Abstract
Many debilitating diseases, including neurodegenerative diseases, involve apoptosis. Several methods have been developed for visualizing apoptotic cells in vitro or in fixed tissues, but few tools are available for visualizing apoptotic cells in live animals. Here we describe a genetically encoded fluorescent reporter protein that labels apoptotic cells in live zebrafish embryos. During apoptosis, the phospholipid phosphatidylserine (PS) is exposed on the outer leaflet of the plasma membrane. The calcium-dependent protein Annexin V (A5) binds PS with high affinity, and biochemically purified, fluorescently labeled A5 probes have been widely used to detect apoptosis in vitro. Here we show that secreted A5 fused to yellow fluorescent protein specifically labels apoptotic cells in living zebrafish. We use this fluorescent probe to characterize patterns of apoptosis in living zebrafish larvae and to visualize neuronal cell death at single-cell resolution in vivo.—Van Ham, T. J., Mapes, J., Kokel, D., Peterson, R. T. Live imaging of apoptotic cells in zebrafish.
Keywords: neuronal cell death, in vivo microscopy, vertebrate model, neurotoxicity, p53, DNA damage response
Multicellular animals dispose of unneeded or damaged cells in an ordered fashion by apoptosis. During embryogenesis, genetically controlled apoptosis eliminates many cells to morphologically sculpt the embryo. For example, 20–80% of neurons die via apoptosis during development of the vertebrate nervous system (1). In mice, loss of function of a protease involved in execution of apoptosis, the CPP32 caspase enzyme, causes a large excess of neurons (2). Dysregulation of apoptosis is implicated in several incurable diseases, including neurodegenerative diseases and cancer. Many neurodegenerative diseases are characterized by a progressive loss of neurons, likely by apoptotic cell death, but how this initiates and progresses in vivo is very poorly understood (3, 4). Although several methods allow labeling of apoptotic cells in vitro or in fixed tissue, visualizing apoptotic cells in live animals has remained a challenge.
Dying cells progress through a series of stereotypical morphological changes including blebbing of the plasma membrane, altered cellular morphology (rounding), cell shrinkage, and nuclear condensation followed by nuclear fragmentation and chromosomal DNA degradation (5). Several methods have been developed for labeling apoptotic cells with fluorescent nucleic acid binding dyes, such as acridine orange, ethidium bromide, and propidium iodide (6, 7). A standardized technique to detect apoptotic cells in fixed tissue or fixed cells is terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), which is based on end labeling of DNA degradation products enzymatically or by a fluorescent probe (8).
Another well-established method to detect apoptotic cells in vitro is based on loss of membrane asymmetry during apoptosis (9). During apoptosis, the normal asymmetric distribution of phospholipids in the cell membrane is lost, and phosphatidylserine (PS) is exposed on the outer leaflet of the lipid bilayer. The calcium-dependent protein Annexin V (A5) binds PS with high affinity, and biochemically purified, fluorescently labeled A5 probes have been widely used to detect apoptotic cells in vitro (10–12).
The zebrafish (Danio rerio) has proved to be a very valuable vertebrate model organism with which to study embryonic development and is amenable to genetic screening and chemical biology (13–15). Most vertebrate organ systems, including the heart and central nervous system, develop within days after fertilization (13, 16). The occurrence of apoptotic cells during the first 4 d of zebrafish embryonic development has been studied and quantified previously by using in situ TUNEL (17). This study showed transient high levels of apoptosis in various cells and tissues during specific phases of development (16, 18). Because the zebrafish is very well suited for live fluorescence imaging due to the optical transparency during early development (19), a transgenic fluorescent marker allowing in vivo imaging of apoptotic cells would greatly benefit research into the in vivo dynamics of apoptotic cell death.
We demonstrate that secreted A5 protein fused to yellow fluorescent protein (YFP) (secA5-YFP) labels apoptotic cells in live zebrafish. Labeled cells exhibit several other characteristics of apoptotic cells, and the pattern of apoptotic cells observed by live imaging was similar to previous findings using TUNEL. Labeling of apoptotic cells increases rapidly on DNA-damaging treatment, and this is abolished by loss of function of the p53 tumor suppressor gene. Finally, apoptotic neurons, including axons and dendrites, can be visualized in the zebrafish embryonic brain by live imaging.
MATERIALS AND METHODS
Zebrafish maintenance
TuAB zebrafish embryos were used for all experiments. Fish embryos were incubated at 28°C in HEPES-buffered E3 in the dark. The p53 mutant used contains a mutation leading to a substitution of a methionine for a lysine at residue 214 (M214K), leading to a transactivation-dead p53 protein (20). The zebrafish line expressing mCherry in the central nervous system (Mue4465_13) was previously published (21) and was a gift from Reinhard Koester and Martin Distel (Helmholz Institute, Munich, Germany).
Cloning
The UAS-Annexin-V-YFP construct (dk70) was created using the Gateway cloning system (Invitrogen, Carlsbad, CA, USA) and the Michael L. Nonet laboratory (Washington University, St. Louis, MO, USA) bleeding heart (pBH) vectors. To achieve secretion of the A5 protein a short signal peptide was used, based on the 18-aa sequence previously used in Caenorhabditis elegans (22). Human A5 was amplified from pet22b:A5 (gift from Mark Schlissel, University of California, Berkeley, CA, USA) using a forward primer containing the protein secretion signal (forward: 5′-atgcataaggttttgctggcactgttctttatctttctggcaccagcaggcaccatggcacaggttctcagaggcac) and a reverse primer containing the Attb2 gateway site (reverse: 5′-ggggACCacttt gtacaagaaagctgggtcgtcatcttctccacagagcagc). The PCR product was subsequently amplified using a forward primer to add the Attb1 site (forward: 5′-ggggACAAGTTTGTACAAAAAAGCAGGCTtaACCatgcataaggttttgctggc) and the same reverse primer. This PCR product was cloned into the Gateway donor vector and subsequently into the pBH-UAS-gtwy-YFP vector. Expression of the secA5-YFP transgene was mediated by using the Gal-4-UAS system. A strong ubiquitous promotor based on the zebrafish TATA-box binding protein (TBP) promoter (23) was used to drive expression of the Gal4 protein from the pCH-Gtwy-G4m vector (pCH-TBP-G4m; gift from Chetana Sachidanandan, Institute of Genomics and Integrative Biology, Delhi, India; unpublished results). Gateway recombination reactions were performed according to the manufacturer’s instructions (Invitrogen). Gateway vectors (pBH-UAS-gtwy-YFP and pCH-Gtwy-G4m) were a gift from the Michael L. Nonet laboratory.
Microinjections
Approximately 2 nl of DNA (at concentrations of 15 ng/μl) was microinjected into 1-cell-stage fertilized zebrafish (Tuebingen AB) embryos. At 1 d following fertilization [24 hours postfertilization (hpf)], the injected embryos were used for experiments. We created secA5-YFP transgenic zebrafish using the Tol2 method as described previously (24) by coinjecting transposase mRNA with UAS-secA5-YFP plasmid, containing Tol2 sites, and selection of transgenics based on the red heart marker (cmlc2-RFP) indicating transgene expression. To induce secA5-YFP expression in transgenic animals, P/F0 animals were crossed with animals stably expressing the pCH-TBP-G4m plasmid.
Irradiation/UV treatment
Embryos at 24 hpf were subjected to UV (120 mJ/cm2) for ∼15 s in 6-cm dishes containing buffered E3 (Spectrolinker, XL-1500; Spectronics Corporation, Westbury, NY, USA).
Quantification of apoptotic cells
To quantify apoptosis, the number of secA5-YFP+ cells was counted in ≥10 embryos (embryo proper) using a fluorescence dissection stereomicroscope (Discovery V8; Zeiss, Oberkochen, Germany). The number of secA5-YFP+ cells was counted at multiple time points in the same group of embryos. Images were taken using an AxioCam MRc camera and AxioVision 4.8 software (Zeiss).
Chemicals
Chemicals used in these studies were dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA). Camptothecin (CPT), etoposide, doxorubicin, colchicine, and MPTP were obtained from Sigma. DTT was obtained from Promega (Madison, WI, USA). Chemical treatments were performed in E3 yielding a DMSO concentration of 1% in 96-well plates.
Staining procedures and imaging
Dechorionated embryos (30 hpf) were fixed in fresh 4% paraformaldehyde in PBST (PBS, 0.05% Tween) overnight at 4°C, dehrydrated using methanol (3×5 min), and stored overnight at −20°C, rehydrated using decreasing concentration of methanol in PBST, and permeabilized using sodium citrate (0.1%) at room temperature for 15 min. Fixed and permeabilized embryos were rehydrated and treated for 15 min with 1 μg/ml proteinase K, followed by several washes in PBST and then a 1 h incubation at 37°C in a red fluorescent (TMR-red) TUNEL cell death detection reagent (in situ cell death Detection Kit-TMR Red; Roche, Basel, Switzerland) and incubated in PBST containing DAPI to counterstain nuclei (4′, 6-diamidino-2′-phenylindol, dichloride; Thermo Scientific/Pierce, Rockford, IL, USA). Embryos taken up in PBST containing DAPI were imaged whole mount using confocal microscopy. Fixed embryos were imaged on a Zeiss LSM5 Pascal confocal microscope using ×40 water dipping objective (Zeiss) and 405-, 488-, 514-, and 543-nm laser lines.
Live imaging
Live imaging of embryonic zebrafish brain was performed essentially as described previously (25); briefly, zebrafish larvae in E3 medium containing tricaine (0.01 mg/ml) were mounted into small vertical holes in a thin 0.7% agarose layer covering a 3.5-cm glass-bottom culture dish (MatTek, Ashland, MA, USA). Time-lapse images were captured on an inverted microscope (Axio Observer Z1; Zeiss) using the LSM700 scanning system (Zeiss, 488- and 561-nm laser lines) and a ×40/0.6 objective (LD Plan-Neofluar; Zeiss).
Image and time-lapse processing
All images and movies were processed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA; http://rsbweb.nih.gov/ij/). Confocal images and supplemental videos are all maximum projections of z stacks. Time-lapse images were recorded every ∼5 min; supplemental videos are maximum projections of z stacks played back at 5 frames/s.
RESULTS
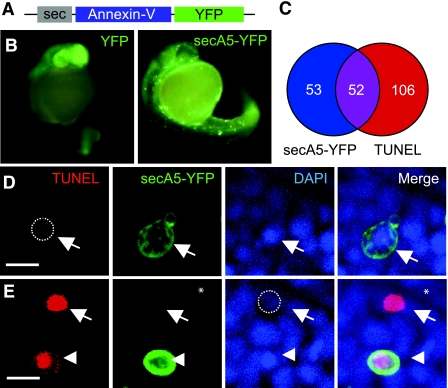
To create a genetically encoded A5-based marker of apoptotic cells in zebrafish, we fused human A5 to YFP. Because A5 binding to apoptotic cells occurs extracellularly, we attached a secretion signal peptide preceding the human A5 gene fused to YFP (secA5-YFP) (Fig. 1A). To obtain secA5-YFP transgene expression in zebrafish, we took advantage of the UAS-Gal4 system (26). Zebrafish embryos expressing secA5-YFP from microinjected UAS-secA5-YFP DNA driven by ubiquitously expressed Gal4 (TBP promoter-Gal4/TBP-Gal4) showed a diffuse expression pattern, reflecting the extracellular accumulation of secA5-YFP (Fig. 1B). Here secA5-YFP also localized to several distinct rings around cells in the embryo, which we hypothesized were apoptotic cells (Fig. 1B and Supplemental Fig. S1). We compared secA5-YFP labeling with several other markers of apoptosis including DNA fragmentation (TUNEL), nuclear condensation (DAPI), and altered cellular morphology. We found that ∼50% of the cells labeled by secA5-YFP were also TUNEL positive in vitro (Fig. 1C, D, E). By counterstaining with DAPI, we also observed that the majority of secA5-YFP-labeled (secA5-YFP+) cells exhibited nuclear condensation, another hallmark of apoptosis (Fig. 1D, E). Almost all of the secA5-YFP+ cells we observed showed altered and rounded morphology consistent with apoptotic cells (5, 27). We observed several nonrounded cells that were secA5-YFP+, which quickly acquired a rounded morphology, indicating that secA5-YFP binding can occur before loss of normal morphology during apoptosis. Cells with the brightest TUNEL signal typically showed no secA5-YFP labeling and no nuclear staining (Fig. 1C–E). One explanation for this observation is that secA5-YFP labels cells in early stages of apoptosis, and that in the final stages of apoptosis where DNA fragmentation is greatest, apoptotic cells lack a defined cell membrane and nuclear architecture (10, 28). In line with this possibility, we often observed secA5-YFP+ debris near these high-intensity TUNEL spots (Fig. 1E). Thus, cells labeled with secA5-YFP are apoptotic and exhibit several hallmarks of apoptotic cells, including DNA fragmentation, nuclear condensation, altered morphology, and loss of membrane asymmetry; and secA5-YFP labeling allows quantification of apoptotic cells in embryonic animals.
Figure 1.
secA5-YFP labels apoptotic cells in live zebrafish embryos. A) Schematic representation of secreted secA5-YFP fusion construct. B) Representative fluorescence images of 1-d-old microinjected embryos ubiquitously expressing YFP or secA5-YFP. C) Venn diagram showing population of cells TUNEL+ (red) and secA5-YFP+ (blue) and those overlapping (purple). D, E) Images showing TUNEL, DAPI, and secA5-YFP labeling of paraformaldehyde-fixed 1-d-old secA5-YFP-expressing embryos. Arrows in panel D indicate a secA5-YFP-labeled cell with condensed chromatin that is negative for TUNEL; arrows in panel E show a TUNEL+ cell that is negative for secA5-YFP labeling and has no DAPI staining. Arrowheads in panel E show a secA5-YFP-labeled cell with condensed chromatin and TUNEL+ staining. Scale bar = 10|gmm.
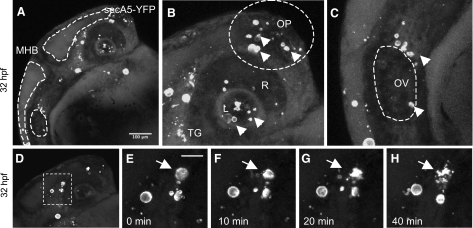
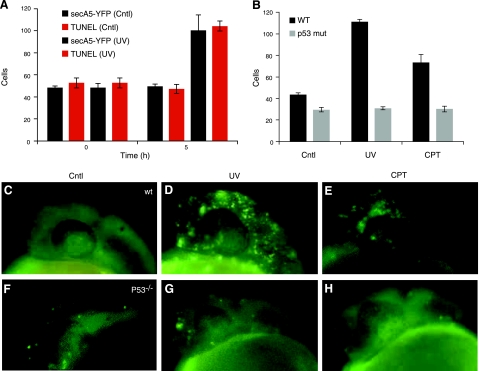
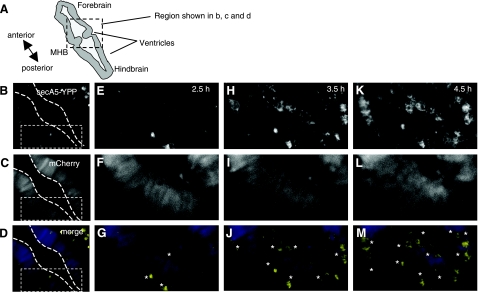
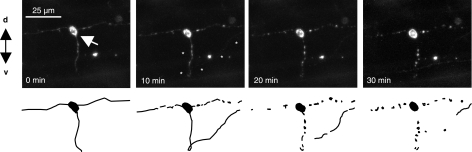
Apoptosis occurs in many tissues during specific phases of embryonic development, including the tailbud, the eye (retina and lens), the olfactory placode, and the otic vesicle. To study apoptotic cells during embryogenesis, we analyzed several secA5-YFP embryos during early development. We consistently observed a cluster of labeled cells rostral to the otic vesicle around 24–36 hpf (Fig. 2A, C). Apoptotic cells were also present within the lens, followed by the appearance of a larger number of apoptotic cells in the retina around 34 hpf (Fig. 2A, B). The anterior region of the brain containing the olfactory placode contained many apoptotic cells as well at 32 hpf (Fig. 2B). Interestingly, many of the labeled cells analyzed in the embryo were observed to form several large blebbings and to fragment (Fig. 2D–H). Rohon Beard neurons are primary sensory neurons with large cell bodies located dorsally within the spinal cord that project long branched dendritic arbors (29, 30). Many RB neurons are eliminated by apoptosis within 72 hpf. In transgenic secA5-YFP-expressing fish after 48 hpf not many labeled apoptotic cells could be observed throughout the trunk except several large (∼10 μm), round cells in the dorsal part of the spinal cord, likely reflecting apoptotic RB neurons (Supplemental Fig. S2). Thus, secA5-YFP transgenic zebrafish can be used to study developmental apoptotic cells at a high spatial and temporal resolution by live imaging. %Many forms of cellular damage can activate the apoptotic pathway by activation of the p53 tumor suppressor gene. To study the cell death response to DNA damage, we analyzed secA5-YFP apoptotic cells in 1-d-old embryos subjected to DNA-damaging UV irradiation. UV-irradiated embryos showed a 2-fold increase in the number of apoptotic cells compared to control embryos in vivo (Fig. 3A, C, D and Supplemental Fig. S3A). UV-irradiated embryos also contained twice as many TUNEL positive cells in vitro (Fig. 3A). By contrast, when we studied secA5-YFP apoptotic cells in p53 mutant animals treated with UV, the apoptotic response to DNA damage was abolished, and no ectopic cell death could be detected (20) (Fig. 3B–D, F, G and Supplemental Fig. S3B). We also examined cell death caused by several small molecules, including neurotoxins. We exposed secA5-YFP animals to a number of proapoptotic compounds, including CPT, etoposide, doxorubicin, colchicine, DTT, and MPTP. All of these compounds caused ectopic apoptosis (data not shown and Supplemental Fig. S4). CPT caused the strongest increase in the number of apoptotic cells and at concentrations lower than 1 μM. CPT causes DNA damage by inhibiting topoisomerase I (TOPI), and therefore the apoptotic response to CPT is likely also dependent on p53. Indeed, when we tested the effect of CPT in p53 mutant animals, the induction of ectopic apoptosis was lost (Fig. 3B, E, H). Thus, secA5-YFP can be used to quantify ectopic cell death in live animals, and to trace onset, dynamics, and progression of apoptosis. %We observed many apoptotic cells in the brain and spinal cord in CPT-treated animals (Supplemental Fig. S4). CPT and TOPI inhibition have been reported previously to cause neuronal cell death. For example, CPT causes apoptosis in cultured cortical neurons, and genetic and CPT-mediated TOPI inhibition cause apoptosis in the Drosophila brain (31, 32). To study the dynamics of ectopic cell death in the zebrafish embryonic brain, we took advantage of the strong induction of cell death by CPT. We used zebrafish transgenically expressing a red fluorescent protein (mCherry) in the nervous system and coexpressed secA5-YFP ubiquitously in the same animals. These animals were treated with CPT at ∼24 hpf and subjected to confocal imaging. We observed a large induction of labeled apoptotic brain cells within 6 h, showing individually labeled cells and rapidly changing cellular morphology (Fig. 4 and Supplemental Videos S1–S3). Thus, the secA5-YFP system allows dynamic live visualization of apoptotic cells within the embryonic central nervous system. %Because we observed that several cells showed changing morphology after secA5-YFP binding, secA5-YFP may allow us to study alterations in neuronal morphology during apoptosis in vivo. We have studied secA5-YFP labeling of axons and dendrites during development (Fig. 5 and Supplemental Video S4). Neurons labeled by secA5-YFP exhibit rapid fragmentation and dissolution of axons and dendrites, leaving only the rounded cell body behind (Fig. 5). The fragmentation coincides with alteration in morphology of the cell body (rounding), and these events appear to occur in synchrony. Because we observed secA5-YFP labeling before major alterations in neuronal morphology on several occasions, this suggests that membrane asymmetry occurs before these major alterations in neuronal morphology during apoptosis. Thus, secA5-YFP can be used to visualize dynamic alterations in neuronal axons and dendrites during the progression of cell death. Such features cannot be observed using TUNEL or other established apoptotic markers.
Figure 2.
secA5-YFP apoptotic cells during zebrafish embryogenesis. A–C) Dorsal is up, anterior to the right. Head region of 32-hpf transgenic secA5-YFP embryo (A), showing labeled cells mostly in anterior parts of the brain, around and within the eye (lens, retina, and trigeminal ganglia) (B) and near the otic vesicle (C). Mid-hindbrain boundary (MHB) shown between 2 brain ventricles indicated by dotted lines. D) Embryo shown in panel A, indicating area shown in subsequent panels. E–H) Area indicated in panel D at 0 min (E), 10 min (F), 20 min (G), and 40 min (H). MHB, mid-hindbrain boundary; L, lens; R, retina; OP, olfactory placode; OV, otic vesicle; SC, spinal cord; NC, notochord. Scale bars = 100 |gmm (A); 10 |gmm (E–H). Arrows and arrowheads indicate labeled apoptotic cells.
Figure 3.
p53-dependent ectopic apoptosis induced by UV and camptothecin. A) Quantification of secA5-YFP cells in control and UV-treated live embryos, and in vitro quantification of TUNEL+ cells in fixed control and UV-treated embryos. B) Quantification of secA5-YFP cells in wild-type and p53 mutant 1-d-old animals 5 h after UV irradiation or CPT treatment. C–H) Representative images of secA5-YFP cells in 1-d-old wild-type (C–E) and p53 mutant animals (F–H) after 5 h of control (C, F), UV irradiation (D, G) or CPT treatment (E, H). Error bars = sem; n = 10.
Figure 4.
Live imaging of ectopic apoptosis in the zebrafish embryonic brain. Time-lapse still images of mid-hindbrain area (dorsal view) in 26-hpf animals treated with CPT (0.5 |gmM). A) Schematic representation of zebrafish embryonic brain; boxed region is region shown in panels B–D. B) secA5-YFP-labeled cells. C) Neuronal mCherry-labeled cells. D) Merged fluorescence (yellow indicates secA5-YFP; blue indicates mCherry). B–M) Images shown, 0 (B–D), 2:30 (E–G), 3:30 (H–J), and 4:30 (K–M), are 2, 4.5, 5.5, and 6.5 h after treatment with camptothecin, respectively. Anteroposterior (A-P) axis is from upper left corner to lower right corner. MHB, mid-hindbrain boundary.
Figure 5.
secA5-YFP labels neuronal processes during apoptosis. Top panels: confocal time-lapse still images show spinal cord interneuron [likely a commissural primary ascending (CoPA) interneuron; see Hale et al., ref. (35) labeled by secA5-YFP. Dorsal is up, anterior to the right. Scale bar = 25 |gmm. Asterisks denote appearance of ascending part of axon. Bottom panels: schematic representation of fragmentation of neuronal arbors.
DISCUSSION
We present a method to visualize apoptotic cells in living zebrafish by a genetically encoded marker. The cells labeled using secA5-YFP show several characteristics of apoptotic cells. These features include condensed chromatin, TUNEL staining, blebbing, and fragmentation of the cells. Our data indicate secA5-YFP labeling can occur before major alterations in cell morphology. As indicated by secA5-YFP labeling of the many apoptotic cells in different temporal and spatial patterns during development, individual apoptotic cells can be tracked from appearance to fragmentation, and the disappearance of cell corpses. Therefore, this secA5-YFP system allows a thorough analysis of the dynamics of apoptosis occurring in vivo and will thereby allow a better understanding of the spatio-temporal dynamics of apoptosis during vertebrate development. This approach may aid as well in the characterization of zebrafish mutants recovered from forward genetic screens because mutant phenotypes, including behavioral phenotypes, may be related to neurodegeneration and apoptosis (33).
Acridine orange (AO) is a vital dye often used as a marker of apoptotic cells in zebrafish (7). Recently it was shown that AO can also be used in live imaging to observe cell death in the zebrafish embryonic spinal cord in a model of inherited cognitive disease related to mutant tau (34). Although this method may label many of the same apoptotic cells as labeled by secA5-YFP, there are several advantages of the secA5-YFP system. The secA5-YFP marker can be used noninvasively to quantify apoptosis in embryos within their chorions. It also allows detection of morphological changes occurring during apoptosis, such as cell shrinkage, fragmentation, and loss of neuronal axons and dendrites. In addition, secA5-YFP bound to apoptotic cells can be detected in fixed tissue and can therefore also be combined with immunohistochemistry and ultrastructural studies, and fluorescent proteins of different spectral properties can be used in place of YFP to accommodate specific imaging requirements. It will be of interest to assess the potential for visualizing apoptosis at later stages in secA5-YFP transgenic animals, and to study whether it reports on neurodegeneration of spinal neurons in response to overexpression of mutant tau or proteins related to neurodegenerative disease (34).
In all, this genetically encoded secA5-YFP system allows for quantitative, in vivo imaging of apoptotic cells during development and creates the opportunity to perform genetic or small molecule screening to find new modifiers of apoptosis. For example, finding genetic targets or small molecules that enable irradiation-induced apoptosis in the absence of p53 would be of great therapeutic interest, given that over 50% of cancers are p53 deficient. Second, we can now visualize apoptotic neurons as they arise in the central nervous system during development, in response to dominant neurodegenerative disease mutations (such as mutant alpha-synuclein and superoxide dismutase) or in response to neurotoxic chemicals.
Supplementary Material
Acknowledgments
The authors thank the members of the R.T.P. laboratory for technical assistance and advice, Lindsay Cade for graphical expertise, Ding Xue (University of Colorado, Boulder, CO, USA) for helpful comments, David Langenau and Myron Ignatius (Massachusetts General Hospital Cancer Center, Charlestown, MA, USA) for p53 mutant animals, Chetana Satchidanandan (Institute of Genomics and Integrative Biology, Delhi, India) and Martin Distel and Reinhard Koester (Helmholtz Institute, Munich, Germany) for transgenic animals, the Koichi Kawakami laboratory (National Institute of Genetics, Mishima, Japan) for reagents, Anne Hart for use of equipment, the Michael L. Nonet laboratory (Washington University, St. Louis, MO, USA) for plasmids, and Iain Drummond (Massachusetts General Hospital, Charlestown, MA, USA) for advice on microscopy.
This work was supported by National Institutes of Health (NIH) grants NS063733 and MH086867. This work was supported in part by the NIH/National Cancer for Research Resources Centers of Biomedical Research Excellence in Obesity and Cardiovascular Disease, grant P20 RR021954.
REFERENCES
- 1.Gordon N. (1995) Apoptosis (programmed cell death) and other reasons for elimination of neurons and axons. Brain Dev. 17, 73– 77 [DOI] [PubMed] [Google Scholar]
- 2.Kuida K., Zheng T. S., Na S., Kuan C.-Y., Yang D., Karasuyama H., Rakic P., Flavell R. A. (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384, 368– 372 [DOI] [PubMed] [Google Scholar]
- 3.Mattson M. P. (2000) Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell. Biol. 1, 120– 129 [DOI] [PubMed] [Google Scholar]
- 4.Vila M., Przedborski S. (2003) Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 4, 365– 375 [DOI] [PubMed] [Google Scholar]
- 5.Kerr J. F., Wyllie A. H., Currie A. R. (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239– 257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lecoeur H. (2002) Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases. Exp. Cell. Res. 277, 1– 14 [DOI] [PubMed] [Google Scholar]
- 7.Hammerschmidt M., Pelegri F., Mullins M. C., Kane D. A., van Eeden F. J. M., Granato M., Brand M., Furutani-Seiki M., Haffter P., Heisenberg C.-P., Jiang Y.-J., Kelsh R. N., Odenthal J., Warga R. M., Nüsslein-Volhard C. (1996) Dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123, 95– 102 [DOI] [PubMed] [Google Scholar]
- 8.Gavrieli Y., Sherman Y., Ben-Sasson S. A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119, 493– 501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207– 2216 [PubMed] [Google Scholar]
- 10.Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545– 1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koopman G., Reutelingsperger C. P., Kuijten G. A., Keehnen R. M., Pals S. T., van Oers M. H. (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84, 1415– 1420 [PubMed] [Google Scholar]
- 12.Van Genderen H., Kenis H., Lux P., Ungeth L., Maassen C., Deckers N., Narula J., Hofstra L., Reutelingsperger C. (2006) In vitro measurement of cell death with the annexin A5 affinity assay. Nat. Protoc. 1, 363– 367 [DOI] [PubMed] [Google Scholar]
- 13.Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253– 310 [DOI] [PubMed] [Google Scholar]
- 14.Van Eeden F. J., Granato M., Odenthal J., Haffter P. (1999) Developmental mutant screens in the zebrafish. Methods Cell Biol. 60, 21– 41 [DOI] [PubMed] [Google Scholar]
- 15.Zon L. I., Peterson R. T. (2005) In vivo drug discovery in the zebrafish. Nat. Rev. Drug Disc. 4, 35– 44 [DOI] [PubMed] [Google Scholar]
- 16.Kimmel C. B. (1993) Patterning the brain of the zebrafish embryo. Annu. Rev. Neurosci. 16, 707– 732 [DOI] [PubMed] [Google Scholar]
- 17.Cole L. K., Ross L. S. (2001) Apoptosis in the developing zebrafish embryo. Dev. Biol. 240, 123– 142 [DOI] [PubMed] [Google Scholar]
- 18.Schoenwolf G. C. (1981) Morphogenetic processes involved in the remodeling of the tail region of the chick embryo. Anat. Embryol. (Berl.) 162, 183– 197 [DOI] [PubMed] [Google Scholar]
- 19.Solnica-Krezel L., Stemple D. L., Driever W. (1995) Transparent things: cell fates and cell movements during early embryogenesis of zebrafish. Bioessays 17, 931– 939 [DOI] [PubMed] [Google Scholar]
- 20.Berghmans S., Murphey R. D., Wienholds E., Neuberg D., Kutok J. L., Fletcher C. D. M., Morris J. P., Liu T. X., Schulte-Merker S., Kanki J. P., Plasterk R., Zon L. I., Look A. T. (2005) tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. U. S. A. 102, 407– 412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Distel M., Wullimann M. F., Koster R. W. (2009) Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc. Natl. Acad. Sci. U. S. A. 106, 13365– 13370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link C. D. (1995) Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 92, 9368– 9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burket C. T., Montgomery J. E., Thummel R., Kassen S. C., LaFave M. C., Langenau D. M., Zon L. I., Hyde D. R. (2008) Generation and characterization of transgenic zebrafish lines using different ubiquitous promoters. Transgenic Res. 17, 265– 279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami K. (2004) Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 77, 201– 222 [DOI] [PubMed] [Google Scholar]
- 25.Lowery L. A., Sive H. (2005) Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development 132, 2057– 2067 [DOI] [PubMed] [Google Scholar]
- 26.Scheer N., Campos-Ortega J. A. (1999) Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 80, 153– 158 [DOI] [PubMed] [Google Scholar]
- 27.Robertson A. M., Bird C. C., Waddell A. W., Currie A. R. (1978) Morphological aspects of glucocorticoid-induced cell death in human lymphoblastoid cells. J. Pathol. 126, 181– 187 [DOI] [PubMed] [Google Scholar]
- 28.Collins J. A., Schandi C. A., Young K. K., Vesely J., Willingham M. C. (1997) Major DNA fragmentation is a late event in apoptosis. J. Histochem. Cytochem. 45, 923– 934 [DOI] [PubMed] [Google Scholar]
- 29.Grunwald D. J., Kimmel C. B., Westerfield M., Walker C., Streisinger G. (1988) A neural degeneration mutation that spares primary neurons in the zebrafish. Dev. Biol. 126, 115– 128 [DOI] [PubMed] [Google Scholar]
- 30.Lamborghini J. E. (1987) Disappearance of Rohon-Beard neurons from the spinal cord of larval Xenopus laevis. J. Comp. Neurol. 264, 47– 55 [DOI] [PubMed] [Google Scholar]
- 31.Song J., Parker L., Hormozi L., Tanouye M. A. (2008) DNA topoisomerase I inhibitors ameliorate seizure-like behaviors and paralysis in a Drosophila model of epilepsy. Neuroscience 156, 722– 728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris E. J., Geller H. M. (1996) Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J. Cell Biol. 134, 757– 770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granato M., van Eeden F. J., Schach U., Trowe T., Brand M., Furutani-Seiki M., Haffter P., Hammerschmidt M., Heisenberg C.-P., Jiang Y.-J., Kane D. A., Kelsh R. N., Mullins M. C., Odenthal J., Nüsslein-Volhard C. (1996) Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123, 399– 413 [DOI] [PubMed] [Google Scholar]
- 34.Paquet D., Bhat R., Sydow A., Mandelkow E.-M., Berg S., Hellberg S., Fälting J., Distel M., Köster R. W., Schmid B., Haass C. (2009) A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J. Clin. Invest. 119, 1382– 1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hale M. E., Ritter D. A., Fetcho J. R. (2001) A confocal study of spinal interneurons in living larval zebrafish. J. Comp. Neurol. 437, 1– 16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.