Abstract
Background and objectives: Children and adolescents with chronic kidney disease (CKD) are at high risk for cardiovascular morbidity and mortality. A systemic arteriopathy and cardiomyopathy has been characterized in pediatric dialysis patients by the presence of morphologic and functional abnormalities.
Design, setting, participants, & measurements: The Cardiovascular Comorbidity in Children with CKD (4C) Study is a multicenter, prospective, observational study aiming to recruit more than 600 children, aged 6 to 17 years, with initial GFR of 10 to 45 ml/min per 1.73 m2. The prevalence, degree, and progression of cardiovascular comorbidity as well as its association with CKD progression will be explored through longitudinal follow-up. The morphology and function of the heart and large arteries will be monitored by sensitive noninvasive methods and compared with aged-matched healthy controls. Multiple clinical, anthropometric, biochemical, and pharmacologic risk factors will be monitored prospectively and related to the cardiovascular status. A whole-genome association study will be performed to identify common genetic variants associated with progression of cardiovascular alterations and/or renal failure. Monitoring will be continued as patients reach end-stage renal disease and undergo different renal replacement therapies.
Results: While cardiovascular morbidity in adults is related to older age and additional risk factor load (e.g., diabetes), the role of CKD-specific factors in the initiation and progression of cardiac and vascular disease are likely to be characterized with greater sensitivity in the pediatric age group.
Conclusions: The 4C study is expected to provide innovative insight into cardiovascular and renal disease progression in CKD.
Children and adolescents with chronic kidney disease (CKD) are at high risk for cardiovascular events (1). In young adults with end-stage renal disease, cardiovascular mortality is increased 500- to 1000-fold compared with the general population (2).
Young CKD patients usually do not present with clinical symptoms of cardiovascular disease (CVD). Ischemic heart disease and myocardial infarction are very rare in childhood. However, even children may present with subclinical CVD, and significant structural and functional changes of the heart and the large arteries can be detected by sensitive methods (3,4).
Recently, noninvasive measurements of vascular morphology and function such as carotid artery intima-media thickness (cIMT), pulse wave velocity (PWV), and the pulse wave augmentation index (AI) have been established as valid surrogate markers of arteriopathy in adult CKD patients (5). Similar to echocardiographic findings (6), these surrogate markers are highly predictive for future cardiovascular events.
The pediatric population appears uniquely suited to study the effects of CKD on the cardiovascular system due to the virtual absence of vascular morbidity related to aging, diabetes, and smoking. However, the factors underlying early cardiovascular morbidity in CKD and their relative contribution are poorly understood. Moreover, the natural evolution of cardiovascular lesions and their relationship to kidney disease progression are largely unknown due a lack of prospective observational studies in a sufficiently large cohort of pediatric CKD patients.
To improve our understanding of the causes and consequences of cardiovascular comorbidity in children with CKD, a consortium of pediatric nephrologists in Europe has joined to perform a long-term prospective observational study monitoring the cardiovascular health of children as they advance through successive stages of CKD. The Cardiovascular Comorbidity in Children with CKD (4C) Study will follow up to 625 patients in more than 50 pediatric nephrology units in 14 European countries (http://www.4c-study.org).
Materials and Methods
Objectives
The 4C Study will systematically evaluate the prevalence, clinical symptoms, and progression of cardiovascular and kidney disease and assess the impact of potential risk factors, as well as the influence of genetic variables on disease progression.
Measurements of morphologic and functional characteristics of the heart and large arteries will serve as surrogate end points for CVD in this study.
The morphologic end points are the progression of cIMT and left ventricular mass index (LVMI). The functional end points are progression of local (stiffness of carotid artery) and systemic arterial stiffness (PWV) and myocardial dysfunction (tissue Doppler).
Possible associations of these end points with multiple potential clinical, anthropometric, biochemical, and pharmacologic risk factors will be explored. We aim to develop and validate a clinically applicable individual cardiovascular risk score.
Furthermore, possible genetic risk factors for early manifestation of progressive vascular lesions and progressive kidney disease might be identifiable by genome-wide single-nucleotide polymorphism (SNP)-screening and haplotype analysis.
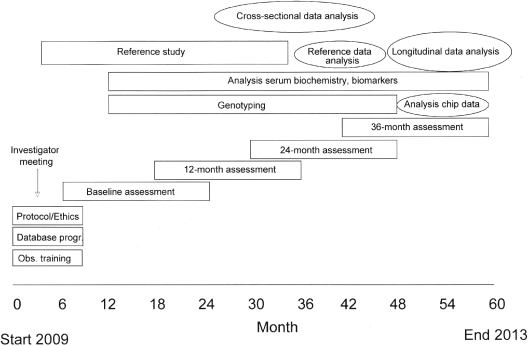
Study Organization (Figure 1)
Figure 1.
Study flow chart.
Patients meeting the inclusion criteria will be enrolled by local investigators. The course of estimated GFR (7) and proteinuria will be followed every 6 months. Every 12 months, the patients will undergo a detailed cardiovascular assessment comprising sonographic assessment of cIMT and elasticity, PWV, AI, left ventricular mass and contractility, 24-hour BP monitoring, as well as profiling of serum markers. A blood sample for DNA analysis will be obtained at a single time point.
Eight investigators will serve as regional coordinators. Each coordinator will be responsible for the acquisition of data and biomaterials in 5 to 10 study centers and will perform the sonographic assessments and pulse wave studies at the individual centers once per year. To guarantee optimal reproducibility and quality of the investigations, the coordinators will be thoroughly instructed in the use of the diagnostic methodologies in several joint training sessions before start of the study. Echocardiographic assessments will be performed either by a local cardiologist or by the regional coordinator. Biosamples will be collected by the regional coordinators and stored in a central biorepository. Regular investigator meetings will be held to synchronize study activities, discuss results, and exchange experiences.
Patient follow-up will be continued when end-stage renal disease is attained and renal replacement therapy started. The evolution of cardiovascular risk factors, function, and morphology will be evaluated prospectively by structured subanalysis in patients undergoing maintenance dialysis (4C-D substudy) and pre-emptive or postdialysis transplantation (4C-T substudy).
The 4C Study is funded by the KfH Foundation for Preventive Medicine. This nonprofit organization provides support for selected prospective observational studies in the field of CKD in Germany (http://www.kfh-stiftung-praeventivmedizin.de/). A core set of clinical and laboratory data will be collected in each funded study and submitted to a central database for integrative analysis. All studies are reviewed annually by a steering committee.
Study Population
The 4C Study is a prospective observational cohort study in pediatric CKD patients. Inclusion criteria are age 6 to 17 years and estimated GFR 10 to 45 ml/min per 1.73 m2. Exclusion criteria are the presence of active systemic vasculitis, renal vascular anomalies, coexisting primary cardiovascular anomalies, and anomalies of the limbs preventing diagnostic procedures. Children who reach end-stage renal disease will be continuously followed while on renal replacement therapy.
It is planned to follow the patients throughout childhood until transition to an adult treatment center. The study will be continued for at least 3 years, with a possible extension to 8 years if funding can be secured.
After the end of the funded period, it is planned that patients will be followed in a registry of cardiovascular and renal disease progression, in which any locally available clinical, anthropometric, and biochemical screening parameters will continue to be documented through attainment of adult age.
Clinical and Laboratory Methods
Cardiovascular Monitoring (Table 1)
Table 1.
Synopsis of data obtained in the 4C Study
| Cardiovascular monitoring (annual intervals) |
|
| Clinical and anthropometric measurements | |
| At time of enrollment: | Baseline evaluation (e.g., patient data, renal and extra-renal diagnoses, gestational age, birth weight and length, cardiovascular medical history of patient and family). |
| At 6-month intervals: | Height, weight, BMI, pubertal stage, current medication. |
| At 12-month intervals: | Body composition analysis (by multifrequency bioelectrical impedance analysis). |
| Monitoring of renal function: | |
| At 6-month intervals: | Estimated GFR (plasma Cystatin C clearance), serum creatinine, urinary protein excretion. |
| Biochemical and biomarker monitoring (blood testing) | |
| At 12-month intervals: | Serum lipid profile, ultrasensitive CRP, uric acid, 1,84-/7,84-PTH, 25-OH-Vit.D3, 1,25-Vit.D3, osteoprotegerin, fetuin-A, homocysteine, ADMA, adiponectin, leptin. |
| Biomarkers of oxidative and carbonyl stress. |
Carotid sonography will be performed using local ultrasound devices. Device settings and measurement parameters as well as the examination procedures will be standardized according to international radiologic consensus recommendations (8). All sonography findings will be documented digitally and cross-validated in a blinded manner. This will guarantee a maximum of objectivity and minimize the residual interobserver-variability.
ECGs and echocardiographies will be performed by the local pediatric cardiologists, standardized according to the guidelines of the American Society of Echocardiography. Whenever possible, additional tissue doppler imaging will be performed. All evaluations will be video-documented and centrally analyzed.
All centers are equipped with ambulatory BP monitoring devices (Spacelab ABPM device 90207-2Q; Spacelabs Medical, Issaquah, WA).
Standard operating procedures for ABPM have been placed on the study web site for continuous reference by participating centers.
Coronary calcification will be evaluated by multislice computed tomography (CT) scan if available in the individual centers (optional).
Biochemical Monitoring, Analysis of Biomarkers for CVD
Blood samples will be aliquoted, and analyses of biochemical parameters will be performed in a central laboratory (Synlab, Heidelberg). Biomarker analyses will be performed in several specialized laboratories.
Genome-Wide Association Study
Genome-wide screening will be performed using the Affymetrix 6.0 SNP Chip containing more than one million SNP markers for haplotype analysis. Hybridization and analysis of the chips will be performed at the Cologne Center of Genomics.
Data Acquisition and Handling
All clinical data obtained at the 6-month patient visits are regularly transmitted to the central database by online data entry via the study web site. Laboratory results will also be transmitted electronically and stored in the central database on a central server at Heidelberg University Computing Facilities. The web site and database will be on a secure server containing a public and a password-protected domain.
Patient data will be pseudonymized at the local study centers and will be transferred in an anonymized fashion to the central database. Likewise, transmission of the core data set shared with the other studies within the network of the KfH Foundation for Preventive Medicine will be in anonymized form. All data management will be performed according to the principles of confidentiallity and the regulations of national data protection laws (e.g., German Bundesda-tenschutzgesetz [BDSG]).
Reference Data—Examinations in Healthy Controls
Reference values for cIMT measurements are available for subjects aged 10 to 20 years (9). For children aged 6 to 9 years, a reference cohort is currently being studied cross-sectionally.
No pediatric reference data for PWV and AI exist to date. Normative values will be obtained in approximately 1500 healthy children and adolescents aged 6 to 20 years. Healthy children will be enrolled from local schools in German, Turkish, Polish, Italian, and English cities.
For the analysis of 24-hour BP profiles, validated normative values are available from 1000 healthy European children examined with the same methodology (10).
Age-related data will be expressed in SD scores (SDS).
Statistical Procedures
The following working hypotheses will be tested:
-
(1) Patients with rapidly progressive CVD can be distinguished from patients with slow progression or stable cardiovascular function (rapid versus slow progressors).
This hypothesis will be addressed by comparing two subgroups of patients distinguished by the average change in cIMT. To that end, the observed intra-individual cIMT differences at the annual month examination time points will be averaged. In a pilot study, patients with progressive cardiovascular morbidity showed a mean increase in cIMT by 0.7 ± 0.5 SDS per year as compared with 0.0 ± 0.5 SDS change per year in stable patients (11). Making a conservative assumption of an average annualized change of 0.35 ± 1 SDS in the progressive versus 0.0 ± 1 SDS in the stable group, approximately 173 individuals per group will be required to detect a difference between groups as significant by two-sided t test. Accounting for a 15% cumulative dropout during 3 years of follow-up, 400 subjects will be required to test this hypothesis.
-
(2) Functional alterations of the vessels and myocardium precede structural alterations.
This hypothesis will be tested by correlating time-averaged functional parameters (PWV, systolic/diaystolic myocardial function) at the 0- and 12-month observations with time-averaged morphologic parameters (cIMT, LVMI) at the 24- and 36-month observations. Multiple linear regression analysis will be performed controlling for patient age, GFR, and time averaged BP. Inclusion of 376 patients will allow to explain a minimal variance in morphologic outcomes of 2.5% by functional parameters with 90% power and 1.5% alpha error.
-
(3) The progression of cardiovascular comorbidity is associated with the progression of CKD and its associated biochemical abnormalities.
Two statistical strategies will be used to identify anthropometric and biochemical predictors (biomarkers; Table 1) of progressive cardiovascular damage. Time-averaged means will be analyzed by two approaches. First, multiple linear regression analysis will be performed to assess independent relationships of the potential risk factors on vascular end points (mean change in PWV, cIMT, myocardial circumferential shortening, LVMI. Assuming that correction for the baseline value of each end point will account for 35% of total variance, inclusion of 540 patients will allow to detect independent effect sizes down to 2.5% variance at 80% statistical power if a maximum of 20 potential independent predictor variables are included in each single analysis. In a second approach, logistic regression analysis will be performed to express predictor effect sizes by odds ratios. Patients with progressor and nonprogressor status will be compared for vascular parameters. Assuming that the baseline probability of the progressor status is 0.33 and that regression of any particular independent variable of interest with the other covariates yields an R2 of 0.15, inclusion of 488 patients will allow detection of odds ratios of 1.35 and greater at an error probability of 5% and a sensitivity of 80%.
(4) Individual common genetic variants affect the risk of progressive cardiovascular comorbidity in children with CKD.
(5) Individual common genetic variants affect the risk of renal failure progression in children with CKD.
A genome-wide association study will be performed to identify individual SNPs, haplotypes, or DNA copy number variations. Multiple equilibration disequilibrium testing will be performed in comparing cardiovascular progressor versus nonprogressor, and renal failure progressor versus nonprogressor status, respectively. The detectable relative risk of progression will be dependent on the marker allele frequency and the cohort size studied. If 500 patients are included, genetic markers associated with a relative risk of at least 1.45 will be detected with 80% power and an error probability of 5% if their allele frequency is at least 20% and those with a relative risk of at least 1.6 if allele frequency is 10%.
Determination of Sample Size
As detailed above, the estimated required number of patients to test working hypotheses 1 to 3 ranges between 376 and 540. Assuming a dropout of 15% within 3 years of follow-up, a minimum of 620 patients will be required.
This patient number will provide limited sensitivity to detect genetic markers of progressive cardiovascular lesions and renal failure. Therefore, it is planned to combine the 4C genotype data set with other pediatric CKD cohorts (The Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients [ESCAPE] study, North American Chronic Kidney Disease in Children [CKiD] study) to optimize statistical power. For testing of genetic markers of renal failure progression, combination with the available ESCAPE and CKiD cohorts will increase the sample size to 1500 subjects.
Ethical Aspects
The patient information and consent forms (translated into the national language) have been reviewed and approved by the local Institutional Review Boards in each participating institution.
Appropriate measures will be used to guarantee maximal data confidentiality. All patient-related clinical data will be pseudonymized locally. Blood samples for genetic testing will be double-coded. Neither the laboratories nor the central office are able to personally identify individual patients, and no investigator outside the central office will be able to correlate genetic results with clinical or laboratory data.
Discussion
Previous studies in children have shown that CKD-related risk factors are strongly associated with the prevalence and severity of cardiovascular changes.
In 156 children with CKD stage II to IV, the prevalence of left ventricular hypertrophy (LVH) was 35%, and age-corrected left ventricular mass was correlated with the degree of renal dysfunction (12). In the only prospective study performed so far in 31 CKD children (stage II to IV), 32% of patients developed LVH within 2 years (13). These findings confirm single-center experience reporting an LVH prevalence of 20% to 25% for CKD stage II to IV and 70% in CKD stage V patients (14). At attainment of end-stage renal disease and during dialysis treatment, 70% to 90% of pediatric patients present with LVH, which usually persists even after successful renal transplantation (15). LVH in children and young adults has been associated with male gender, obesity, and the severity of hyperparathyroidism and its treatment (16,17). Furthermore, 25% of 130 children with CKD stage II to IV presented with subclinical systolic dysfunction. This was associated with concentric LVH (18). In pediatric dialysis patients, systolic dysfunction can be often detected by use of tissue doppler imaging (19,20).
B-Mode ultrasound evaluation of the cIMT is an independent predictor of the risk of cardiovascular events. High-resolution ultrasound allows highly reproducible measurements of cIMT (21). Normative values for cIMT are available for adolescents (22). Increased cIMT has been described in young adults with childhood onset end-stage renal disease (23) and in children and adolescents with different stages of CKD, on dialysis and after renal transplantation (20,22). The cIMT was highest in dialysis patients, but found significantly increased in all stages of CKD and after transplantation (22). In this study, cIMT correlated with the mean past calcium-phosphate product, cumulative calcium-containing phosphate binder intake and the mean dose of calcitriol (22). Litwin et al. prospectively evaluated the evolution of cIMT in pediatric patients over 12 months and found rapid progression in CKD and dialysis patients and stabilization or partial regression in transplanted children (11).
M-Mode sonography allows measurement of local arterial stiffness, e.g., expressed by the distensibility coefficient of the common carotid artery. The gold standard for assessment of systemic arterial stiffness is the measurement of carotid-femoral PWV (24). In addition, central pulse wave analysis allows the determination of the AI, an indirect measure of arterial stiffness (24). An increase of PWV and AI was found in pediatric patients on dialysis (25) and after transplantation (26). Mitsnefes et al. found significantly increased stiffness of the common carotid artery in 60 children with CKD and on dialysis. Arterial stiffness was correlated with serum calcium, phosphate, parathyroid hormone (PTH), and the cumulative dose of calcium-containing phosphate binders and calcitriol (27).
In several cross-sectional studies in young adults with childhood-onset CKD and children on dialysis, coronary calcifications were found in CT scans at a prevalence of 10% to 92% (23,28–31). In these studies, significant associations of coronary calcifications with the following factors were found: Age, duration of dialysis, markers of (chronic) infection (C-reactive protein [CRP], chlamydia antibodies), calcium/phosphate product, phosphate level, PTH level, and cumulative intake of calcium-containing phosphate binders and active vitamin D metabolites.
Thus, there is evidence for systemic CVD characterized by cardiomyopathy and arteriopathy in pediatric CKD patients (Table 2).
Table 2.
Key features of subclinical (asymptomatic) cardiovascular disease in children with CKD
|
The 4C Study aims to characterize the most useful noninvasive technologies for cardiovascular monitoring, identify the pace at which CVD progresses, and detect the most relevant biochemical and genetic markers of progressive CVD and renal failure in children with CKD. The major challenge to meet these objectives is sample size. Because of the low incidence of CKD in childhood, a large encatchment area needs to be covered requiring multicenter and, in Europe, multinational cooperation. To meet this challenge, the 4C Study consortium will utilize and extend the infrastructure established in the ESCAPE trial, a recently concluded interventional study in children with CKD (32). For the genome-wide search for genetic risk markers of CKD progression, the 4C cohort will be combined with that of the ESCAPE trial and the cohort followed in the CKiD study (33) aiming at a total sample size of 1500 children.
Another challenge to be met by the 4C consortium is the absence or incomplete standardization and validation of most noninvasive cardiovascular monitoring technologies in children. In a joint effort, reference data for the vascular measurements will be obtained in a large representative sample of healthy children matched for age and region of origin.
Taken together, this study will investigate the extent and progression of cardiovascular morbidity using a broad spectrum of methods in the largest cohort of children and adolescents with CKD assembled to date. It is hoped that these data will allow individual risk assessment in pediatric patients and development of preventive strategies for the treatment of cardiovascular comorbidity.
Disclosures
None.
Acknowledgments
The study has been made possible by grants of the KfH Foundation for Preventive Medicine (http://www.kfh-stiftung-praeventivmedizin.de/), the Integrated Treatment and Research Center for Organ Transplantation at Hannover, Germany (funded by German Federal Ministry for Education and Research), and local funds at the Coordinating Centers Warsaw, Ankara (Hacettepe University), Istanbul, Izmir (Ege University), and Adana (Cukurova University).
Principal Investigators of 4C Study: Austria: L.B. Zimmerhackl, Innsbruck; K. Arbeiter, Vienna. Belgium: J. Vande Walle, Gent; Czech Republic: J. Dusek, Praha. France: P. Cochat, Lyon; M. Fischbach, Strasbourg. Germany: U. Querfeld, Berlin; J. Dötsch, Erlangen; R. Büscher, Essen; M. Pohl, Freiburg; A. Melk, Hannover; M. Kemper, Hamburg; F. Schaefer, E. Wühl, Heidelberg; U. John, Jena; B. Hoppe, Cologne; S. Wygoda, Leipzig; M. Konrad, Münster; G. Klaus, Marburg; M. Wigger, Rostock. Hungary: P. Sallay, Budapest. Italy: G. Montini, Bologna; A. Canepa, Genova; S. Testa, Milano; L. Murer, Padova; C.M. Matteucci, Rome; L. Peruzzi, Torino. Lithuania: A. Jankauskiene, Vilnius. Poland: D. Drozdz, Krakow; M. Litwin, A. Niemirska, Warsaw; T. Urasinski, Szczecin; A.M. Zurowska, Gdansk. Portugal: A. Caldas-Afonso, Porto. Serbia: A. Peco-Antic, Belgrade. Sweden: R. Krmar, Stockholm. Switzerland: G. Laube, Zürich; G. Simonetti, Bern. Turkey: A. Anarat, Adana; A.S. Bakkaloglu, Ankara; F. Yalcinkaya, Ankara; E. Baskin, Ankara; N. Cakar, Ankara; O. Soylemezoglu, Ankara; G. Demircin, Ankara; S. Caliskan, Istanbul; N. Canpolat, Istanbul; N. Yildiz, Istanbul; M. Civilibal, Istanbul; A. Kiyak, Istanbul; H. Alpay, Istanbul; G. Ozcelik, Istanbul; S. Emre, Çapa-Istanbul; S. Mir, Bornova Izmir. United Kingdom: R. Shroff, London.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J: Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation 114: 2710–2738, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Left ventricular mass and systolic performance in pediatric patients with chronic renal failure. Circulation 107: 864–868, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J, Rees L: Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18: 2996–3003, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Guerin AP, Pannier B, Metivier F, Marchais SJ, London GM: Assessment and significance of arterial stiffness in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 17: 635–641, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024–2031, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS: Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 21: 93–111, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Jourdan C, Wuhl E, Litwin M, Fahr K, Trelewicz J, Jobs K, Schenk JP, Grenda R, Mehls O, Troger J, Schaefer F: Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens 23: 1707–1715, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F: Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 20: 1995–2007, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Litwin M, Wuhl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Troger J, Mehls O, Schaefer F: Evolution of large-vessel arteriopathy in paediatric patients with chronic kidney disease. Nephrol Dial Transplant 23: 2552–2557, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Matteucci MC, Wuhl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer F: Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol 17: 218–226, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-Year follow-up study. J Pediatr 149: 671–675, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chavers BM, Herzog CA: The spectrum of cardiovascular disease in children with predialysis chronic kidney disease. Adv Chronic Kidney Dis 11: 319–327, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Mitsnefes MM, Schwartz SM, Daniels SR, Kimball TR, Khoury P, Strife CF: Changes in left ventricular mass index in children and adolescents after renal transplantation. Pediatr Transplant 5: 279–284, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Groothoff JW, Gruppen MP, Offringa M, de Groot E, Stok W, Bos WJ, Davin JC, Lilien MR, Van de Kar NC, Wolff ED, Heymans HS: Increased arterial stiffness in young adults with end-stage renal disease since childhood. J Am Soc Nephrol 13: 2953–2961, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Briese S, Wiesner S, Will JC, Lembcke A, Opgen-Rhein B, Nissel R, Wernecke KD, Andreae J, Haffner D, Querfeld U: Arterial and cardiac disease in young adults with childhood-onset end-stage renal disease-impact of calcium and vitamin D therapy. Nephrol Dial Transplant 21: 1906–1914, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Chinali M, de Simone G, Matteucci MC, Picca S, Mastrostefano A, Anarat A, Caliskan S, Jeck N, Neuhaus TJ, Peco-Antic A, Peruzzi L, Testa S, Mehls O, Wuhl E, Schaefer F: Reduced systolic myocardial function in children with chronic renal insufficiency. J Am Soc Nephrol 18: 593–598, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Mitsnefes MM, Kimball TR, Border WL, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Impaired left ventricular diastolic function in children with chronic renal failure. Kidney Int 65: 1461–1466, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Mitsnefes MM, Kimball TR, Border WL, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Abnormal cardiac function in children after renal transplantation. Am J Kidney Dis 43: 721–726, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M: Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 115: 459–467, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Litwin M, Wuhl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Troger J, Mehls O, Schaefer F: Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16: 1494–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F: Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H: Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Covic A, Mardare N, Gusbeth-Tatomir P, Brumaru O, Gavrilovici C, Munteanu M, Prisada O, Goldsmith DJ: Increased arterial stiffness in children on haemodialysis. Nephrol Dial Transplant 21: 729–735, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Briese S, Claus M, Querfeld U: Arterial stiffness in children after renal transplantation. Pediatr Nephrol 23: 2241–2245, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR: Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: Role of calcium-phosphorus metabolism. J Am Soc Nephrol 16: 2796–2803, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Eifinger F, Wahn F, Querfeld U, Pollok M, Gevargez A, Kriener P, Gronemeyer D: Coronary artery calcifications in children and young adults treated with renal replacement therapy. Nephrol Dial Transplant 15: 1892–1894, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Civilibal M, Caliskan S, Adaletli I, Oflaz H, Sever L, Candan C, Canpolat N, Kasapcopur O, Kuruoglu S, Arisoy N: Coronary artery calcifications in children with end-stage renal disease. Pediatr Nephrol 21: 1426–1433, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Civilibal M, Caliskan S, Kurugoglu S, Candan C, Canpolat N, Sever L, Kasapcopur O, Arisoy N: Progression of coronary calcification in pediatric chronic kidney disease stage 5. Pediatr Nephrol 24: 555–563, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Moller K, Wigger M, Peruzzi L, Mehls O, Schaefer F: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]