Abstract
Background and objectives: The risk of developing Wilms tumor (WT) can be present or absent in patients with nephrotic syndrome (NS) caused by WT1 mutations. Here, the genotype/phenotype correlation regarding the outcome and risk for WT in 52 patients from 51 families with NS due to WT1 mutations is described.
Design, setting, participants, & measurements: This study followed 19 patients with mutations in intron 9 splice donor site (KTS mutations), 27 patients with missense mutations, 4 patients with nonsense mutations, 1 patient with a splice site mutation in intron 8, and 1 patient with a deletion.
Results: Twenty-four different WT1 mutations were detected. Sixteen of the 19 patients with KTS mutations were females. These patients had isolated NS if karyotype was 46,XX and Frasier syndrome if karyotype was 46,XY. Patients with KTS mutations presented at a significantly older age and with a slower progression toward chronic kidney disease (CKD) stage 5, compared with missense mutations. Patients with nonsense mutations presented initially with WT. Six patients with missense mutations developed WT after the diagnosis of NS (interval-range from NS onset to WT of 0.1 to 1.4 years).
Conclusions: (1) KTS mutations cause isolated NS with absence of WT in 46,XX females. (2) KTS mutations cause Frasier syndrome with gonadoblastoma risk in 46,XY phenotypic females. (3) KTS mutations cause NS with a slower progression when compared with missense mutations. (4) Missense mutations can occur with and without WT. (5) WT1 analysis is important in young patients with NS for early detection and tumor prophylaxis.
WT1 mutations can lead to three distinct clinical entities that are associated with the glomerular disease of steroid-resistant nephrotic syndrome (SRNS): (1) Denys-Drash syndrome (DDS) (1); (2) Frasier syndrome (FS) (2); and (3) isolated NS (3,4). DDS is classically characterized by the triad of infantile SRNS, ambiguous genitalia, and Wilms tumor (WT) (5,6). The characteristic renal biopsy finding in DDS is diffuse mesangial sclerosis (DMS) (6,7). Incomplete forms of DDS have been reported with early glomerulopathy associated with either major urogenital abnormalities or WT (6). FS is characterized by the association of SRNS with male pseudohermaphroditism. In FS, the onset of NS occurs later than in DDS with typical histologic findings of focal segmental glnomerulosclerosis (FSGS) (6). The term “isolated NS” is used here to denote NS without accompanying WT or major urogenital abnormalities. In more than 95% of cases with NS and WT1 mutations, the mutations are located in exon 8 or 9 of WT1 (4,8–10). FS is caused by mutations in the donor splice-site of intron 9. The predicted consequence of mutations in this site is the heterozygous loss of the three amino acid residues KTS (referred from here as KTS mutations) with a resulting reduction in the ratio of the isoforms +KTS/-KTS (11).
There is still uncertainty about the appropriate management of these patients. An important clinical issue emerges in cases where the diagnosis of SRNS caused by WT1 mutation is made, yet without the diagnosis of a tumor. The known risk of WT in DDS patients and of gonadoblastoma in FS patients creates a challenge to the clinician in terms of surveillance and tumor prevention (12,13). This uncertainty led us to investigate the clinical outcome in a large cohort of 52 patients from 51 different families with WT1 mutations as the cause of NS. Our goal was to describe the genotype/phenotype correlation in this large group of patients. Our hypothesis was that specific mutations may predict isolated NS without the manifestation of WT or gonadoblastoma.
Materials and Methods
Patients and Data Recruitment
Patient recruitment following informed consent and the clinical evaluation has been described previously (14). Upon termination of this study (July 2009), a follow-up questionnaire was sent to the treating physicians with questions regarding tumor occurrence, novel extra-renal manifestations, karyotype if not done before, the development of chronic kidney disease (CKD) stage 5 since recruitment, and kidney transplantation success, if done. Fifty-two patients from 51 families were found to have NS with a heterozygous mutation in the WT1 gene (Supplemental Table 1). Eighteen patients were briefly described in previous mutation analysis of SRNS patients (3,4,15,16), and one patient (A1655) was described in a case report (17) (Supplemental Table 1). Standard steroid treatment and responses to steroid therapy were defined according to previously published guidelines (18,19).
Diagnosis of WT1 Mutation by Direct Sequencing
Mutation analysis was performed by exon-flanking PCR with consecutive direct sequencing of exons 8 and 9 of WT1 as described previously (3,4). WT1 analysis was limited to exons 8 and 9 as the NS-causing mutations of this gene were described in these two exons in more than 95% of the patients (4). The diagnosis of a de novo mutation or segregation from a parent was established when DNA samples of the parents were available. All mutations considered disease causing were absent from 90 healthy control individuals.
Categories of WT1 Mutations for Genotype/Phenotype Correlation
Patients were classified for genotype/phenotype correlation of WT1 mutations according to the type of mutation found. Patients with the KTS mutations in intron 9, IVS9 + 4 C>T or IVS9 + 5 G>A, were classified as one group on the basis of previous data suggesting that these mutations both predominantly cause FS or isolated NS (3,6). All patients with a missense mutation were considered as another group under the suggestion that these mutations mainly cause early onset DDS or isolated DMS (3,20).
Clinical Analysis and Exclusion Criteria for Genotype/Phenotype Correlation
DDS was defined by the minimal diagnostic criteria of disease presentation before 2 yr of age with glomerulopathy and one of the following: (1) urogenital abnormalities; (2) WT (6). The complete form of FS was defined as male pseudohermaphrodism (female phenotype with karyotype 46,XY) and progressive glomerulopathy after the age of 2 years (6). An incomplete form of FS was defined in 46,XY phenotypic male patients with progressive glomerulopathy after the age of 2 years and major urogenital abnormalities (21). Isolated NS was defined by the lack of the syndromic features of DDS or FS.
For genotype/phenotype correlation of age of NS onset, we included all patients with KTS mutations or missense mutations for whom we could obtain the age of onset. We excluded patients with KTS mutations or missense mutations from the evaluation of interval between NS onset and CKD stage 5 (see Figure 1) for one or more of the following reasons: (1) patients without an exact date given of disease onset and/or CKD stage 5 diagnosis; (2) patients who reached CKD stage 5 due to bilateral nephrectomy, which was performed either as a treatment of WT or as a preventive measure.
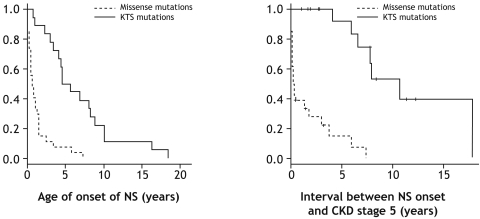
Figure 1.
(Left) NS manifests later in patients with KTS mutations compared with patients with missense mutations in exons 8 or 9 of WT1. Kaplan-Meier survival analysis of interval from birth to onset of NS for 18 patients with splice site mutation in intron 9 (KTS mutations) of WT1 and 27 patients with missense mutations in WT1. The age of NS onset was significantly older in the KTS mutation group compared with patients with missense mutations (t test, P < 0.006). (Right) KTS mutations lead to a slower progression of NS toward CKD stage 5 than missense mutations. Kaplan-Meier survival analysis of interval between onset of NS and CKD stage 5 in 17 patients with splice site mutation in intron 9 (KTS mutations) compared with 26 patients with missense mutations. The vertical latches represent the interval between NS onset and termination of this study for each patient that did not reach CKD stage 5. The interval between onset of NS and CKD stage 5 was significantly longer in the KTS mutation group compared with patients with missense mutations (Log-Rank test, P < 0.006).
Exclusion criteria from genotype/phenotype correlation (see Table 3) of WT, FS, and isolated NS were: (1) patients who died before the age of 5 years without a fully developed syndrome (DDS with WT or FS); (2) patients without WT who had a preventive bilateral nephrectomy before the age of 5 years or within less than 2 years after NS onset.
Table 3.
Genotype/phenotype correlation regarding WT, FS, and isolated NS in 40 patients with NS and WT1 mutations in exons/introns 8 or 9
| Syndrome | +KTS/-KTS Splice Mutations |
Missense Mutations |
Nonsense Mutations |
Number of Patients | ||
|---|---|---|---|---|---|---|
| IVS9 + 4 C>T,Indiv/KT-Gender/Gonads | IVS9 + 5 G>A,Indiv/KT-Gender/Gonads | R394W,Indiv/KT-Gender/Gonads | Other missense,Indiv/KT-Gender/Gonads | R390X,Indiv/KT-Gender/Gonads | ||
| Absence of WT | ||||||
| 2 | 4 | 0 | 0 | 0 | 6 | |
| F1194/M-F/GB | F963/M-M/testicular atrophy | |||||
| A3110 II-1/M-F/GB | F1073/M-M/hypospadias | |||||
| A1688/M-F/GB | ||||||
| A2520/M-F/SG | ||||||
| Isolated NSa | 7 | 5 | 5 | 5 | 0 | 22 |
| F953/F-F/nl | F921/F-F/nl | A2203/F-F/nl | F734/F-F/nl | |||
| F999/F-F/nl | F1280/F-F/nl | A2207/M-M/nl | A644/F-F/nl | |||
| A670/F-F/nl | A562/F-F/nl | A2353/F-F/nl | A1276/F-F/nl | |||
| A1463/F-F/nl | A2074/F-F/nl | A3028/F-F/nl | A1277/F-F/nl | |||
| A1850/?-F/nl | A2328/F-F/nl | A3038/F-F/nl | A1438/?-M/nl | |||
| A1853/F-F/nl | ||||||
| A3110 I-2/F-F/nl | ||||||
| Presence of WT | ||||||
| Complete or incomplete DS | 0 | 0 | 2 | 6 | 4 | 12 |
| A2329/F-F/nl | F1031/F-F/nl | A822/M-M/AG | ||||
| A2407/M-M/nl | A133/F-F/nl | A1251/M-M/AG | ||||
| A1114/M-M/AG | A1655/F-F/nl | |||||
| A1325/F-F/nl | A1998/M-M/AG | |||||
| A2092/F-F/nl | ||||||
| A3021/M-M/AG | ||||||
| Total | 9 | 9 | 7 | 11 | 4 | 40b,c |
AG, ambiguous genitalia; KT, karyotype; nl, normal; NS, nephrotic syndrome; SG, streak gonads; WT, Wilms’ tumor; ?, Karyotype not done.
Isolated NS defined as NS without DDS, FS, or WT. Atrial septal defect, laryngomalacia, left ventricular hypertrophy, aberrant renal artery, and cleft palate were not considered as part of DDS or FS.
Three patients in the total cohort of 52 died prematurely (<2 years from NS onset) without the diagnosis of Wilms tumor and were excluded from this genotype/phenotype correlation.
Nine patients in the total cohort of 52 were excluded from this genotype/phenotype correlation due to prophylactic nephrectomy early in the course of the disease (<2 years of NS onset).
Statistical Analysis
For comparison of NS age of onset between groups with different type of mutations, we fit a t test comparing the log (age) of each group. For comparison of interval from NS onset to CKD stage 5 between groups with different types of mutations, we used the Log-Rank test. For each comparison, P < 0.05 was considered as statistically significant.
Results
Mutation Analysis of 52 Patients from 51 Families with NS and WT1 Mutations
Mutation analysis and clinical data of all 52 patients with NS and WT1 mutation are presented in Supplemental Table 1. In the 51 families, we found 18 families (and 19 patients) with KTS mutations, 27 families with missense mutations, four families with nonsense (truncating) mutations, one family with mutation in the donor splice-site of intron 8, and one family with an in-frame deletion. Overall, 16 patients had the minimal diagnostic criteria of DDS. Five patients had the complete form of DDS (i.e., with a major urogenital malformation and WT). Another 11 patients had the incomplete form of DDS, of which seven patients were found to have only WT and four patients had only a major urogenital malformation without WT.
We detected 24 different mutations in 51 families (Supplemental Table 1). There were two different KTS mutations, 19 missense mutations, one nonsense mutations, one mutation in the donor splice-site of intron 8, and one in-frame deletion. Six of the 24 different mutations in this cohort (25%) are novel (Table 1). The frequency of mutations was uneven by distribution, as the three most common mutations were found in 24 of the 51 families (47%). These were the IVS9 + 5 G>A in nine families, IVS9 + 4 C>T in eight families, and p.R394W in seven families. Segregation of mutations was found only in one family, A3110, with affected mother and daughter (A3110 I-2 and A3110 II-1, respectively). All other mutations examined were mutations that occurred de novo in the affected children.
Table 1.
Six novel WT1 gene mutations in patients with nephrotic syndrome
| Patient | Mutation,a Consequence | Age of NS Onset (years) | Clinical Course | Renal Biopsy |
|---|---|---|---|---|
| A1114 | c.1133C>G, p.T378R | 1.3 | DDS | DMS |
| A1325 | c.1180C>G, p.R394G | 0.5 | NS with WT, early death | DMS |
| A1438 | c.1193T>G, p.L398R | 7.3 | Isolated NS | FSGS |
| A1696 | c.1123_1128delAGGAGA, p.R375_R376del | 0.1 | NS, nephrectomy at age 1.1 years | Not done |
| A1899 | c.1078 T>C, p.C360R | 0.4 | DDS; death of respiratory failure | DMS |
| A2925 | IVS 8 + 1 G>A, splice defect | 0.1 | NS; death of infection | Not done |
DDS, Denys-Drash syndrome; DMS, diffuse mesangial sclerosis; FSGS, focal segmental glomerulosclerosis.
All mutations are heterozygous.
Genotype/Phenotype Correlation for Age of NS Onset in Patients with KTS or Missense Mutations
For genotype/phenotype correlation of age of NS onset, we included 45 of the 46 patients with KTS or missense mutations for whom we could obtain the age of onset. That led to the exclusion of one patient with a KTS mutation. Eighteen patients had KTS mutations, and 27 patients had missense mutations. Kaplan-Meier survival analysis was performed for age of NS onset (Figure 1, left). Patients with KTS mutations in intron 9 had a statistically significant older age of NS onset (median age 5.1 years, range 0.8 to 18.5 years; n = 18) compared with patients with missense mutations (median age 0.8 years, range 0 to 7.3 years; n = 27) with P < 0.006 (t test). We conclude that KTS mutations lead to onset of NS, which is delayed by a median of 4.3 yr compared with missense mutations of WT1 (Figure 1, left).
Genotype/Phenotype Correlation for Interval Between NS Onset and CKD Stage 5 in Patients with KTS or Missense Mutations
Three patients (two with KTS mutations and one with a missense mutation) were excluded from the evaluation of interval between NS onset and CKD stage 5. That led us to evaluate 43 of the 46 patients with KTS or missense mutations. Seventeen of these patients had KTS mutations, and 26 patients had missense mutations. Kaplan-Meier survival analysis was performed for interval between NS onset and CKD stage 5 (Figure 1, right). Ten patients with KTS mutations and four patients with missense mutations did not reach CKD stage 5 upon termination of study (see Figure 1, right, and Supplemental Table 1). Patients with the KTS mutations had a statistically significant longer interval from NS onset to CKD stage 5 (median interval of 8 years) when compared with patients with missense mutations (median interval of 0.2 years) with P < 0.006 (Log-Rank test). We conclude that KTS mutations delay the interval between onset of NS and CKD stage 5 when compared with missense mutations.
NS and WT in Patients with WT1 Mutations
Twelve patients in the cohort had NS with WT (Table 2). The age of WT diagnosis ranged from 0.3 to 1.7 years. In six patients, the diagnosis of WT was made either before or concomitantly with the diagnosis of NS. In these six patients, the interval from WT diagnosis to NS diagnosis was very inhomogeneous, varying from 0 to 13 years. Four of these six patients had the nonsense mutation p.R390X.
Table 2.
Clinical features of 12 NS patients with Wilms tumor and WT1 mutation
| Patient | Gender | Mutation | Age at NS Onset (years) | Age at WT Diagnosis (years) | Kidney Biopsy |
|---|---|---|---|---|---|
| Patients with manifestation of WT before or concomitantly with NS diagnosis | |||||
| A133 | f | p.H401R | 0.3 | 0.3 | No data |
| A822 | m | p.R390X | 1.0 | 1.0 | MCNS |
| A1251 | m | p.R390X | 1.7 | 1.7 | No data |
| A1655 | f | p.R390X | 3.9 | 1.0 | MPGN1 |
| A1998 | m | p.R390X | 4.0 | 1.0 | FSGS |
| A2092 | f | p.D396N | 1.5 | 1.5 | No data |
| Patients with manifestation of NS before WT diagnosis | |||||
| F1031 | f | p.C388R | 0.1 | 1.5 | DMS |
| A1114 | m | p.T378R | 1.3 | 1.4 | DMS |
| A1325 | f | p.R394G | 0.5 | 1.2 | DMS |
| A2329 | f | p.R394W | 0.6 | 1.5 | FSGS |
| A2407 | m | p.R394W | 1.5 | 1.6 | Global sclerosis |
| A3021 | m | p.R394L | 0.2 | 0.7 | no data |
f, female; m, male; MCNS, minimal change nephrotic syndrome; MPGN1, membranoproliferative glomerulonephritis type 1.
The other six patients of the 12 with WT had WT diagnosed after NS onset. In these six patients, WT occurred at 0.6 to 1.6 years of age. The interval range from onset of NS to WT was 0.1 to 1.4 years. In contrast to the six patients with WT diagnosed before NS, who had mostly nonsense mutations, all six patients diagnosed with WT after NS carried missense mutations (Table 2).
Genotype/Phenotype Correlation Regarding WT, FS, and Isolated NS in 40 NS Patients with WT1 Mutations
Clinical data to examine genotype/phenotype correlation regarding WT, FS, or isolated NS was available in 40 of the 52 patients of the cohort (Table 3). Nine patients were excluded from the correlation due to early preventive bilateral nephrectomy (<2 years after disease onset) precluding assessment of WT onset. Another three patients were excluded due to early death (<2 years after disease onset) precluding assessment of WT onset. Of the 18 patients with KTS mutations, 16 were phenotypically females and had either FS (n = 4) or isolated NS (n = 12) (Table 3, first two columns). All four phenotypic females with karyotype 46,XY manifested with FS and streak gonads, while all 12 females with karyotype 46,XX were found to have isolated NS (Table 3). Two male patients, F963 and F1073, both with the KTS mutation IVS9 + 5 G>A, were found to have karyotype 46,XY and FSGS associated with hypospadias and testicular atrophy. These two patients were considered as a variation of FS and are grouped together with FS in Table 3, second column (21). Three of the four phenotypic female patients with FS developed gonadoblastoma.
All four patients with a nonsense mutation had the same mutation p.R390X (Table 3, fifth column). All four patients presented initially with WT with onset of NS either concomitantly or after the diagnosis of WT.
In 18 of the 27 patients with a missense mutation, clinical data were sufficient to assess genotype/phenotype correlation (Table 3, columns 3 and 4). Seven of the 18 patients had the p.R394W missense mutation. The other 11 patients had 11 different missense mutations. Patients with missense mutations presented either with isolated NS (n = 10), with NS and WT only (incomplete form of DDS; n = 6, see Table 3, columns 3 and 4), or with the complete form of DDS (urogenital abnormalities and WT; n = 2, see Table 3, columns 3 and 4). Eight of the 10 patients with isolated NS and a missense mutation had NS onset in the first 2 yr of life (range 0 to 1.5 years; see Figure 1, left).
Discussion
In this study we describe the long-term follow-up and genoptype/phenotype correlation in a large cohort of 52 patients from 51 different families with NS and a mutation in exons 8 and 9 of WT1. To the best of our knowledge, this is so far the largest published cohort of such kind. This study analyzed WT1 mutations only in exons 8 and 9, as mutations in these exons accounts for more than 95% of SRNS cases caused by WT1 mutations (4). Importantly, the relationships describe here are limited to patients with NS and may not be generalized to patients with WT, who have yet to develop NS or patients with WT1 mutation who have not developed NS.
Our main findings on genotype/phenotype correlation may give guidelines regarding the pathologic significance of certain WT1 mutations in NS patients in the following way: (1) KTS mutations are associated with a later onset of NS and slower progression of NS toward CKD stage 5, when compared with missense mutations (Figure 1). These results are compatible with previous data (3,4,20). (2) None of the NS patients with KTS mutations developed WT. One previous report described a patient with a KTS mutation and FS associated with WT, but our data suggest that this association is an exception (22). (3) In NS patients with KTS mutations: If phenotypic female gender and karyotype were in concordance, patients developed isolated NS only. (4) In phenotypic female patients with NS and KTS mutations: If karyotype was 46,XY, patients developed FS with risk of gonadoblastoma. These findings are in agreement with previous literature (4,6). Two phenotypically NS male patients with the mutation IVS9 + 5 G>A and karyotype 46,XY were found to have FSGS associated with hypospadias or testicular atrophy. It was previously reported of two 46,XY male patients with NS and the IVS9 + 4 C>T mutation associated with either hypospadias (23) or normal phenotype (24). Our data suggest that these reports are indeed the exception to the rule in patients with the KTS mutations. Patient A1271 with the mutation IVS9 + 5 G>A had DMS with chordee and right hydrocele of testis but was excluded from the definite clinical classification due to an early preventive bilateral nephrectomy. Although similar DDS phenotype was previously reported in patients with KTS mutations, our data suggest that this is an atypical presentation of KTS mutations (25). (5) All four NS patients with the nonsense mutation (p.R390X) developed WT. Other nonsense mutations of WT1 were previously reported with a variety of phenotypes, almost always with WT occurrence (26,27). (6) NS patients with missense mutations presented in this cohort either in the form of DDS with WT or in the form of isolated NS. Therefore, missense mutations do not allow any conclusion regarding presence or absence of WT risk. None of the NS patients with missense mutations had FS, suggesting that the previous description of a missense mutation in a FS patient is anecdotal (21).
Interestingly, all 12 patients with NS and WT developed the tumor early in life, before the age of 1.7 years. This observation is in agreement with previous findings of an earlier age of tumor detection in patients with WT and WT1 mutations when compared with patients with WT without a WT1 mutation (28).
This study, as previous studies, did not reveal a specific missense mutation that would be associated with a reduced risk to WT occurrence (20,29). Under these circumstances, a preventive bilateral nephrectomy to individuals with CKD stage 5 and missense mutations of WT1 may be warranted. The lack of association in this study may have been influenced by the exclusion from clinical classification of nine patients with missense mutations due to early preventive nephrectomy or death, and by the fact that all missense mutations, other than p.R394W, were found each in only one patient.
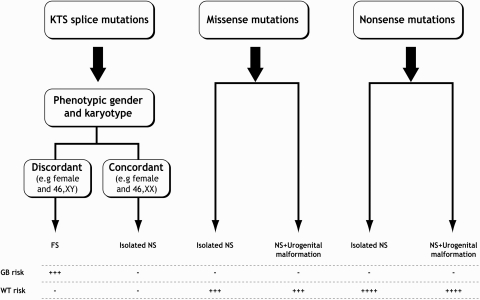
Once a WT1 mutation is found in a patient with NS, this raises a concern of increased risk to develop WT or gonadoblastoma (6,12). The clinician and the patient then need to make a decision regarding the options of surveillance and possible prevention of a tumor. Potential benefits of periodic screening for WT diagnosis are early detection and treatment of WT, although there is no definite evidence that this strategy will decrease overall mortality or tumor stage (30). Potential drawbacks are anxiety for the patient and his/her family and unnecessary tests or procedures (31). In many centers, a bilateral preventive nephrectomy is performed in cases of NS associated with WT1 mutation, especially when the patient has reached CKD stage 5 (12). Indeed in our cohort, nine patients had a preventive bilateral nephrectomy. The initial suggestion for a preventive nephrectomy in patients with DDS was published in the pre-era of genetic screening for WT1 mutations (32). The drawbacks of a preventive nephrectomy are the additive risk of a bilateral nephrectomy procedure and the potential loss of residual renal function in CKD stage 5 patients (33,34). In light of our findings, we suggest a flow chart, for the risk evaluation and surveillance of patients with NS and WT1 mutations, that can provide guidance when counseling parents of children with NS and WT1 mutations (Figure 2).
Figure 2.
Tumor risk assessment in patients with nephrotic syndrome and WT1 mutations in exons 8 or 9. GB, gonadoblastoma.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank the patients and their parents for their participation in this study.
Following are the members of the Study Group of the Gesellschaft für Pädiatrische Nephrologie (GPN): A. Arbeiter (Essen), A. Bakkalogulu (Ankara), M. Benz (Munich), D. Bockenhauer (London), R. Bogdanovic (Belgrade), V. Chandha (Richmond), R. Ettenger (Los Angeles), C. Ghossein (Chicago), A. Goldberg (Chicago), J. Heiliczer (Philadelphia), D. Hooper (Cincinnati), B. Hoppe (Cologne), R. Jenkins (Portland), B. Kaplan (Philadelphia), M.J. Kemper (Hamburg), M. Konrad (Munster), R. London (Chicago), C. Mache (Graz), O. Mansoor (Miami), M. Mayr (Basel), T. Neuhaus (Zurich), C. Plank (Erlangen), G. Reusz (Budapest), C. Rinat (Jerusalem), T. Seeman (Prague), M. Strecker (San Francisco), K. Taranta-Janusz (Bialystoc), F. Weigel (Jena), and A. Zolotnitskaya (New York).
F. H. is an investigator of the Howard Hughes Medical Institute, the Frederick G. L. Huetwell professor, and a Doris Duke Distinguished Clinical Scientist, and is supported by grants from the National Institutes of Health (NIH; P50-DK039255, R01-DK076683), the KMD Foundation, and the Thrasher Research Fund.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Genotype–Phenotype Correlations: Filling the Void,” on pages 1542–1543.
References
- 1.Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, Striegel JE, Houghton DC, Junien C, Habib R, Fouser L: Germline mutations in the Wilms’ tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 67: 437–447, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Barbaux S, Niaudet P, Gubler MC, Grünfeld JP, Jaubert F, Kuttenn F, Fékété CN, Souleyreau-Therville N, Thibaud E, Fellous M, McElreavey K: Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 17: 467–470, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Ruf RG, Schultheiss M, Lichtenberger A, Karle SM, Zalewski I, Mucha B, Everding AS, Neuhaus T, Patzer L, Plank C, Haas JP, Ozaltin F, Imm A, Fuchshuber A, Bakkaloglu A, Hildebrandt F; APN Study Group: Prevalence of WT1 mutations in a large cohort of patients with steroid-resistant and steroid-sensitive nephrotic syndrome. Kidney Int 66: 564–570, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Mucha B, Ozaltin F, Hinkes BG, Hasselbacher K, Ruf RG, Schultheiss M, Hangan D, Hoskins BE, Everding AS, Bogdanovic R, Seeman T, Hoppe B, Hildebrandt F; Members of the APN Study Group: Mutations in the Wilms’ tumor 1 gene cause isolated steroid resistant nephrotic syndrome and occur in exons 8 and 9. Pediatr Res 59: 325–331, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Drash A, Sherman F, Hartmann WH, Blizzard RM: A syndrome of pseudohermaphroditism, Wilms’ tumor, hypertension, and degenerative renal disease. J Pediatr 76: 585–593, 1970 [DOI] [PubMed] [Google Scholar]
- 6.Niaudet P, Gubler MC: WT1 and glomerular diseases. Pediatr Nephrol 21: 1653–1660, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Habib R, Loirat C, Gubler MC, Niaudet P, Bensman A, Levy M, Broyer M: The nephropathy associated with male pseudohermaphroditism and Wilms’ tumor (Drash syndrome): A distinctive glomerular lesion—report of 10 cases. Clin Nephrol 24: 269–278, 1985 [PubMed] [Google Scholar]
- 8.Little M, Wells C: A clinical overview of WT1 gene mutations. Hum Mutat 9: 209–225, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Mrowka C, Schedl A: Wilms’ tumor suppressor gene WT1: From structure to renal pathophysiologic features. J Am Soc Nephrol 11: S106–S115, 2000 [PubMed] [Google Scholar]
- 10.Baird PN, Santos A, Groves N, Jadresic L, Cowell JK: Constitutional mutations in the WT1 gene in patients with Denys-Drash syndrome. Hum Mol Genet 1: 301–305, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P, Gessler M: Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1+/-KTS splice isoforms. Hum Mol Genet 7: 709–714, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Hu M, Zhang GY, Arbuckle S, Graf N, Shun A, Silink M, Lewis D, Alexander SI: Prophylactic bilateral nephrectomies in two paediatric patients with missense mutations in the WT1 gene. Nephrol Dial Transplant 19: 223–226, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Love JD, DeMartini SD, Coppola CP: Prophylactic bilateral salpingo-oopherectomy in a 17-year-old with Frasier syndrome reveals gonadoblastoma and seminoma: A case report. J Pediatr Surg 41: e1–e4, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F: Arbeitsgemeinschaft Für Pädiatrische Nephrologie Study Group: Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F: Arbeitsgemeinschaft für Paediatrische Nephrologie Study Group: Nephrotic syndrome in the first year of life: Two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119: e907–e919, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Gbadegesin R, Hinkes BG, Hoskins BE, Vlangos CN, Heeringa SF, Liu J, Loirat C, Ozaltin F, Hashmi S, Ulmer F, Cleper R, Ettenger R, Antignac C, Wiggins RC, Zenker M, Hildebrandt F: Mutations in PLCE1 are a major cause of isolated diffuse mesangial sclerosis (IDMS). Nephrol Dial Transplant 23: 1291–1297, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bockenhauer D, van’t Hoff W, Chernin G, Heeringa SF, Sebire NJ: Membranoproliferative glomerulonephritis associated with a mutation in Wilms’ tumour suppressor gene 1. Pediatr Nephrol 24: 1399–1401, 2009 [DOI] [PubMed] [Google Scholar]
- 18.International study of kidney disease in children: Primary nephrotic syndrome in children: Clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity: A report of the International Study of kidney disease in children. Kidney Int 20: 765–771, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Arbeitsgemeinschaft für Pädiatrische Nephrologie: Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1: 380–383, 1988 [PubMed] [Google Scholar]
- 20.Schumacher V, Schärer K, Wühl E, Altrogge H, Bonzel KE, Guschmann M, Neuhaus TJ, Pollastro RM, Kuwertz-Bröking E, Bulla M, Tondera AM, Mundel P, Helmchen U, Waldherr R, Weirich A, Royer-Pokora B: Spectrum of early onset nephrotic syndrome associated with WT1 missense mutations. Kidney Int 53: 1594–1600, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kohsaka T, Tagawa M, Takekoshi Y, Yanagisawa H, Tadokoro K, Yamada M: Exon 9 mutations in the WT1 gene, without influencing KTS splice isoforms, are also responsible for Frasier syndrome. Hum Mutat 14: 466–470, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Barbosa AS, Hadjiathanasiou CG, Theodoridis C, Papathanasiou A, Tar A, Merksz M, Györvári B, Sultan C, Dumas R, Jaubert F, Niaudet P, Moreira-Filho CA, Cotinot C, Fellous M: The same mutation affecting the splicing of WT1 gene is present on Frasier syndrome patients with or without Wilms’ tumor. Hum Mutat 13: 146–153, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Tajima T, Sasaki S, Tanaka Y, Kusunoki H, Nagashima T, Nonomura K, Fujieda K: 46,XY phenotypic male with focal segmental glomerulosclerosis caused by the WT1 splice site mutation. Horm Res 60: 302–305, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Melo KF, Martin RM, Costa EM, Carvalho FM, Jorge AA, Arnhold IJ, Mendonca BB: An unusual phenotype of Frasier syndrome due to IVS9 + 4 C>T mutation in the WT1 gene: Predominantly male ambiguous genitalia and absence of gonadal dysgenesis. J Clin Endocrinol Metab 87: 2500–2505, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Denamur E, Bocquet N, Mougenot B, Da Silva F, Martinat L, Loirat C, Elion J, Bensman A, Ronco PM: Mother-to-child transmitted WT1 splice-site mutation is responsible for distinct glomerular diseases. J Am Soc Nephrol 10: 2219–2223, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Heathcott RW, Morison IM, Gubler MC, Corbett R, Reeve AE: A review of the phenotypic variation due to the Denys-Drash syndrome-associated germline WT1 mutation R362X. Hum Mutat 19: 462, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Schumacher V, Schneider S, Figge A, Wildhardt G, Harms D, Schmidt D, Weirich A, Ludwig R, Royer-Pokora B: Correlation of germ-line mutations and two-hit inactivation of the WT1 gene with Wilms tumors of stromal-predominant histology. Proc Natl Acad Sci USA 15: 3972–3977, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royer-Pokora B, Beier M, Henzler M, Alam R, Schumacher V, Weirich A, Huff V: Twenty-four new cases of WT1 germline mutations and review of the literature: Genotype/phenotype correlations for Wilms tumor development. Am J Med Genet A 15: 249–257, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Jeanpierre C, Denamur E, Henry I, Cabanis MO, Luce S, Cécille A, Elion J, Peuchmaur M, Loirat C, Niaudet P, Gubler MC, Junien C: Identification of constitutional WT1 mutations, in patients with isolated diffuse mesangial sclerosis, and analysis of genotype/phenotype correlations by use of a computerized mutation database. Am J Hum Genet 62: 824–833, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao A, Rothman J, Nichols KE: Genetic testing and tumor surveillance for children with cancer predisposition syndromes. Curr Opin Pediatr 20: 1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 31.American Society of Clinical Oncology: American Society of Clinical Oncology policy statement update: Genetic testing for cancer susceptibility. J Clin Oncol 21: 2397–2406, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Eddy AA, Mauer SM: Pseudohermaphroditism, glomerulopathy, and Wilms tumor (Drash syndrome): Frequency in end-stage renal failure. J Pediatr 106: 584–587, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Guzzo I, Mancini E, Wafo SK, Ravà L, Picca S: Residual renal function and nutrition in young patients on chronic hemodialysis. Pediatr Nephrol 24: 1391–1397, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Krediet RT: How to preserve residual renal function in patients with chronic kidney disease and on dialysis. Nephrol Dial Transplant 21[Suppl 2]: ii42–ii46, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.