Abstract
Background and objectives: Sleep disorders, including sleep-disordered breathing and periodic limb movements during sleep, are associated with an increased risk for cardiovascular diseases, which are the leading causes of death in patients with ESRD. This study investigated the association between sleep disorders and mortality in patients with ESRD.
Design, setting, participants, & measurements: Thirty patients on maintenance hemodialysis, who were clinically stable for >2 months, underwent overnight polysomnography to evaluate sleep parameters.
Results: All patients were followed for a median of 48 months (range: 14 to 62 months), and 14 of them died during the follow-up period. Among the sleep parameters, the percent of sleep time with arterial oxygen saturation <90% (T <90%), mean arterial oxygen saturation, and periodic limb movement index score were associated with significant increases in the risk of death. However, associations of the apnea–hypopnea index or oxygen desaturation index with mortality were NS. The hazard ratios (95% confidence intervals) for death per one SD increment in the log-transformed T <90% and periodic limb movement index score were 2.10 (1.06 to 4.15) and 2.48 (1.11 to 5.52), respectively, after adjusting for age.
Conclusions: We found that nocturnal hypoxemia and periodic limb movement during sleep, rather than apnea itself, were associated with an increased risk for death in patients with ESRD. However, conclusions from this study should be drawn with caution, because they are limited by the small sample size.
Sleep disorders, including sleep-disordered breathing (SDB) and periodic limb movements during sleep (PLMS), are common features of ESRD. SDB, which includes sleep apnea as its most extreme variant, is characterized by repeated episodes of apnea and hypopnea, resulting in decreased blood oxygen saturation. The prevalence of SDB in patients with ESRD is 40 to 80%, which is much higher than in the general population (1–3). PLMS are stereotypical, involuntary limb movements that occur during sleep, commonly involving the lower extremities. PLMS may affect 50 to 70% of patients with ESRD (4,5).
Sleep disorders are related to an increased risk for cardiovascular complications in ESRD, which are the leading causes of death in this population. Studies of patients with ESRD have shown that nocturnal hypoxemia is associated with left-ventricular hypertrophy (6), coronary artery disease (3), and a higher risk for cardiovascular events (7). However, limited evidence exists as to whether sleep disorders increase the risk for death in patients with ESRD. A recent study of dialysis patients using a sleep questionnaire failed to demonstrate an association between sleep apnea and mortality (8), while another study using self-reported sleep quality scales found that the risk of death was significantly higher for dialysis patients with poor sleep quality (9). Although polysomnography is the gold standard for diagnosing sleep disorders, few studies using polysomnography have investigated this possible association.
We conducted a cohort study of patients who were undergoing long-term hemodialysis. Polysomnography was performed to diagnose sleep disorders and to evaluate sleep parameters, and the blood concentrations of B-type natriuretic peptide (BNP), cardiac troponin-T, and fetuin-A were measured as cardiovascular biomarkers. Our goal was to investigate the possible associations between sleep disorders and mortality in patients with ESRD.
Materials and Methods
Subjects
This study was conducted in a single hemodialysis unit in Korea. Fifty stable patients, who were undergoing maintenance hemodialysis for at least 2 months, were screened to participate in the study. To be enrolled, the patients were required to be more than 18 years of age, without malignancies, and clinically stable (i.e., no symptoms of congestive heart failure, angina pectoris, or infectious disease) for more than 2 months. Thirty of fifty screened patients underwent polysomnography between October 2003 and January 2005 to evaluate sleep parameters. Of the 20 excluded, 15 refused polysomnography, three had cancer, and two could not undergo polysomnography secondary to transfer. Only the results for the 30 participating patients were analyzed. This study protocol was approved by our institutional review board, and written informed consent was obtained from each enrolled patient.
Sleep Study
The polysomnographic studies were performed using a validated recording unit (Embla System; Medcare, Reykjavik, Iceland). We recorded electroencephalography (C3-A2, C4-A1, O1-A2, O2-A1), electrooculography (bilateral outer canthi), electromyography (bilateral anterior tibialis and mentalis muscles), electrocardiography, airflow (bilateral nares and mouth), respiratory effort (chest and abdomen), and arterial oxygen saturation (SaO2; finger pulse oximetry). All variables were recorded continuously on a computerized data acquisition system and stored on an optical disc for later analysis.
All polysomnographies were scored manually, according to established criteria. Apnea was defined as the absence of airflow for more than 10 seconds. Hypopnea was defined as a partial reduction in respiratory airflow to below 50% of the sleeping baseline level for more than 10 seconds, with or without an associated fall in SaO2. The apnea hypopnea index (AHI) was defined as the number of apnea and hypopnea episodes per hour of sleep. To assess the severity of hypoxemia induced by SDB, we measured the duration of SaO2 <90% and expressed it as a percent of the total sleeping time (T <90%) (10). The oxygen desaturation index (ODI) was defined as the number of events per hour in which the SaO2 decreased by 4% or greater from the baseline level (11). The mean SaO2 during sleep was calculated by averaging the high and low values for each 30-second epoch. Periodic limb movements were defined as more than four consecutive leg movements lasting 0.5 to 5 seconds in 5 to 90 seconds. The periodic limb movement index (PLMI) was defined as the number of periodic limb movements per hour. PLMS was diagnosed when the PLMI was 15 or greater, and the limb movements were not related to respiratory events.
Biochemical Analysis
The results of monthly serum biochemical determinations were collected for each of the 6 months immediately following each sleep study. Routine serum chemistry variables including cholesterol, albumin, and phosphate were analyzed using standard methods. The C-reactive protein concentration was measured every 3 months using a latex agglutination method (Denka, Tokyo, Japan) with a detection limit of 0.01 mg/dl.
The BNP concentration was determined in EDTA-anticoagulated whole blood within 2 hours of sample collection. BNP concentrations were measured once using a fluorescence immunoassay (Triage BNP test; Biosite, San Diego, CA) with a detection range of 5 to 5000 pg/ml.
Three predialysis blood samples were collected and stored at 6-month intervals to determine fetuin-A and troponin-T levels. Serum fetuin-A was determined with a human fetuin-A enzyme linked immunosorbent assay kit (BioVendor GmbH, Heidelberg, Germany) using a two-site sandwich technique. Cardiac troponin-T concentrations were measured using an electrochemiluminescence immunoassay (Elecsys Troponin T STAT Immunoassay; Roche Diagnostics, Indianapolis, IN) with a detection limit of 0.01 ng/ml.
Follow-up
Patients were followed until death, transplantation, or study termination, and the follow-up continued until December 2008. The primary endpoint was survival time measured from the date of polysomnography. The secondary endpoint was measured as the time to the first cardiovascular event, which was defined as myocardial infarction, stroke, hospitalization for congestive heart failure, coronary revascularization, amputation for ischemic gangrene, or sudden cardiac death. Each event was reviewed and assessed by a physician unaware of the polysomnography data. As part of the review process, all available medical information concerning each death was collected. This information included study and hospitalization records.
Statistical Analysis
The results of biochemical determinations were averaged to obtain simple or geometric means for each measurement. Comparisons between deceased and surviving patients were conducted using an unpaired t test or the Mann-Whitney U-test, as appropriate. The distribution of categorical variables among the groups was assessed using a Fisher exact test. Spearman rank test was used to assess the correlations between the sleep parameters and the clinical and biochemical risk factors. The sleep data were categorized into the lowest, middle, and highest thirds. The Kaplan-Meier method was used to construct cumulative time-to-event curves, and the intergroup differences were evaluated using the log-rank test. Cox regression models were used to control for the effects of other clinical/biochemical variables on mortality. Multivariate models were restricted to one sleep parameter and one potential confounding variable at a time due to the small sample size. All statistical analyses were performed with the SPSS software package (version 13.0; SPSS Inc, Chicago, IL). Two-sided P values of <0.05 were considered statistically significant.
Results
At baseline, the mean age of the 30 participants (21 men and 9 women) was 55.8 ± 10.6 years. The causes of ESRD included chronic glomerulopathy in eight patients, diabetic nephropathy in 15 patients, hypertensive nephropathy in three patients, and polycystic kidney disease in one patient. The causes of renal failure were unknown in three patients. Eight patients had a history of cardiovascular disease, including acute myocardial infarction, congestive heart failure, stroke, and peripheral vascular disease. Twenty-eight patients were receiving antihypertensive treatment, and 28 patients were receiving erythropoietin treatment. Hypnotics had been intermittently administered to four patients for insomnia but were discontinued for at least 7 days before the polysomnographic study. All of the patients were treated with conventional hemodialysis using bicarbonate. The duration of each dialysis session was 4 hours.
For the polysomnographic studies, the median AHI of the 30 dialysis patients was 22.0 (range: 0.7 to 66.9), and 25 of them had SDB with an AHI greater than 5.0. Episodes of obstructive, central, and mixed apnea and hypopnea were distributed among the patients with SDB. Nine patients had primarily obstructive apnea, and four patients had primarily central apnea. Another nine patients had both obstructive and central apnea, and the remaining three patients had mainly hypopnea with a few episodes of apnea. No patient had Cheyne-Stokes respiration. The median PLMI was 36.9 (range: 0.9 to 207.8), and 21 patients had PLMS of varying severities.
All patients were followed for a median of 48 months (range: 14 to 62 months), from the date of polysomnography until death, transplantation, or study termination. Of the 30 patients studied, 14 died during the follow-up period. In 12 of these patients, the causes of death were cardiovascular- or infection-related events. Suicide was the cause of death in one patient, and gastrointestinal bleeding associated with gastric cancer was the cause in another. Table 1 shows the results of comparisons of baseline characteristics between patients in the deceased and surviving groups. Among the sleep parameters, the T <90% and the PLMI scores were higher and the mean SaO2 was lower in the deceased patients than in the survivors. The ODI showed a trend toward lower values in the deceased patients, although the difference was not statistically significant. Furthermore, the AHI score was not different between the two groups.
Table 1.
Comparison of baseline characteristics between deceased and surviving patients
| Deceased (n = 14) | Survivors (n = 16) | P | |
|---|---|---|---|
| Survival time (months; median [range]) | 33 (15 to 53) | 57 (28 to 62) | <0.001 |
| Clinical characteristics | |||
| Age (years; mean ± SD) | 60.3 ± 11.9 | 51.8 ± 7.7 | 0.026 |
| Males (n) | 10 | 11 | 1.000 |
| Diabetics (n) | 8 | 7 | 0.715 |
| Previous CV event (n) | 6 | 2 | 0.101 |
| Smokers (n) | 4 | 1 | 0.157 |
| Systolic BP (mmHg; mean ± SD) | 142.5 ± 9.6 | 144.3 ± 7.6 | 0.578 |
| Diastolic BP (mmHg; mean ± SD) | 77.9 ± 8.4 | 82.7 ± 6.4 | 0.089 |
| Body mass index (kg/m2; mean ± SD) | 21.8 ± 2.7 | 23.5 ± 3.9 | 0.195 |
| Dialysis duration (months; median [range]) | 44 (27 to 118) | 8 (2 to 108) | 0.064 |
| Biochemical characteristics | |||
| Albumin (g/dl; mean ± SD) | 3.84 ± 0.13 | 3.86 ± 0.18 | 0.810 |
| Total cholesterol (mg/dl; mean ± SD) | 155 ± 31 | 154 ± 31 | 0.946 |
| Phosphate (mg/dl; mean ± SD) | 5.08 ± 0.90 | 5.19 ± 0.84 | 0.224 |
| C-reactive protein (mg/dl; median [range]) | 0.22 (0.03 to 0.86) | 0.17 (0.03 to 0.72) | 0.166 |
| Fetuin-A (mg/dl; median [range]) | 27.0 (22.0 to 32.7) | 29.9 (24.9 to 41.2) | 0.005 |
| Troponin-T (ng/ml; median [range]) | 0.07 (0.01 to 0.76) | 0.04 (0.01 to 0.28) | 0.193 |
| BNP (pg/ml; median [range]) | 894 (161 to 5000) | 525 (50 to 2960) | 0.022 |
| Kt/V (mean ± SD) | 1.55 ± 0.32 | 1.45 ± 0.19 | 0.288 |
| nPCR (g/kg per d; mean ± SD) | 1.10 ± 0.28 | 1.17 ± 0.21 | 0.420 |
| Sleep parameters | |||
| Total sleep time (minutes; median [range]) | 376 (181 to 474) | 391 (242 to 491) | 0.400 |
| Sleep efficiency (%; median [range]) | 83.6 (40.3 to 97.6) | 85.9 (61.7 to 96.9) | 0.667 |
| AHI (/h; median [range]) | 26.8 (0.9 to 40.8) | 19.0 (0.7 to 66.9) | 0.355 |
| ODI (/h; median [range]) | 19.2 (0.4 to 36.0) | 7.9 (0.5 to 50.8) | 0.058 |
| T <90% (%; median [range]) | 5.0 (0.0 to 24.0) | 0.3 (0.0 to 17.2) | 0.003 |
| Mean SaO2 (%; median [range]) | 93.3 (87.6 to 98.1) | 96.6 (91.8 to 98.1) | 0.003 |
| PLMI (/h; median [range]) | 62.9 (4.5 to 207.8) | 17.4 (0.9 to 161.0) | 0.022 |
CV, cardiovascular; nPCR, normalized protein catabolic rate.
Correlations between sleep parameters and baseline clinical and biochemical risk factors are shown in Table 2. The AHI score correlated significantly with age, the presence of diabetes, and the blood level of fetuin-A. The AHI also correlated with ODI and T <90% but did not correlate with PLMI. The ODI correlated with patient age, fetuin-A level, and protein catabolic rate. The T <90% correlated with age, a previous cardiovascular event, and fetuin-A, troponin-T, and BNP levels. However, the PLMI score did not correlate significantly with any clinical or biochemical risk factor.
Table 2.
Correlations of sleep parameters with clinical and biochemical risk factors (n = 30)a
| AHI | ODI | T <90% | PLMI, /h | |
|---|---|---|---|---|
| Clinical risk factors | ||||
| Age | 0.367b | 0.365b | 0.398b | 0.308 |
| Gender | 0.357 | 0.324 | 0.291 | 0.088 |
| Diabetes | 0.389b | 0.227 | 0.139 | 0.050 |
| Previous CV event | 0.218 | 0.287 | 0.445b | 0.261 |
| Body mass index | 0.059 | 0.205 | 0.073 | −0.156 |
| Dialysis duration | 0.003 | 0.057 | 0.307 | 0.308 |
| Biochemical risk factors | ||||
| Albumin | −0.078 | −0.317 | −0.127 | −0.101 |
| C-reactive protein | −0.045 | 0.110 | 0.225 | 0.358 |
| Fetuin-A | −0.660c | −0.664c | −0.475c | −0.291 |
| Troponin-T | 0.282 | 0.284 | 0.389b | 0.273 |
| BNP | 0.068 | 0.239 | 0.372b | 0.310 |
| Kt/V | −0.046 | −0.276 | −0.316 | −0.076 |
| nPCR | −0.310 | −0.380b | −0.341 | −0.103 |
| Sleep parameters | ||||
| AHI | 0.779c | 0.404b | 0.019 | |
| ODI | 0.779c | 0.778c | 0.128 | |
| T <90% | 0.404b | 0.778c | 0.466c | |
| PLMI | 0.019 | 0.128 | 0.466c |
Values are expressed as correlation coefficients.
P < 0.05.
P < 0.01.
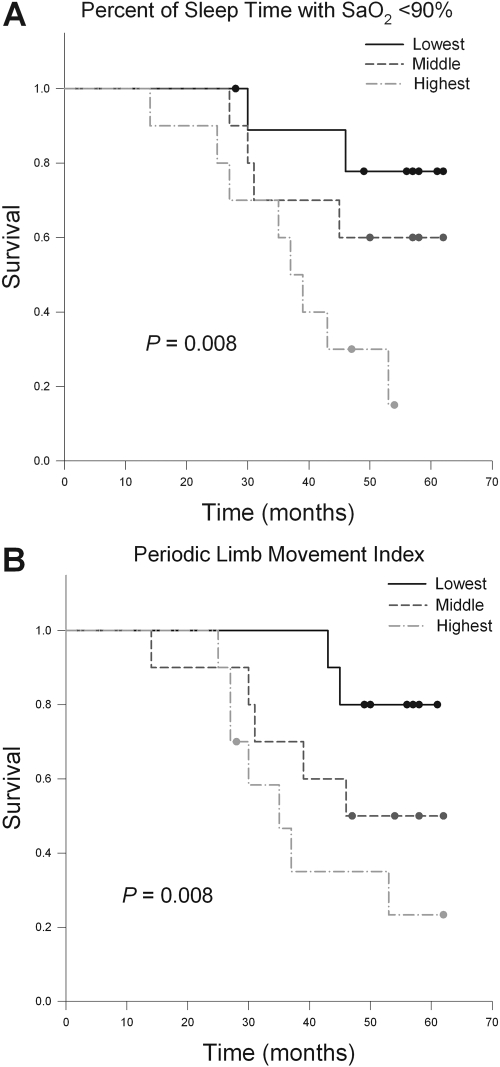
The Kaplan-Meier survival curves for patients grouped by T <90% are shown in Figure 1A. The T <90% was associated with mortality (P = 0.008): the mean survival times (95% confidence intervals [CI]) for the lowest, middle, and highest tertiles of T <90% were 57 months (50 to 64), 51 months (41 to 60), and 38 months (30 to 46), respectively. The mean SaO2 during sleep was also associated with mortality (P = 0.001): the mean survival times (95% CI) for the lowest, middle, and highest mean SaO2 tertiles were 36 months (28 to 44), 51 months (43 to 59), and 58 months (52 to 65), respectively. The Kaplan-Meier survival curves for patients grouped by PLMI are shown in Figure 1B. The PLMI was associated with mortality (P = 0.008): the mean survival times (95% CI) for the lowest, middle, and highest PLMI tertiles were 57 months (53 to 62), 47 months (37 to 57), and 40 months (31 to 50), respectively. However, the AHI or ODI failed to significantly predict mortality in the Kaplan-Meier survival analyses.
Figure 1.
Kaplan-Meier survival curves for patients with the lowest (0 to 0.3%), middle (0.5 to 4.2%), and highest (4.5 to 24.0%) tertiles of the percent of sleep time with an SaO2 <90% (A) and for patients with the lowest (0.9 to 16.4/h), middle (18.4 to 55.0/h), and highest (70.8 to 207.8/h) tertiles of the periodic limb movement index (B).
Fourteen patients suffered from at least one cardiovascular event during the study period. The T <90%, mean SaO2, and PLMI score were also associated with significant increases in the risk of experiencing a cardiovascular event (P = 0.035, P = 0.005, and P = 0.010, respectively). By contrast, the association of AHI or ODI with cardiovascular events was NS.
The independent prognostic values of T <90%, mean SaO2, and PLMI were assessed using Cox regression models, which included one sleep parameter and one potential confounder at a time (Table 3). The clinical/biochemical variables that correlated significantly with a sleep parameter or were different between deceased and surviving patients were introduced as covariates. In most cases, controlling for the effect of each variable did not result in a loss of statistical significance for T <90% or mean SaO2 as a predictor of mortality. However, the hazard ratio for T <90% became nonsignificant when controlling for fetuin-A, and the hazard ratio for mean SaO2 was slightly attenuated and became borderline significant after controlling for a previous cardiovascular event or BNP. The relationship between the PLMI score and mortality remained robust and statistically significant in all cases.
Table 3.
Cox regression analyses demonstrating the association between sleep parameters and death from any cause (n = 30)a
| Covariate | T <90%b | Mean SaO2 | PLMIb |
|---|---|---|---|
| Unadjusted | 2.40 (1.26 to 4.58) | 0.56 (0.36 to 0.88) | 2.49 (1.20 to 5.13) |
| Age | 2.10 (1.06 to 4.15) | 0.58 (0.34 to 0.97) | 2.48 (1.11 to 5.52) |
| Dialysis duration | 2.36 (1.20 to 4.65) | 0.56 (0.34 to 0.90) | 2.37 (1.13 to 4.98) |
| Diabetes | 2.39 (1.25 to 4.56) | 0.57 (0.36 to 0.89) | 2.47 (1.20 to 5.12) |
| Previous CV event | 2.16 (1.08 to 4.35) | 0.63 (0.37 to 1.07) | 2.55 (1.16 to 5.59) |
| Fetuin-A | 1.77 (0.88 to 3.58) | 0.49 (0.26 to 0.91) | 2.36 (1.08 to 5.18) |
| Troponin-Tb | 2.27 (1.16 to 4.46) | 0.61 (0.38 to 0.99) | 2.38 (1.13 to 5.03) |
| BNPb | 2.23 (1.13 to 4.40) | 0.66 (0.41 to 1.06) | 2.44 (1.13 to 5.26) |
Values are expressed as hazard ratios with 95% CI per one SD increment of each sleep parameter.
Log-transformed values were used for the analyses.
Discussion
In this cohort, PLMI was the strongest and most consistent predictor of death in patients with ESRD. This finding provides additional evidence supporting the only other study of patients with ESRD that investigated the association between PLMS and mortality (12). Additionally, the T <90% and mean SaO2, parameters representing the severity of nocturnal hypoxemia, were also associated with an increased risk for mortality. To our knowledge, this is the first study showing an association between nocturnal hypoxemia and mortality in patients with ESRD. However, AHI, the diagnostic criterion standard for sleep apnea, was not a significant predictor of mortality.
Polysomnography, the gold standard for diagnosing sleep disorders, is especially useful in dialysis patients because uremic symptoms can mask sleep symptoms. The only previous study based on polysomnography that tested the association between sleep disorders and mortality in ESRD was a case series of 29 patients with disrupted sleep or daytime sleepiness (12). That study showed that PLMS, rather than SDB, was an independent predictor of mortality. In agreement with that study, we also found that the PLMI, rather than the AHI, was related to mortality. However, T <90%, a marker of nocturnal hypoxemia, significantly correlated with AHI and was a predictor of mortality in our patients. A few differences existed among the subjects included in the previous study and the present one. The previous study involved only patients who had problems regarding sleep or day time sleepiness and were referred to the sleep center. In the present study, we selected patients irrespective of the presence of sleep symptoms, such that some showed no evidence of SDB. Furthermore, the median survival time of patients was different between the two studies: our patients survived and were followed-up for a longer time than patients in the previous study. The longer survival time, extended follow-up time, and the wider range of SDB severity might have allowed us to identify the significant effect of nocturnal hypoxemia on mortality in our study.
We also found that T <90% was significantly correlated with the cardiovascular biomarkers fetuin-A, BNP, and troponin-T, and associated with an increase in the risk of experiencing a cardiovascular event. Nocturnal hypoxemia has long been implicated as a cardiovascular risk factor in dialysis patients. Previous studies of patients with ESRD have demonstrated that nocturnal hypoxemia during sleep is linked to left ventricular concentric hypertrophy (6) and incident cardiovascular events (7). Overall, these observations provide evidence for a link between nocturnal hypoxemia and cardiovascular diseases in patients with ESRD. Therefore, we believe that severe nocturnal hypoxemia triggered by SDB is associated with an increased risk of cardiovascular complications, which may increase the risk of death in ESRD patients.
In particular, the low fetuin-A concentration was strongly linked to sleep parameters, indicating severe SDB. Fetuin-A is a negative acute phase protein that inhibits vascular calcification. Studies of patients with ESRD have shown that lower levels of fetuin-A are associated with a higher prevalence of severe coronary calcification (13,14). Additionally, we previously showed that severe SDB was associated with prevalent severe coronary calcification (3). These findings suggest that SDB, fetuin-A level, and vascular calcification are interrelated and/or overlapping, and that they represent a common pathway leading to adverse outcomes in ESRD. Further studies will be needed to confirm these associations and to explore whether SDB treatment has a beneficial effect on such cardiovascular risk factors in patients with ESRD.
In this study, the PLMI score was associated with both a poor cardiovascular outcome and mortality. A hypothesis regarding the relationship between PLMS and outcome considers the effect on nocturnal BP. General population studies have reported that PLMS is associated with repetitive sleep-time elevations in BP and heart rate (15,16). Overactivity of the sympathetic nervous system caused by PLMS seems to be responsible for the consequent increase in BP variability and nondipper BP pattern. Several large-scale studies of nonrenal patients have found that sleep-time BP, determined by ambulatory monitoring, is a much better predictor of cardiovascular risk and mortality than daytime BP (17,18). Moreover, a cohort study of 168 nondiabetic hemodialysis patients has shown that the night/day systolic BP ratio is the sole BP variable associated with all-cause and cardiovascular mortality (19). Therefore, the possible link between PLMS and sleep-time hypertension can at least partially explain the association between PLMS and poor outcomes.
To date, no reliable guidelines have been established for the treatment of sleep disorders in patients with ESRD. Although data are limited, benzodiazepines and dopamine agonists are often used to treat PLMS. However, periodic limb movements improve only slightly in dialysis patients by treatment with pergolide, a dopamine agonist (20). Furthermore, a recent study has reported that benzodiazepine or zolpidem use is common in incident dialysis patients and may be associated with greater mortality (21). Optimal uremia control, in the form of nocturnal hemodialysis, may have a promising therapeutic effect (22,23). Improvement in SDB following conversion from conventional to nocturnal hemodialysis has the potential to reduce mortality risk. However, whether sleep disorder treatment in patients with ESRD affects the high morbidity and mortality of this disease has yet to be shown.
The results of our study should be interpreted with caution because of certain limitations. First, the sample size was small, so the power to detect associations with statistical analyses was limited. Specifically, all possible confounders could not be concurrently introduced in the multivariate analysis. For more complete analyses, a sufficient number of events must be observed for each covariate considered. Second, subjects were on long-term hemodialysis at a single center, which can make it difficult to draw general conclusions and can lead to survivor or other selection biases. Third, this observational study does not allow for conclusions about causal relationships. Clearly, properly designed interventional studies will establish whether sleep disorders and mortality are causally linked in patients with ESRD.
In conclusion, this study provided clinical evidence that sleep disorders are associated with the risk of death and cardiovascular events in patients with ESRD. Further studies are needed to elucidate the mechanistic and causal relationships between sleep disorders and mortality in patients with ESRD.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Jurado-Gamez B, Martin-Malo A, Alvarez-Lara MA, Munoz L, Cosano A, Aljama P: Sleep disorders are underdiagnosed in patients on maintenance hemodialysis. Nephron Clin Pract 105: c35–42, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Markou N, Kanakaki M, Myrianthefs P, Hadjiyanakos D, Vlassopoulos D, Damianos A, Siamopoulos K, Vasiliou M, Konstantopoulos S: Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung 184: 43–49, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Jung HH, Han H, Lee JH: Sleep apnea, coronary artery disease, and antioxidant status in hemodialysis patients. Am J Kidney Dis 45: 875–882, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Rijsman RM, de Weerd AW, Stam CJ, Kerkhof GA, Rosman JB: Periodic limb movement disorder and restless legs syndrome in dialysis patients. Nephrology (Carlton) 9: 353–361, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Hohl-Radke F, Aedtner F, Domrose U, Neumann KH, Staedt J: [Restless legs syndrome in patients on dialysis for kidney insufficiency: Effect of medication]. Dtsch Med Wochenschr 133: 71–75, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Candela V, Labate C, Tassone F: Left ventricular hypertrophy and nocturnal hypoxemia in hemodialysis patients. J Hypertens 19: 287–293, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Zoccali C, Mallamaci F, Tripepi G: Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol 13: 729–733, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Argekar P, Griffin V, Litaker D, Rahman M: Sleep apnea in hemodialysis patients: Risk factors and effect on survival. Hemodial Int 11: 435–441, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R: Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23: 998–1004, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb JD, Schwartz AR, Marshall J, Ouyang P, Kern L, Shetty V, Trois M, Punjabi NM, Brown C, Najjar SS, Gottlieb SS: Hypoxia, not the frequency of sleep apnea, induces acute hemodynamic stress in patients with chronic heart failure. J Am Coll Cardiol 54: 1706–1712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou D, Grote L, Peker Y, Lindblad U, Hedner J: Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep 29: 367–374, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Benz RL, Pressman MR, Hovick ET, Peterson DD: Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis 35: 1052–1060, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Moe SM, Reslerova M, Ketteler M, O'Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX: Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 67: 2295–2304, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Coen G, Manni M, Agnoli A, Balducci A, Dessi M, De Angelis S, Jankovic L, Mantella D, Morosetti M, Naticchia A, Nofroni I, Romagnoli A, Gallucci MT, Tomassini M, Simonetti G, Splendiani G: Cardiac calcifications: Fetuin-A and other risk factors in hemodialysis patients. ASAIO J 52: 150–156, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA: Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology 68: 1213–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS: Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol 118: 1923–1930, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E: Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 46: 156–161, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G: Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: Follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 111: 1777–1783, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Tripepi G, Fagugli RM, Dattolo P, Parlongo G, Mallamaci F, Buoncristiani U, Zoccali C: Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int 68: 1294–1302, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Pieta J, Millar T, Zacharias J, Fine A, Kryger M: Effect of pergolide on restless legs and leg movements in sleep in uremic patients. Sleep 21: 617–622, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Winkelmayer WC, Mehta J, Wang PS: Benzodiazepine use and mortality of incident dialysis patients in the United States. Kidney Int 72: 1388–1393, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hanly PJ, Pierratos A: Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med 344: 102–107, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hanly PJ, Gabor JY, Chan C, Pierratos A: Daytime sleepiness in patients with CRF: impact of nocturnal hemodialysis. Am J Kidney Dis 41: 403–410, 2003 [DOI] [PubMed] [Google Scholar]