Abstract
Background and objectives: Approximately two-thirds of kidney transplant recipients with no previous history of diabetes experience inpatient hyperglycemia immediately after kidney transplant surgery; whether inpatient hyperglycemia predicts future new onset diabetes after transplant (NODAT) is not established.
Design, setting, participants, & measurements: A retrospective study was conducted to determine the risk conferred by inpatient hyperglycemia on development of NODAT within 1 year posttransplant. All adult nondiabetic kidney transplant recipients between June 1999 and January 2008 were included. Posttransplant inpatient hyperglycemia was defined as any bedside capillary blood glucose ≥ 200 mg/dl or insulin therapy during hospitalization. NODAT was defined as HbA1C ≥ 6.5%, fasting venous serum glucose ≥ 126 mg/dl, or prescribed diet or medical therapy for diabetes mellitus.
Results: The study cohort included 377 patients. NODAT developed in 1 (4%) of the 28 patients without inpatient hyperglycemia, 4 (18%) of the 22 patients with inpatient hyperglycemia but not treated with insulin, and in 98 (30%) of the 327 of the patients who were diagnosed with inpatient hyperglycemia and were treated with insulin. In adjusted analyses, requirement of insulin therapy during hospitalization posttransplant was associated with a 4-fold increase in NODAT (relative risk 4.01; confidence interval, 1.49 to 10.7; P = 0.006).
Conclusion: Development of inpatient hyperglycemia after kidney transplantation in nondiabetic patients significantly increased the risk of NODAT. Additionally, we observed a significantly increased risk of cardiovascular events in patients who developed NODAT.
New onset diabetes mellitus after transplantation (NODAT) is a frequent and serious complication after kidney transplantation (1–3) with negative consequences on long-term graft and patient outcomes, quality of life, and health care costs (1,2,4–6). Rates of NODAT have been reported as high as 50% (7), but more recent studies suggest a 1-year cumulative incidence between 15% and 25% (1–3). Some of the reasons for the wide dispersion in the reported incidence of NODAT include lack of uniformity in diagnostic criteria and variations in immunosuppression protocols and study cohorts.
While NODAT traditionally is diagnosed during the outpatient phase of care (i.e., after hospital discharge), hyperglycemia among patients with no previous history of diabetes may become evident as early as in the immediate posttransplant in-patient period. Our recent report is among the first to describe the issues related to inpatient hyperglycemia in the immediate posttransplant period after kidney transplantation (8). In that analysis, 87% of 319 nondiabetic patients admitted for their first kidney transplant had at least one episode of inpatient hyperglycemia following surgery, and 66% were administered insulin on the day of hospital discharge (8). It is not clearly known whether inpatient hyperglycemia identified during the period immediately after kidney transplantation among patients with no history of diabetes is a transient problem that results from stresses associated with surgery, hospitalization, and immunosuppression, or whether it poses a risk for future NODAT. If inpatient hyperglycemia is associated with future NODAT among patients with no history of diabetes before transplant, then based on our previous analyses, a substantial number of patients could be at risk for developing NODAT. Recognition of this risk for NODAT while patients are still in the hospital could allow planning for early outpatient intervention or even prevention. We conducted a retrospective analysis in a cohort of kidney transplant recipients with no known history of diabetes mellitus to explore the prognostic value of inpatient hyperglycemia for development of future NODAT. Additionally, we analyzed selected transplant outcomes among patients who had evidence of posttransplant inpatient hyperglycemia.
Materials and Methods
Study Cohort
We conducted a retrospective observational study of all adult, nondiabetic patients undergoing a first kidney transplant at the Mayo Clinic Arizona between June 1999 and January 2008. All patients had at least a 1-year follow-up posttransplant. After Institutional Review Board approval, we identified the study cohort by conducting a systematic chart review. Available data included demographics (i.e., age, sex, and race/ethnicity), family history of type 2 diabetes mellitus (T2DM), dialysis history (duration and modality), pretransplant hepatitis C seropositivity, donor type (living or deceased donor), immunosuppression therapy after transplantation, and body mass index (BMI) pre- and posttransplant. Diabetes status before transplantation was documented as reported in the form submitted to United Network for Organ Sharing (no prior diagnosis of diabetes mellitus and not on prescribed diet or medications for diabetes mellitus). Additionally, all patients had a fasting plasma glucose < 126 mg/dl and hemoglobin A1C (HbA1C) < 6.5% before transplant.
Postoperative Management after Kidney Transplantation
Patients typically undergo kidney transplantation within a few hours of hospital admission. After transplantation, patients are given intravenous fluid with normal saline (replacing 1 ml per 1 ml urine output for the first 24 hours, after which the rate is reduced to 0.5 ml per 1 ml urine output), which is discontinued once the patient is able to tolerate adequate oral intake. A clear liquid diet is prescribed on postoperative day 1, and the diet is advanced as tolerated to an appropriate diet (usually a regular diet for those with no history of diabetes before transplant). Before June 2003, we used a steroid-based maintenance immunosuppression. After June 2003, we used a rapid steroid withdrawal maintenance immunosuppression except in those patients who require prednisone for nontransplant indications or who are at high risk for rejection (complement-dependent lymphocytotoxic crossmatch and/or flow cytometric crossmatch positive and/or donor-specific antibody positivity); thus, the cohort included both patients prescribed and not prescribed maintenance prednisone. The standard immunosuppression includes induction therapy with either rabbit antithymocyte Ig or basiliximab among all patients on rapid steroid withdrawal protocol and some patients who were continued on maintenance steroids. All patients receive a 5-day tapering course of glucocorticoids (methylprednisolone intravenous 500 mg on day 1, 250 mg on day 2, and 125 mg on day 3, followed by oral prednisone 60 mg on day 4 and 30 mg on day 5, then discontinued if on the rapid steroid withdrawal group). Patients who require ongoing steroid therapy receive the same initial 5-day corticosteroid with a subsequent tapering of prednisone over 8 to 12 weeks to maintenance of 5 mg prednisone. Tacrolimus is initiated when there is more than a 30% drop in serum creatinine. The day of tacrolimus initiation is variable for each patient. However, all patients, including those with delayed graft function, begin therapy with tacrolimus before discharge. Mycophenolate mofetil and tacrolimus are the maintenance immunosuppressants.
Definition of Inpatient Hyperglycemia Post-Kidney Transplant
Bedside capillary blood glucose monitoring is typically conducted on patients four times a day using an ACCU-CHEK® Inform (Roche Diagnostics, Indianapolis, IN), an instrument that scans and records patient identification from a bar code, followed by direct downloading to our laboratory database. Insulin is begun at the discretion of the physician and may be prescribed as short acting insulin only or as a basal-bolus regimen of long plus short acting insulin. Our institution utilizes different blood glucose thresholds at the discretion of the care provider to initiate administration of corrective insulin therapy.
Available bedside capillary blood glucose tests are from the first, last, and median day of hospitalization. Inpatient hyperglycemia posttransplantation was defined in two ways: (1) bedside capillary glucose ≥ 200 mg/dl on at least one measurement or (2) the administration of insulin therapy at any time during the hospital stay. The kidney transplant database was linked by patient identifiers to our electronic laboratory database to retrieve information on bedside glucose values and insulin administration by methods described previously (8–10).
Definition of NODAT
Historically there is lack of uniformity in definition of NODAT across studies; very few centers use the American Diabetes Association (ADA) diagnostic criteria, while other centers diagnose NODAT only after the introduction of insulin or oral therapy. We used composite criteria to define NODAT: Either HbA1C ≥ 6.5%, fasting venous serum glucose ≥ 126 mg/dl, or receiving diet or medical therapy for diabetes mellitus between 1 month and 1 year posttransplantation. An HbA1C level of ≥ 6.5% has been adopted by the ADA as a diagnostic criterion for diabetes (11). Additionally, we also conducted analyses defining NODAT as: (1) HbA1C ≥ 6.5% or receiving diet or medical therapy for diabetes mellitus between 1 month and 1 year posttransplantation and (2) fasting venous serum glucose ≥ 126 mg/dl or receiving diet or medical therapy for diabetes mellitus between 1 month and 1 year posttransplantation. For purposes of this manuscript, unless specified, NODAT refers to our composite criteria definition of NODAT. HbA1C determinations were performed on a Roche Cobas 6000 chemistry analyzer (Roche Diagnostics). The HbA1C determination is based on turbidimetric immunoinhibition (TINIA) using a hemolyzed whole blood sample. All hemoglobin variants that are glycated at the B-chain N-terminus and which have antibody-recognizable regions identical to that of HbA1C are measured in this assay. HbA1C is standardized against the National Glycohemoglobin Standardization Program.
The time period (between 1 and 12 months posttransplant) for development of NODAT was chosen because patients are clinically stable and on stable doses of immunosuppression by 4 weeks after transplantation, and this period excludes patients who may have developed transient hyperglycemia only in the immediate posttransplant due to stress of surgery and high-dose corticosteroid therapy. Additionally, the highest incidence of NODAT occurs within the first year posttransplant (2,4).
Data Analyses
We conducted baseline descriptive analyses of the study cohort. Normally distributed continuous variables were compared using t tests, and non-normally distributed variables were compared using the Wilcoxon rank sum test. The χ2 test was used to compare proportions. Both unadjusted and adjusted logistic regression models were used to determine risk conferred by inpatient hyperglycemia (anytime during hospital stay) and patient characteristics on the development of future NODAT. Because 66% of patients in the previous study had hyperglycemia or were prescribed insulin during the last day of the hospital stay (8), we also assessed the relationship of hyperglycemia at discharge and NODAT. Finally, we assessed the association of the total number of units of insulin required during the hospitalization to the development of NODAT.
The incidence rates of postsurgical wound complications (including wound dehiscence, wound infection, and wound hematoma), infections within 4 month posttransplant, cardiovascular events, and overall graft and patient survival among patients with and without hyperglycemia were studied in relation to inpatient hyperglycemia and NODAT. Statistical analyses were conducted with Stata® statistical software, version 10.0 (StataCorp LP, College Station, TX).
Results
From June 1999 to January 2008, 377 previously nondiabetic patients underwent kidney transplantation. Patient characteristics are described in Table 1. Median hospital stay after transplantation was 4 days. The maintenance immunosuppression is predominantly calcineurin-based: 93% and 86% were prescribed tacrolimus at 4 and 12 months posttransplant, respectively.
Table 1.
Descriptive analyses of study cohort
| Variable | Study Cohort, n = 377 |
|---|---|
| Age (mean ± SD), years | 49 ± 15 |
| Female gender, % | 44 |
| White, % | 71 |
| African American, % | 7 |
| American Indian, % | 7 |
| Hispanic | 13 |
| Family history of type 2 diabetes mellitus, % | 15 |
| Hemodialysis pretransplant, % | 63 |
| Peritoneal dialysis pretransplant, % | 12 |
| Recipient of preemptive TX, % | 25 |
| Hepatitis C seropositivity, % | 4 |
| Deceased donor, % | 35 |
| Pre-TX BMI > 30, % | 25 |
| Pre-TX triglyceride >200 mg/dl, % | 57 |
| Prednisone use at 1 month, % | 43 |
| Prednisone use at 4 months, % | 48 |
| Prednisone use at 12 months, % | 49 |
| Tacrolimus use at 4 months, % | 93 |
| Tacrolimus use at 12 months, % | 86 |
| Rapamune use at 12 months, % | 6 |
The 1 year (between 1 month and 1 year posttransplantation) cumulative incidence of NODAT using the composite definition was 27% (n = 103). Using the second definition of NODAT, either HbA1C ≥ 6.5% or receiving diet or medical therapy for diabetes mellitus, the incidence was 17% (n = 65). Using the third definition of NODAT, either fasting venous serum glucose ≥ 126 mg/dl or receiving diet or medical therapy for diabetes mellitus, the incidence was 25% (n = 96). As shown in Table 2, 349 (92.5%) patients met our definition of inpatient hyperglycemia, 327 (86.7%) were prescribed insulin on at least one occasion during the posttransplant hospitalization period, and 249 (66%) patients were hyperglycemic or prescribed insulin therapy on the day of hospital discharge. There were no significant differences in the demographic distribution, pretransplant BMI, triglyceride, and family history for T2DM among those who did or did not experience inpatient hyperglycemia.
Table 2.
Relationship of inpatient hyperglycemia and new onset diabetes mellitus after transplantation using various criteria to define NODAT
| Category | Total Number of Patients | Total Cases with NODATa (%) | Total Cases with NODATb (%) | Total Cases with NODATc (%) | Total Cases with NODATd (%) |
|---|---|---|---|---|---|
| Absence of inpatient hyperglycemia | 27 | 2 (7%) | 2 (7%) | 2 (7%) | 0 (0%) |
| Random blood sugar > 200 mg/dl but no insulin administered | 23 | 3 (13%) | 1 (4%) | 3 (13%) | 1 (14%) |
| Insulin administered during hospitalization | 327 | 98 (30%) | 62 (19%) | 91 (28%) | 48 (15%) |
| Total | 377 | 103 (27%) | 65 (17%) | 96 (25%) | 49 (13%) |
Cumulative composite definition of NODAT: NODAT was defined as either hemoglobin A1C ≥ 6.5% or fasting venous serum glucose ≥ 126 mg/dl, or receiving diet or medical therapy for diabetes mellitus between 1 month and 1 year posttransplantation.
NODAT defined as either hemoglobin A1C ≥ 6.5% or receiving diet or medical therapy for diabetes mellitus between 1 month and 1 year posttransplantation.
NODAT defined as either fasting venous serum glucose ≥ 126 mg/dl or receiving diet or medical therapy for diabetes mellitus between 1 month and 1 year posttransplantation.
NODAT defined as initiation of diet or medical therapy for diabetes mellitus between 1 month and 1 year posttransplantation.
Only a small number of patients without inpatient hyperglycemia (1 of 28, or 4%) developed NODAT, compared with 102 of 349 (29%) of those with inpatient hyperglycemia (Table 2). Only 22 patients with inpatient hyperglycemia did not receive insulin therapy, of whom 4 (18%) developed NODAT.
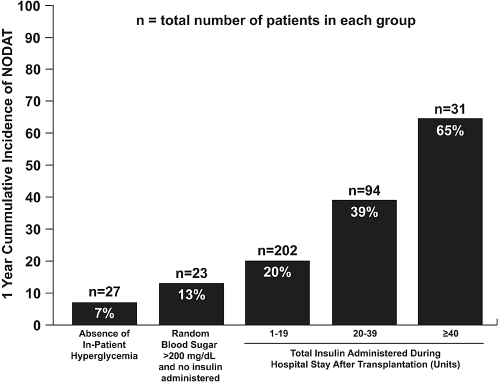
In a separate analysis, we examined the relationship between cumulative insulin doses received in the hospital with NODAT risk. Insulin requirements during hospitalization were positively associated with risk of NODAT (Figure 1). There was a 2.4 higher risk (relative risk [RR], 2.4; 95% interval [CI], 1.5 to 3.9; P < 0.001) of future development of NODAT if a patient had required more than or equal to a total of 20 units of insulin during hospitalization compared with those not requiring insulin.
Figure 1.
Relationship of blood glucose and insulin requirement immediately after kidney transplantation to the incidence of NODAT.
In unadjusted analyses (Table 3), inpatient posttransplant hyperglycemia was significantly associated with NODAT. Similarly, the presence of hyperglycemia or insulin therapy at discharge was significantly associated with NODAT. Greater age, steroid therapy, and greater BMI also increased the risk for NODAT (Table 3). Gender, race, pretransplant dialysis modality (hemodialysis [HD] versus peritoneal dialysis [PD]), hepatitis C seropositivity, and mean trough tacrolimus level at 1, 4, and 12 months were not statistically significant predictors.
Table 3.
Unadjusted association of various variables and NODAT
| Variable | Univariate OR (95% CI), P value |
|---|---|
| Inpatient hyperglycemia defined as increase in blood sugar ≥ 200 mg/dl or requirement of insulin therapy during hospitalization | 5.07 (1.17 to 21.8), 0.03 |
| Requirement of insulin at any time during hospitalization | 3.85 (1.48 to 9.99), 0.006 |
| Hyperglycemia (requirement of insulin or random blood sugar > 200 mg/dl) during last day of hospitalization | 3.06 (1.62 to 5.77), 0.001 |
| Age per 1 year increase | 1.03 (1.01 to 1.05), <0.001 |
| Presence of family history | 1.63 (0.93 to 2.86), 0.09 |
| On steroid maintenance at discharge | 1.62 (1.03 to 2.56), 0.04 |
| On steroid at first month posttransplant | 1.67 (1.06 to 2.63), 0.03 |
| On steroid at fourth month posttransplant | 1.69 (1.07 to 2.66), 0.03 |
| On steroid at 1 year posttransplant | 1.86 (1.17 to 2.94), 0.009 |
| Pretransplant BMI, per 1 unit increase | 1.05 (1.01 to 1.09), 0.015 |
| BMI at 1 month posttransplant | 1.05 (1.01 to 1.09), 0.03 |
| BMI at 4 month posttransplant | 1.04 (0.99 to 1.08), 0.12 |
| BMI at 12 month posttransplant | 1.04 (1.01 to 1.08), 0.02 |
OR, odds ratio; CI, confidence interval; BMI, body mass index.
In adjusted analyses, inpatient posttransplant hyperglycemia was significantly associated with an over 4-fold increase in NODAT (Table 4). Greater age, BMI, and steroid therapy also remained as significant predictors. Similarly, hyperglycemia at hospital discharge also predicted NODAT (RR, 3.00; CI, 1.5 to 5.98), adjusted for age, pretransplant BMI, on steroid therapy, tacrolimus trough levels, and presence of family history of T2DM.
Table 4.
Adjusted association of various variables and NODATa
| Variable | Multivariate OR (95% CI),cP value |
|---|---|
| Inpatient hyperglycemia during hospitalizationb | 4.01 (1.49 to 10.7), 0.006 |
| Age per year | 1.03 (1.02 to 1.05), <0.001 |
| Presence of steroid maintenance | 2.11 (1.29 to 3.45), 0.003 |
| Presence of family history of T2DM | 1.67 (0.93 to 3.03), 0.08 |
| Pretransplant BMI, per unit difference | 1.05 (1.01 to 1.09), 0.04 |
Composite definition of NODAT.
Inpatient hyperglycemia defined requirement of insulin therapy during hospitalization.
Adjusted for age, pretransplant BMI, on steroid maintenance, mean tacrolimus trough at 4 and 12 months, and family history of T2DM.
There were no significant differences in incidence rates of surgical wound complications, infectious complications, and 1- and 3-year patient survival among those with and without inpatient hyperglycemia. Similarly, there was no statistically significant increase in the rates of surgical wound complications, infections, and 1- and 3-year patient survival among those who did and did not develop NODAT. However, cardiovascular events (computed as a composite of angina, myocardial infarction, new onset arrhythmia, or sudden death attributed to cardiovascular causes) within 1 year occurred in 13% (13/103) of the patients who developed NODAT versus 6% (16/274) (P = 0.03) of patients without NODAT and among 8% of patients with inpatient hyperglycemia versus 4% (P = 0.29) of those without inpatient hyperglycemia.
Discussion
Kidney transplantation is the best therapy for end-stage renal disease (12). However, NODAT undermines the benefits of transplantation by decreasing allograft and patient survival and quality of life (1,2,4,5). Moreover, it has been estimated that NODAT incurs an extra incremental Medicare payment of $21,500 per patient by 2-years posttransplant (4). We found a high prevalence of hyperglycemia in this population without a known history of diabetes. The reason for the high prevalence of inpatient hyperglycemia and need for insulin is likely a combination of stress and medications, but the pathophysiology of hyperglycemia in the acute care setting in this population has not been studied, to our knowledge, and certainly warrants investigation.
Although investigators have typically diagnosed and studied NODAT after hospital discharge (1–7,13). Kuypers et al. evaluated hyperglycemia on the first postoperative day after kidney transplantation as a risk factor for future NODAT (14). They found, by multivariate analysis, that hyperglycemia on the first postoperative day was not a risk factor for NODAT in patients on maintenance prednisone. They also performed a 2-hour oral glucose tolerance test on the fifth postoperative day in this cohort and found, by multivariate analysis, that impaired fasting glucose, impaired glucose tolerance, and diabetic values by the World Health Organization (WHO) criteria were all predictive of future NODAT. Although our study also captured information regarding hyperglycemia in the early posttransplant period—the day of admission to the day of hospital discharge—we did not evaluate those data separately. Rather, we found that nearly two-thirds of patients with no previous history of diabetes develop inpatient hyperglycemia during hospitalization, between transplantation and hospital discharge (8), and that hyperglycemia during this period increases risk of NODAT. An additional difference is that our cohort included both patients prescribed and not prescribed maintenance prednisone. The independent effect of maintenance prednisone on NODAT is reported (Table 4). To understand the importance of inpatient hyperglycemia as a potential independent risk factor for development of NODAT and other complications, we defined inpatient hyperglycemia in two ways: (1) inpatient hyperglycemia at anytime posttransplant during the hospitalization or (2) inpatient hyperglycemia on day of discharge. We observed that, regardless of our definition, inpatient hyperglycemia conferred increased risk of NODAT (Table 5). Cumulative inpatient insulin dose was also associated with a higher risk of developing NODAT. Those patients with greater insulin requirements could represent individuals with higher degrees of insulin resistance, more advanced beta cell impairment in synthesis or secretion of insulin, or persons with greater susceptibility to the diabetogenic action of immunosuppressant therapy. Future studies exploring mechanistic pathways (possibly including changes in insulin sensitivity, insulin secretion, hepatic glucose production, and skeletal remodeling) may further our understanding of why inpatient hyperglycemia occurs and by what path it may progress to NODAT. To our knowledge, the relationship between race/ethnicity and inpatient hyperglycemia risk has not been studied either in general or in the newly transplanted, hospitalized patient. It is possible that a race/ethnicity effect was not observed because of the low representation of high risk ethnic diabetes groups or because other factors (such as stress and immunosuppresants) were more powerful factors and superseded the risk imposed by race ethnicity.
Table 5.
Adjusted association of various definitions of inpatient hyperglycemia and NODATa
| Variable | Multivariate OR (95% CI),bP value |
|---|---|
| Inpatient hyperglycemia defined as increase in blood sugar ≥ 200 mg/dl or prescription of insulin therapy during hospitalization | 4.75 (1.08 to 20.9), 0.04 |
| Inpatient hyperglycemia defined prescription of insulin therapy during hospitalization | 4.01 (1.49 to 10.7), 0.006 |
| Hyperglycemia (prescription of insulin or random blood sugar > 200 mg/dl) on the day of hospital discharge | 2.93 (1.52 to 5.63), 0.001 |
Composite definition of NODAT.
Each association above adjusted for age, pretransplant BMI, whether on steroid maintenance, mean tacrolimus trough at 4 and 12 months, and family history of T2DM.
Because of the high risk inpatient hyperglycemia poses for development of NODAT, identification of patients while still in the hospital should allow construction of a patient cohort for prospective clinical trials to test interventions that might reduce the development of the disease during the follow-up period. Additionally, this also justifies the resources utilized toward diabetes education during their inpatient stay. Although transplant patients have not been included in T2DM prevention studies, intensive lifestyle interventions can delay or prevent the onset of T2DM in adults who are not transplant recipients (15–18). The kidney transplant population—and perhaps all patients undergoing solid organ transplantation—may benefit from similar strategies designed to prevent onset of NODAT. Additionally, the inpatient setting is an optimal place for early recognition and treatment of hyperglycemia and to begin efforts to educate the patient in the self-management skills that may be utilized in the outpatient arena (19).
There are some limitations to our study. First, our electronic database information used to define inpatient hyperglycemia does not necessarily distinguish between a preprandial versus a postprandial bedside glucose. However, ADA guidelines recommend no excursions of glucose > 200 mg/dl, even after meals, so our definition of hyperglycemia is consistent with that of the ADA (20). Additionally, a recent recommendation defines inpatient hyperglycemia as a glucose > 140 mg/dl (21). Hence, our cut-off of ≥200 mg/dl may under-represent the number of patients with inpatient hyperglycemia. Second, we used a composite criterion to define development of NODAT (fasting venous glucose ≥ 126 mg/dl or HbA1C ≥ 6.5% or administration of therapy for diabetes). HbA1C ≥ 6.5% as a diagnostic criteria for diabetes is now a standard diagnostic criterion (11). Our composite definition of NODAT includes criteria used for clinical care.
In conclusion, our analysis found that inpatient hyperglycemia after kidney transplantation significantly increases the risk of NODAT. We also found that NODAT significantly increases the risk of cardiovascular events in the first year after transplantation. Whether hyperglycemia increases risk of future NODAT in recipients of other solid organs will need to be studied in those models. In the kidney transplant model, further studies will be necessary to understand the mechanisms by which inpatient posttransplant hyperglycemia develops and how it progresses to NODAT. Finally, the finding that inpatient hyperglycemia confers elevated risk of NODAT should allow design and testing of strategies to prevent NODAT in high-risk patients and may be the next logical step in prevention of this serious complication of kidney transplantation.
Disclosures
None.
Acknowledgments
This publication was made possible by grant number 1 KL2 RR024151 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Additionally, this research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM: Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 62: 1440–1446, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Nam JH, Mun JI, Kim SI, Kang SW, Choi KH, Park K, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC, Huh KB: Beta-Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation 71: 1417–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC: Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 3: 590–598, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ducloux D, Kazory A, Chalopin JM: Posttransplant diabetes mellitus and atherosclerotic events in renal transplant recipients: A prospective study. Transplantation 79: 438–443, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cosio FG, Kudva Y, van der Velde M, Larson TS, Textor SC, Griffin MD, Stegall MD: New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int 67: 2415–2421, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC: Posttransplantation diabetes: A systematic review of the literature. Diabetes Care 25: 583–592, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chakkera HA, Weil EJ, Castro J, Heilman RL, Reddy KS, Mazur MJ, Hamawi K, Mulligan DC, Moss AA, Mekeel KL, Cosio FG, Cook CB: Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol 4: 853–859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook CB, Castro JC, Schmidt RE, Gauthier SM, Whitaker MD, Roust LR, Argueta R, Hull BP, Zimmerman RS: Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum. J Hosp Med 2: 203–211, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Knecht LA, Gauthier SM, Castro JC, Schmidt RE, Whitaker MD, Zimmerman RS, Mishark KJ, Cook CB: Diabetes care in the hospital: Is there clinical inertia? J Hosp Med 1: 151–160, 2006 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 33 (Suppl 1): S62–S69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Mathew JT, Rao M, Job V, Ratnaswamy S, Jacob CK: Post-transplant hyperglycaemia: A study of risk factors. Nephrol Dial Transplant 18: 164–171, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kuypers DR, Claes K, Bammens B, Evenepoel P, Vanrenterghem Y: Early clinical assessment of glucose metabolism in renal allograft recipients: Diagnosis and prediction of post-transplant diabetes mellitus (PTDM) Nephrol Dial Transplant 23: 2033–2042, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 20: 537–544, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V: The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49: 289–297, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Roman SH, Chassin MR: Windows of opportunity to improve diabetes care when patients with diabetes are hospitalized for other conditions. Diabetes Care 24: 1371–1376, 2001 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association: Postprandial blood glucose. Diabetes Care 24: 775–778, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE: American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 15: 353–369, 2009 [DOI] [PubMed] [Google Scholar]