacodynamics of the widely used antioxidant N-acetylcysteine (NAC) after oral administration in patients with ESRD.
Design, setting, participants, & measurements: Twenty-four ESRD patients were randomly assigned to receive 600 or 1200 mg of sustained-release NAC orally every 12 hours for 14 days. Seven healthy control subjects received NAC 600 mg in the same manner. Blood samples were obtained on days 1 and 15 for determination of NAC pharmacokinetics and pharmacodynamics.
Results: Significant dose-related increases in NAC plasma concentrations were observed in ESRD patients with no change in total clearance; a doubling of the dose resulted in a 2-fold increase in NAC area under the plasma concentration-time curve (AUC). However, NAC clearance was reduced by 90% in ESRD, leading to a 7-fold larger AUC and 13-fold longer half-life compared with healthy control subjects. NAC administration resulted in a significant reduction in total homocysteine plasma concentrations in ESRD and healthy subjects, but had no effect on several other oxidative stress markers.
Conclusions: These findings indicate that the total clearance of oral NAC is significantly reduced in ESRD patients, leading to marked increases in systemic exposure, and suggest that NAC may have a limited role in the chronic treatment of oxidative stress-related illness.
End-stage renal disease (ESRD) is associated with systemic oxidative stress, which is an important nontraditional risk factor for the development of cardiovascular disease (1), now the leading cause of morbidity and mortality in patients with ESRD (2). Consequently, interventions aimed at reducing oxidative stress may be beneficial in ESRD, ultimately lowering the rate of cardiovascular events and improving survival.
N-Acetylcysteine (NAC) is an aminothiol-containing antioxidant that has been used therapeutically for five decades (3). It has been used extensively as a mucolytic agent, in the treatment of acetaminophen toxicity, as a cytoprotective agent during cancer chemotherapy, and in the prevention of contrast-induced nephropathy (3,4). The mechanism of NAC's antioxidant activity likely stems from its oxygen free-radical scavenging properties and/or its role as a source of cysteine, necessary for the biosynthesis of glutathione (4,5). The utility of antioxidant compounds like NAC to suppress oxidative stress and exert sustained, long-term beneficial effects in humans is based on the premise that they can be administered chronically in doses that are safe, effective, and well-tolerated. The pharmacokinetic (PK) disposition of NAC has been studied in subjects with normal renal function (6–9), and during intermittent intravenous administration with hemodialysis in ESRD patients (10), while only limited NAC pharmacodynamic (PD) assessments (e.g., endothelial function and plasma concentrations of homocysteine, malondialdehyde, or asymmetric dimethylarginine) have been carried out in ESRD subjects without simultaneous PK determination (11–16). To date, NAC PK and PD have not been comprehensively evaluated in ESRD patients after multiple-dose oral administration, which represents the most practical and cost-effective route of administration for chronic therapeutic use. Thus, the purpose of this study was to examine the PK and PD of NAC after multiple-dose oral administration to patients with ESRD.
Materials and Methods
Study Subjects
After providing written informed consent, all subjects underwent a screening evaluation within the 28-day period before the study day that was based on a complete medical history, physical examination, medication history, and conventional biochemical tests. Eligibility criteria included nonsmoking status, age 18 to 75 years, normal hepatic function, and a negative pregnancy test for women of child-bearing potential, which was repeated and confirmed negative before drug dosing on the study day. ESRD subjects were also required to be on a stable, thrice weekly in-center hemodialysis regimen and have a fistula or graft for blood access. Kidney function in control subjects was estimated via the four-variable Modification of Diet in Renal Disease (MDRD) Study equation (17). Subjects who were medically unstable, had a known allergy or hypersensitivity to NAC, had liver disease, were pregnant or lactating, were seropositive for hepatitis B, hepatitis C, or HIV, used a central venous catheter for blood access, or who were treated with a dialyzer containing vitamin E-bonded membranes were excluded.
Study Design
This was an open-labeled, prospective, randomized, parallel, dose-ranging study of the multiple-dose PK and PD of NAC in hemodialysis patients. The study adhered to the Declaration of Helsinki and was approved by the University of Louisville and Maine Medical Center Institutional Review Boards. Hemodialysis subjects were randomized to either 600 mg orally every 12 hours or 1200 mg (2 × 600 mg tablets) orally every 12 hours of a commercially available sustained-release formulation of NAC (600 mg tablets; Jarrow Formulas, Inc., Los Angeles, CA). To determine the impact of ESRD on NAC PK and PD, we also enrolled healthy subjects in a treatment group that received NAC 600 mg orally every 12 hours.
The PK disposition of NAC was assessed after 14 days of multiple-dose treatment. ESRD patients were studied the day after a regularly scheduled hemodialysis day, and all subjects were studied the morning after an overnight fast. On the 15th day, a final dose of NAC was administered with 240 ml tepid water at approximately 8:00 a.m. An indwelling catheter for blood collection was placed in an antecubital vein. Venous blood samples (n = 13 × 5 ml, total blood volume = 65 ml) were collected immediately before and at 15, 30, 45, 60 minutes, and 1.5, 2, 2.5, 3, 4, 6, and 8 hours after administering the last dose of NAC. Subjects were given a small snack at 10:00 a.m. followed by light meals thereafter. Concomitant medications were held until 2 hours after administering the last dose of NAC. Blood samples were collected at baseline on day 1 and predose on day 15 for determination of homocysteine (HCY), cysteine (CYS), asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine (ARG), total glutathione (GSH), and protein carbonyl concentrations. All blood samples were centrifuged within 1 hour of collection at 2500 rpm at 4°C for 15 minutes. Plasma was harvested and stored at −70°C until analysis.
Bioanalytical Methods
Total glutathione was measured using a colorimetric assay (Bioxytech GSH/GSSG-412; Oxis Research, Portland, OR). Total plasma concentrations of NAC, HCY, and CYS were determined by HPLC with fluorescence detection as described previously (18,19). The linear standard curve for NAC ranged from a lower limit of quantitation of 0.325 to 32.5 μg/ml, and the intra- and interassay coefficients of variation were <10%. ADMA, SDMA, and ARG concentrations were also determined by HPLC using a previously published method (20). Plasma protein carbonyl concentrations were determined by ELISA (OxiSelect; Cell Biolabs, Inc., San Diego, CA).
PK and PD Analyses
NAC PK parameters after multiple dosing were estimated from plasma-concentration data using noncompartmental methods (WinNonlin Professional, version 4.1; PharSight Corp., Mountain View, CA). The baseline (i.e., predose) plasma concentration (C0), Cmax, tmax, and the last plasma concentration measured (the 8-hour plasma concentration, C8) were obtained directly from the individual plasma-concentration time profiles and recorded as observed. The terminal elimination rate constant (λz) was estimated by linear regression of the terminal phase of the logarithmic plasma-concentration time curve. The terminal disposition half-life (t½) was calculated by dividing 0.693 by λz. Area under the plasma concentration-time curve over the 8-hour plasma-sampling period (AUC0-8) was calculated with the log-linear trapezoidal rule. The principle of superposition was used to account for the AUC from the previous doses (21). The AUC from time 0 to infinity (AUC0-∞) for the last dose was calculated as AUC0-8 + C8/λz − C0/λz, where C8 was the 8-hour plasma concentration. The total clearance (CL/F) of NAC after oral administration, where F is the bioavailability, was calculated as dose/AUC0-∞.
The NAC PD parameters assessed were total plasma concentrations of HCY, CYS, ADMA, SDMA, ARG, GSH, and protein carbonyls. Baseline values of each parameter were compared with those observed after 14 days of NAC treatment. In addition, concentrations of HCY and CYS were explored during the 8-hour plasma-sampling period on day 15 (at time 0, 1.5, 3, and 8 hours after NAC administration). Relationships between the percentage change of each parameter from baseline and NAC AUC0-8 and between plasma NAC concentrations and concentrations of HCY and CYS during the 8-hour plasma-sampling period on day 15 were explored. The AUC0-8 for HCY and CYS was calculated with the log-linear trapezoidal rule.
Statistical Analysis
NAC PK parameters were compared between groups by the unpaired two-sided t test. Between and within-group (i.e., baseline versus day 15) comparisons of HCY, CYS, ADMA, SDMA, ARG, total glutathione, and protein carbonyl concentrations were made using an unpaired or paired two-sided t test, as appropriate. Relationships between NAC AUC0-8 or concentrations and PD parameters were evaluated using Spearman's rho correlation coefficient (rs). All statistical calculations were performed with Prism 5.0b (GraphPad Software, San Diego, CA). Data are presented as mean ± SD. A P value of <0.05 was considered statistically significant for all comparisons.
Determination of the target sample size was based on the reported between-subject coefficient of variation for NAC AUC in hemodialysis patients of approximately 30% (10). The sample size of n = 12 ESRD subjects per NAC dose was estimated a priori to have 80% power (two-sided type I error of 0.05) to detect a 36% difference in NAC AUC between ESRD subjects receiving NAC 600 mg versus NAC 1200 mg. In addition, the sample size of n = 7 healthy control subjects and n = 12 ESRD subjects per dose group was estimated to have 82% power (two-sided type I error of 0.05) to detect a 44% difference in NAC AUC between control subjects and each ESRD group. Power calculations were carried out with G*Power (v. 3.0.10) (22).
Results
Study Subjects
A total of 24 patients with ESRD undergoing chronic hemodialysis and seven healthy control subjects with normal kidney function participated in this study. Subject demographic characteristics are presented in Table 1. NAC was well-tolerated by all subjects at all doses. There were no serious adverse events reported and no instances of discontinuation due to adverse clinical or laboratory findings. One ESRD subject randomized to NAC 600 mg voluntarily withdrew from the study (not due to adverse event) after baseline samples were drawn on day 15 and was not included in the PK study. Baseline day 1 samples were not available on two ESRD patients randomized to NAC 1200 mg, so these subjects were not included in all day 1 versus day 15 comparisons of PD parameters. One ESRD subject randomized to NAC 1200 mg was found to be hyperhomocysteinemic at baseline, with HCY plasma concentrations 5-fold higher than the mean concentration for the remaining subjects in the dose group, and was excluded from all HCY analyses as an outlier.
Table 1.
Demographic characteristics of study subjects
| Control Subjects NAC 600 mg | ESRD Subjects |
||
|---|---|---|---|
| NAC 600 mg | NAC 1200 mg | ||
| N | 7 | 12 | 12 |
| Age (yr) | 44.9 ± 10.2 | 59.5 ± 14.0a | 49.8 ± 16.6 |
| Sex (M/F) | 2/5 | 8/4 | 7/5 |
| Race (C/A/H) | 5/1/1 | 8/4/0 | 4/8/0 |
| Height (cm) | 171.3 ± 13.7 | 171.7 ± 9.0 | 171.2 ± 14.6 |
| Weight (kg) | 68.9 ± 14.3 | 77.9 ± 13.7 | 95.6 ± 31.8a |
| eGFR (ml/min per 1.73 m2) | 89.3 ± 17.4 | NA | NA |
C, Caucasian; A, African American; H, Hispanic; eGFR, estimated glomerular filtration rate; NA, not applicable. Data expressed as mean ± SD.
P < 0.05 versus control.
Pharmacokinetics
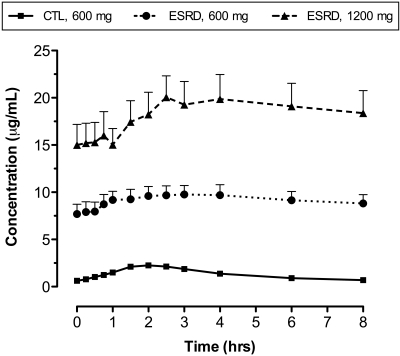
Mean NAC plasma concentration-time curves observed in ESRD and control subjects are depicted in Figure 1, and the corresponding PK parameters are presented in Table 2. Significant dose-related increases in the maximum plasma concentration (Cmax) and AUC values were observed in ESRD patients with NAC 1200 mg versus NAC 600 mg (Figure 1). A doubling of the dose resulted in a 2-fold increase in Cmax and AUC (P < 0.05; Table 2), and NAC clearance was unchanged. As expected with oral sustained-release formulations, the plasma concentration-time curves were flattened, and the time at which Cmax occurred (tmax) was prolonged. Significant PK differences were also found between ESRD patients and healthy subjects. The total clearance (CL/F) of oral NAC 600 mg was reduced by 90% from 56.1 ± 12.7 L/h in control subjects to 4.9 ± 3.5 L/h in ESRD subjects (P < 0.0001), leading to a 7-fold larger NAC AUC0-8 (P < 0.0001) and a 13-fold longer half-life (P < 0.01; Table 2). Cmax increased by 4-fold (P < 0.0001), and tmax was not significantly different. Similar changes in CL/F and half-life were observed in ESRD subjects receiving NAC 1200 mg.
Figure 1.
NAC (mean ± SEM) plasma concentration-time curves observed in ESRD subjects after oral administration of sustained-release NAC 600 mg (circles) or NAC 1200 mg (triangles) and healthy control subjects after administration of NAC 600 mg (squares).
Table 2.
NAC PK parameter estimates
| Parameter | Control Subjects NAC 600 mg | ESRD Subjects |
|
|---|---|---|---|
| NAC 600 mg | NAC 1200 mg | ||
| Cmin (μg/ml) | 0.6 ± 0.4 | 7.5 ± 3.5a | 14.2 ± 8.0a,b |
| Cmax (μg/ml) | 2.5 ± 0.6 | 10.2 ± 3.6a | 20.4 ± 8.8a,b |
| tmax (h) | 1.9 ± 0.6 | 2.6 ± 1.5 | 3.0 ± 1.7a |
| t1/2 (h) | 3.7 ± 0.8 | 51.3 ± 36.7a | 35.4 ± 14.5a |
| CL/F (L/h) | 56.1 ± 12.7 | 4.9 ± 3.5a | 5.7 ± 6.2a |
| AUC0-8 (h·μg/ml) | 10.6 ± 2.3 | 73.6 ± 25.6a | 147.7 ± 65.4a,b |
| AUC0-∞ (h·μg/ml) | 11.2 ± 2.5 | 185.4 ± 117.4a | 374.5 ± 263.5a,b |
Cmin, minimum plasma concentration; t, terminal disposition half-life; AUC, area under the plasma concentration-time curve from 0 to 8 hours (0-8) or extrapolated to infinity (0-∞). Data expressed as mean ± SD.
P < 0.05 versus control.
P < 0.05 versus ESRD (NAC 600 mg).
Pharmacodynamics
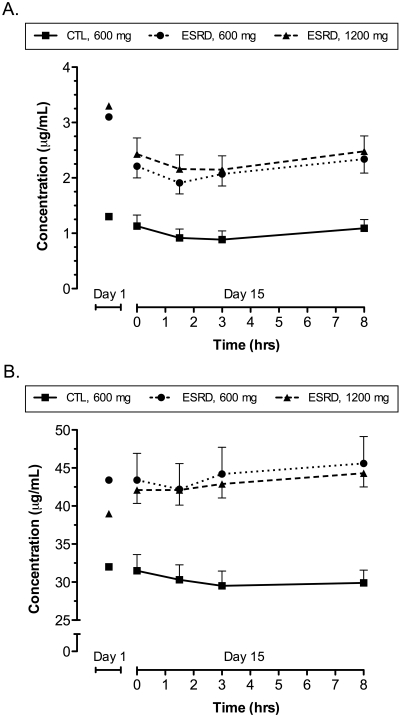
Plasma HCY, CYS, ADMA, SDMA, and protein carbonyl concentrations were significantly higher in all hemodialysis patients than in healthy subjects at baseline (i.e., entry to the study; Table 3). Plasma concentrations of HCY were significantly decreased in healthy control and ESRD subjects after administration of 14 days of NAC 600 or 1200 mg (Table 3). Concentrations of ARG were significantly decreased in ESRD, but not control subjects, only after administration of NAC 600 mg. NAC administration had no effect on CYS, ADMA, SDMA, total glutathione, or protein carbonyl concentrations. A significant relationship was observed between the percentage change of HCY concentrations after 14 days of NAC treatment and NAC AUC0-8 (rs = 0.43, P < 0.05). No other PD parameters exhibited significant relationships with NAC AUC0-8. Mean HCY and CYS plasma concentration-time curves observed during the 8-hour sampling period on day 15 are depicted in Figure 2A and B, respectively. The AUC0-8 of HCY and CYS were not significantly different in ESRD subjects receiving NAC 600 mg compared with those receiving NAC 1200 mg. A significant inverse relationship was observed between plasma concentrations of NAC and HCY (rs = −0.40, P < 0.05), but not CYS (P = NS), in healthy control subjects during the 8-hour sampling period on day 15; no significant relationships were observed between NAC and HCY or CYS in ESRD patients.
Table 3.
PD parameters at baseline on day 1 versus day 15 (predose)
| Parameter | Control Subjects |
ESRD Subjects |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| NAC 600 mg |
NAC 600 mg |
NAC 1200 mg |
|||||||
| Day 1 | Day 15 | %Δ | Day 1 | Day 15 | %Δ | Day 1 | Day 15 | %Δ | |
| HCY (μg/ml) | 1.3 ± 0.6 | 1.1 ± 0.5a | 12.4 ± 8.9 | 3.1 ± 1.2b | 2.4 ± 0.8a | 21.5 ± 16.9 | 3.3 ± 1.4b | 2.4 ± 1.1a | 24.8 ± 7.1 |
| CYS (μg/ml) | 32.0 ± 5.2 | 31.5 ± 5.6 | 1.8 ± 5.4 | 43.4 ± 8.6b | 44.3 ± 11.6 | −1.7 ± 1.6 | 39.0 ± 4.1b | 40.8 ± 5.7 | −4.8 ± 10.9 |
| ADMA (μM) | 0.41 ± 0.08 | 0.38 ± 0.08 | 5.4 ± 18.5 | 0.58 ± 0.07b | 0.58 ± 0.09 | 1.4 ± 11.0 | 0.59 ± 0.09b | 0.60 ± 0.06 | −1.2 ± 11.7 |
| SDMA (μM) | 0.49 ± 0.09 | 0.48 ± 0.11 | 0.3 ± 12.1 | 2.26 ± 0.66b | 2.19 ± 0.59 | 1.5 ± 17.1 | 2.35 ± 0.50b | 2.14 ± 0.34 | 7.5 ± 10.1 |
| ARG (μM) | 96.4 ± 28.9 | 86.4 ± 16.4 | 6.7 ± 21.1 | 86.0 ± 17.5 | 74.8 ± 16.2a | 12.5 ± 12.8 | 69.0 ± 20.6 | 72.5 ± 26.0 | −8.6 ± 36.4 |
| GSH (μM) | 1060 ± 122 | 1059 ± 56 | −0.9 ± 11.6 | 986 ± 204 | 967 ± 131 | −0.8 ± 21.8 | 1121 ± 161 | 1105 ± 118 | 0.9 ± 6.5 |
| CARB (nM/mg protein) | 3.17 ± 0.50 | 3.17 ± 1.11 | −1.0 ± 34.4 | 4.33 ± 1.32b | 3.95 ± 1.13 | 5.6 ± 23.0 | 5.07 ± 1.54b | 4.26 ± 1.28 | 10.5 ± 31.2 |
%Δ, percentage change from day 1. For conversion of HCY to SI units, 7.397 mg/L = 1 μmol/L. For conversion of CYS to SI units, 8.251 mg/L = 1 μmol/L. Data expressed as mean ± SD.
P < 0.05 versus day 1.
P < 0.05 versus control subjects day 1.
Figure 2.
Plasma concentration-time curves (mean ± SEM) of (A) HCY and (B) CYS observed after oral administration of sustained-release NAC 600 mg (circles) or 1200 mg (triangles) to ESRD subjects and NAC 600 mg to healthy control subjects (squares) for 14 d. Baseline concentrations observed on day 1 are also depicted.
Discussion
NAC is an aminothiol-containing antioxidant drug widely used in the prevention or treatment of a number of diseases. Recent strategies aimed at ameliorating oxidative stress-related complications in ESRD include administration of NAC (12,15,23). To date, NAC PK and PD have not been comprehensively and simultaneously assessed in ESRD patients. In this study, we observed NAC plasma concentrations similar to previously reported concentrations ranging from 2.6 to 28 μg/ml in ESRD patients receiving 1200 mg oral NAC twice daily for 30 days (14). Significant dose-related differences in AUC and Cmax were observed in subjects receiving 600 mg versus 1200 mg, but NAC clearance was not impacted by dose (Table 2). However, significant differences in NAC PK were evident in ESRD patients compared with healthy subjects. NAC total clearance was reduced by 90%, leading to a dramatically longer half-life and increased exposure in ESRD subjects (Table 2). PK parameters in control subjects were consistent with previously reported findings in healthy subjects (6,7). NAC total clearance estimates of 4.9 ± 3.5 L/h and 5.7 ± 6.2 L/h in ESRD subjects receiving oral NAC 600 mg and NAC 1200 mg, respectively, are higher than reported in the only other PK study in ESRD patients (10); Soldini and colleagues observed a NAC clearance in ESRD subjects after intermittent intravenous administration (2 g during the first 3 hours of each dialysis session) of 1.25 ± 0.94 L/h (10). The difference in clearance may be due to the route of administration, poor bioavailability, and the contribution of gastrointestinal first pass effects to the elimination of oral NAC, as it appears to be extensively metabolized to cysteine, glutathione, and inorganic sulfite in the gut wall (7). As reported previously, the PK of NAC were highly variable between subjects (6,10,24), with greater variability observed in ESRD subjects than in control subjects.
The reasons for the marked differences in NAC PK between ESRD and control subjects are not clear. Reduced renal clearance is a contributory factor, since it comprises 30% of total body clearance of NAC, but it cannot fully explain the 90% reduction in NAC clearance observed in the present study (6). Altered nonrenal clearance might also be a factor. Nonrenal clearance accounts for 70% of total body clearance of NAC (25), and it is well known that kidney disease can affect nonrenal drug clearance mediated by metabolic enzymes and drug transporters (26). For example, we recently demonstrated that nonrenal clearance of fexofenadine, a substrate of the organic anion transporting polypeptide and P-glycoprotein transporters, is significantly reduced in ESRD patients (27). NAC is extensively metabolized in the gastrointestinal tract and the liver, where it is deacetylated to cysteine by the cytosolic enzyme N-acylamino acid amidohydrolase (acylase I) (28). It is possible that the amount or activity of acylase I is reduced in ESRD. Moreover, NAC uptake by red blood cells is mediated via the anion exchange protein, AE1 (29), and levels of this protein are decreased in hemodialysis patients (30). Therefore, it is also possible that cellular uptake of NAC is reduced, lowering the amount of intracellular NAC available for acylase I metabolism. Given the contribution of nonrenal clearance to the total clearance of NAC, our results clearly show that further study is warranted to elucidate the effect of ESRD on NAC nonrenal clearance pathways.
Plasma concentrations of HCY, but no other PD parameters, were significantly decreased after 14 days of oral NAC administration in control subjects and at both doses of NAC in hemodialysis patients (Table 3). Significant reductions in HCY plasma concentrations after oral (31–33) and intravenous (11,15,34) administration of NAC have been reported previously. Our HCY findings are consistent with those of Friedman et al. who demonstrated a 19% reduction in HCY plasma concentration in ESRD subjects treated with oral NAC 1200 mg twice daily for 4 weeks (14). Others have reported nearly 90% reductions in HCY concentrations after intermittent, intravenous infusion of high-dose NAC during hemodialysis (11,15). However, earlier findings suggest that this approach has short-lived effects, with HCY concentrations returning to baseline values within 24 hours (34). As depicted in Figure 2A, treatment with multiple-dose sustained-release oral NAC resulted in persistently lower steady-state HCY concentrations compared with baseline values that fluctuated relatively little over a dosing interval. Sustained effects on HCY or other surrogates of oxidative stress may be an important consideration in choosing the ideal NAC formulation for use as chronic antioxidant therapy.
There are limitations to this study. Diet and intake of B-vitamins were not controlled. Therefore, it is possible that variable B-vitamin status between subjects may have impacted the HCY lowering effect of NAC (14). Although the 8-hour duration of the PK study on day 15 was substantially shorter than the estimated NAC half-life in ESRD subjects, logistical issues relating to patients' three times weekly hemodialysis schedules and transportation necessitated limiting the study to an interdialytic 8-hour time period. Nevertheless, the significantly elevated NAC plasma concentrations and corresponding AUCs in ESRD versus control subjects provide further evidence that the half-life is much longer in ESRD patients and leads to accumulation after multiple dosing. It is also possible that ESRD alters the dissolution, release properties or absorption of NAC, but it is unlikely that this accounts for the reduced NAC clearance and accumulation observed in ESRD patients. Lastly, administration of oral NAC for the relatively short duration of 14 days may have been insufficient for detecting changes in some of the PD parameters measured in this study. This is supported by recent work in which 16 weeks of vitamin E administration were required to achieve a maximal reduction in the oxidant stress marker F2-isoprostanes (35).
In summary, we present the first PK and PD study of NAC after multiple-dose oral administration to patients with ESRD and demonstrate significant dose-related differences in AUC and Cmax in subjects receiving 600 mg versus 1200 mg. Additionally, significant PK differences were found between ESRD patients and healthy subjects. A 90% reduction in the clearance of oral NAC was observed, which led to a marked increase in systemic exposure. NAC administration resulted in a significant reduction in total HCY plasma concentrations in healthy control and ESRD subjects, but had no effect on several other oxidative stress markers. These findings indicate reduced clearance of oral NAC in ESRD patients and suggest that it may have a limited role in the treatment of oxidative stress related illness.
Disclosures
None.
Acknowledgments
The authors thank Justin Utz for his expert technical assistance and Sid Shastri and Jarrow Formulas for providing the sustained release NAC. During this research, Dr. Nolin was a clinical pharmacologist, and Dr. Himmelfarb was Director, Division of Nephrology and Transplantation, Department of Medicine, ME Medical Center, Portland, ME.
This work was supported by National Institutes of Health (NIH) Research Grant 1 R21 DK074161-01, funded by The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and was presented in part at Renal Week 2007, the American Society of Nephrology Annual Meeting; October 31 through November 5, 2007; San Francisco, CA (Nolin TD, Ouseph R, Himmelfarb J, Ward RA: N-acetylcysteine pharmacokinetics and pharmacodynamics in hemodialysis patients [Abstract]. J Am Soc Nephrol 18: 273A, 2007).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Cotgreave IA: N-acetylcysteine: Pharmacological considerations and experimental and clinical applications. Adv Pharmacol 38: 205–227, 1997 [PubMed] [Google Scholar]
- 4.Dodd S, Dean O, Copolov DL, Malhi GS, Berk M: N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin Biol Ther 8: 1955–1962, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Atkuri KR, Mantovani JJ, Herzenberg LA: N-Acetylcysteine—a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7: 355–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgstrom L, Kagedal B, Paulsen O: Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol 31: 217–222, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Olsson B, Johansson M, Gabrielsson J, Bolme P: Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol 34: 77–82, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Borgstrom L, Kagedal B: Dose dependent pharmacokinetics of N-acetylcysteine after oral dosing to man. Biopharm Drug Dispos 11: 131–136, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Brown M, Bjorksten A, Medved I, McKenna M: Pharmacokinetics of intravenous N-acetylcysteine in men at rest and during exercise. Eur J Clin Pharmacol 60: 717–723, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Soldini D, Zwahlen H, Gabutti L, Marzo A, Marone C: Pharmacokinetics of N-acetylcysteine following repeated intravenous infusion in haemodialysed patients. Eur J Clin Pharmacol 60: 859–864, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Thaha M, Yogiantoro M, Tomino Y: Intravenous N-acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end-stage renal disease. Clin Drug Investig 26: 195–202, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Sahin G, Yalcin AU, Akcar N: Effect of N-acetylcysteine on endothelial dysfunction in dialysis patients. Blood Purif 25: 309–315, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Thaha M, Widodo, Pranawa W, Yogiantoro M, Tomino Y: Intravenous N-acetylcysteine during hemodialysis reduces asymmetric dimethylarginine level in end-stage renal disease patients. Clin Nephrol 69: 24–32, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Friedman AN, Bostom AG, Laliberty P, Selhub J, Shemin D: The effect of N-acetylcysteine on plasma total homocysteine levels in hemodialysis: A randomized, controlled study. Am J Kidney Dis 41: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M: Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end-stage renal failure. Circulation 109: 369–374, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Trimarchi H, Mongitore MR, Baglioni P, Forrester M, Freixas EA, Schropp M, Pereyra H, Alonso M: N-acetylcysteine reduces malondialdehyde levels in chronic hemodialysis patients—a pilot study. Clin Nephrol 59: 441–446, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Nolin TD, McMenamin ME, Himmelfarb J: Simultaneous determination of total homocysteine, cysteine, cysteinylglycine, and glutathione in human plasma by high-performance liquid chromatography: Application to studies of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci 852: 554–561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMenamin ME, Himmelfarb J, Nolin TD: Simultaneous analysis of multiple aminothiols in human plasma by high performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 877: 3274–3281, 2009 [DOI] [PubMed] [Google Scholar]
- 20.de Jong S, Teerlink T: Analysis of asymmetric dimethylarginine in plasma by HPLC using a monolithic column. Anal Biochem 353: 287–289, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gibaldi M, Perrier D: Pharmacokinetics, 2nd Ed.New York, Marcel Dekker, Inc., 1982, pp 451–457 [Google Scholar]
- 22.Faul F, Erdfelder E, Lang AG, Buchner A: G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W: The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: A randomized, controlled trial. Circulation 107: 992–995, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Pendyala L, Creaven PJ: Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during a phase I trial. Cancer Epidemiol Biomarkers Prev 4: 245–251, 1995 [PubMed] [Google Scholar]
- 25.Holdiness MR: Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet 20: 123–134, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Nolin TD, Naud J, Leblond FA, Pichette V: Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 83: 898–903, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, Pichette V, Himmelfarb J: ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol 20: 2269–2276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uttamsingh V, Keller DA, Anders MW: Acylase I-catalyzed deacetylation of N-acetyl-L-cysteine and S-alkyl-N-acetyl-L-cysteines. Chem Res Toxicol 11: 800–809, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Raftos JE, Whillier S, Chapman BE, Kuchel PW: Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int J Biochem Cell Biol 39: 1698–1706, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Maduell F, Fernandez J, Diez J: Alterations of the Cl−/NaCO3− anion exchanger in erythrocytes of uraemic patients. Nephrol Dial Transplant 5: 1018–1022, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Hultberg B, Andersson A, Masson P, Larson M, Tunek A: Plasma homocysteine and thiol compound fractions after oral administration of N-acetylcysteine. Scand J Clin Lab Invest 54: 417–422, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Wiklund O, Fager G, Andersson A, Lundstam U, Masson P, Hultberg B: N-Acetylcysteine treatment lowers plasma homocysteine but not serum lipoprotein(a) levels. Atherosclerosis 119: 99–106, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Roes EM, Raijmakers MT, Peters WH, Steegers EA: Effects of oral N-acetylcysteine on plasma homocysteine and whole blood glutathione levels in healthy, non-pregnant women. Clin Chem Lab Med 40: 496–498, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ventura P, Panini R, Pasini MC, Scarpetta G, Salvioli G: N-Acetyl-cysteine reduces homocysteine plasma levels after single intravenous administration by increasing thiols urinary excretion. Pharmacol Res 40: 345–350, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Roberts LJ, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD: The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med 43: 1388–1393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]