Abstract
Multiple sclerosis (MS) is a chronic, debilitating disease of the central nervous system (CNS) characterized by demyelination and axon loss. The proinflammatory cytokine macrophage migration inhibitory factor (MIF) has been shown to be elevated in the cerebrospinal fluid of patients during relapses. The purpose of this study was to evaluate a new small-molecule inhibitor of MIF and its ability to reduce the severity of an animal model of MS, experimental autoimmune encephalomyelitis (EAE). We utilized 2 structurally related isoxazolines, which show in vitro inhibition of MIF tautomerase activity. We found that administration of an inhibitor of MIF to mice with established EAE immediately reduced the severity of clinical signs and expanded a population of regulatory T lymphocytes. We also noted that the inhibitor reduced relapses of disease in a relapsing/remitting model of EAE. An analysis of leukocyte migration into the brain revealed that administration of inhibitor reduced entry of these cells. No effects on inflammatory cytokine production or T-cell activation in the periphery were noted. From these studies, we conclude that a small-molecule inhibitor of MIF reduces the severity of EAE and prevents access of immune cells into the CNS, which could be of therapeutic relevance to MS.—Kithcart, A. P., Cox, G. M., Sielecki, T., Short, A., Pruitt, J., Papenfuss, T., Shawler, T., Gienapp, I., Satoskar, A. R., Whitacre, C. C. A small-molecule inhibitor of macrophage migration inhibitory factor for the treatment of inflammatory disease.
Keywords: experimental autoimmune encephalomyelitis, multiple sclerosis, blood-brain barrier
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating disease of the central nervous system (CNS) characterized by progressive motor and sensory loss (1, 2). MS affects 2 million people worldwide and >400,000 in the United States, 80% of whom are diagnosed with a relapsing-remitting form of disease (1). Typically identified during the second or third decade of life, MS is the leading cause of nontraumatic disability in young adults. Although the cause is unknown, it is generally accepted that MS is associated with myelin-reactive CD4+ T lymphocytes that gain access to the CNS and cause damage to the myelin sheath and nerve axons (3–9).
During episodes of worsening disease in MS, there is an up-regulation of inflammatory cytokines, including IFN-γ and IL-17 (10). Recently the proinflammatory cytokine macrophage migration inhibitory factor (MIF) was identified as elevated in the cerebrospinal fluid of patients during a relapse of MS (11). MIF, first reported in 1966, is produced by lymphocytes and inhibits macrophage trafficking (12). Since then, its up-regulation has been linked to a number of inflammatory diseases, including rheumatoid arthritis, atherosclerosis, diabetes, sepsis, cancer, colitis, and several infectious diseases (13–21). MIF was cloned and found to be a homotrimer of 12.5-kDa subunits, containing oxidoreductase and tautomerase enzymatic activity (22–24). Several studies have suggested substrates for the enzymatic activity, including phenylpyruvic acid, p-hydroxyphenylpyruvic acid, 3,4-dihydroxyphenylaminechrome, and norepinephrinechrome (22, 25). Other work has shown that MIF may function by binding to CD74 and other noncognate ligands, including CXCR2 and CXCR4 (26, 27). Because of its homotrimeric structure, there are multiple binding sites for potential inhibitors of MIF that could disrupt the tertiary structure of the cytokine and thus inhibit its enzymatic activity or binding to CD74 and other ligands.
Most current therapies for MS are anti-inflammatory in their action and act to suppress ongoing disease (28). One evolving area of MS treatment is targeting of adhesion molecules on T lymphocytes (29). Although this approach to therapy has proven efficacious in clinical trials, several patients have developed infectious complications as a result of suppression of immune surveillance in the CNS (30). Determining a safe means to block entry of autoreactive lymphocytes into the brain and spinal cord is an important focus of MS research.
In this study, we evaluate the role of MIF in the progression of an animal model of MS, experimental autoimmune encephalomyelitis (EAE). We show that genetic deletion of MIF lessens the severity of EAE, and administration of a small-molecule inhibitor of MIF halts the progression of disease and reduces migration of leukocytes into the brain and spinal cord of treated animals. Furthermore, administration of an MIF inhibitor expanded a peripheral population of regulatory T lymphocytes. Finally, we show that the expression of MIF facilitates the up-regulation of the adhesion molecule VCAM. Our data suggest that MIF inhibitors provide a novel approach for treating inflammatory disease, including MS.
MATERIALS AND METHODS
Mice and induction of EAE
C57Bl/6 male mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). To induce EAE, animals were immunized with 200 μg myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (Princeton Biomolecules, San Jose, CA, USA) in complete Freund's adjuvant (containing 200 μg Mycobacterium tuberculosis Jamaica strain), injected intradermally in each of 4 flanks. Pertussis toxin (List Biological Labs, Campbell, CA, USA) was injected as an additional adjuvant intraperitoneally (i.p.) on the day of immunization and 48 h later (200 ng). Female SJL mice were purchased from Jackson Laboratories and immunized with 150 μg proteolipid protein (PLP) peptide 139–151 (Princeton Biomolecules) in complete Freund's adjuvant. All animals were observed daily for clinical signs and scored as follows: 0 = no paralysis, 1 = limp tail or ataxia, 2 = limp tail with ataxia, 3 = partial hind-limb paralysis, 4 = complete hind-limb paralysis, and 5 = death. Cumulative disease index was calculated as the sum of daily clinical scores from each animal during the observation period and reported as an average within each group. Mice were maintained on a 12-h light/dark cycle and given food and water ad libitum. All animal procedures were performed in accordance with institutional review board-approved animal use protocols.
Drug administration
Orally available small-molecule inhibitors of MIF, CPSI-1306, and CPSI-2705 (gifts from Cytokine PharmaSciences, King of Prussia, PA, USA; patent application no. 20050250826) or vehicle control (15% DMSO in 0.5% methylcellulose and H2O) was given orally (p.o.) daily as described in the results. The inhibitor was administered at 1.0 mg/kg, and the total volume administered daily was 50 μl.
Flow cytometry
Single-cell suspensions derived from lymph nodes draining the sites of immunization (inguinal, axillary, brachial, cervical, popliteal, and periaortic) and spleens were stained for CD3, CD4, CD8, CD11b, CD11c, CD19, and CD25 using directly conjugated antibodies (BD Biosciences, San Jose, CA, USA). Isotype control mAbs (BD Biosciences) were matched for each fluorochrome. Cells were labeled at 1 × 106 cells/tube, incubated for 30 min at 4°C, and analyzed using a BD FACSCalibur flow cytometer (BD Biosciences). Intracellular expression of FoxP3 was measured using APC-labeled mAb (R&D Systems, Minneapolis, MN, USA) and processed according to the manufacturer's instructions.
Lymphoid cell proliferation and cytokine measurement
Leukocytes were obtained from lymph nodes and spleens and cultured in RPMI 1640 (containing 10% FBS, 25 mM HEPES, 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 5×10−5M 2-ME) with MOG35–55 (20 μg/ml) or medium alone for 72 h in 96-well round-bottom plates (4×105 cells/well). Cells were pulsed for 12 h with 3H thymidine (1 μCi/well) prior to harvest. Proliferation was evaluated using a Packard BioScience TopCount NKT luminescence counter (Packard Bioscience, Meriden, CT, USA). Supernatants from unpulsed plates were harvested at 72 h and evaluated for the presence of IL-10, IL-17, and IFN-γ by enzyme immunoassay according to the manufacturer's instructions (BD Biosciences). Levels of cytokines were measured using a SPECTRAplus ELISA reader and quantified against a standard curve by SOFTmax PRO software (Molecular Devices, Sunnyvale, CA, USA).
Histopathologic assessment
Immunohistochemical and H&E staining were carried out by the Ohio State University Veterinary Sciences core facility. Animals were perfused with 0.1 M cold PBS at the end of inhibitor treatment. Brains and spinal cords were removed and fixed in 4% paraformaldehyde. H&E sections were graded in blinded fashion from 4 representative sections of the brain and spinal cord for mononuclear cell infiltration: 0 = no inflammation, 1 = few inflammatory cells, 2 = organization of perivascular infiltrates, and 3 = perivascular cuffing (adapted from Fitzgerald et al.; ref. 31).
Immunofluorescent staining
Wild-type and MIF−/− C57Bl/6 mice were perfused intracardially with 0.1 M PBS and 4% paraformaldehyde (PFA) and postfixed in 4% PFA. Spinal cords were flash-frozen in optimal cutting temperature (OCT) compound and cut into 10-μm sections. Purified VCAM-1, ICAM-1, and CD31 antibodies (BD Biosciences) were applied to tissue. Fluorochrome-conjugated secondary antibodies were used for detection. For analysis of staining intensity, MCID elite 6.0 image analysis software (Imaging Research, Waukesha, WI, USA) was used.
Statistical analysis
Statistical significance between groups for cumulative disease index, mean clinical score, and day of onset was calculated using Student's t test. Measures of lymphocyte populations, cytokine production, and proliferation also used the t test. Significance for incidence was calculated using a χ2 test. Significance was recorded as P < 0.05.
RESULTS
MIF-deficient C57Bl/6 mice are protected from EAE
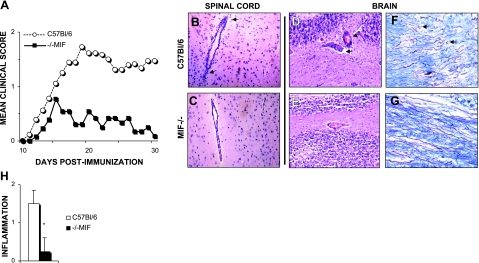
We previously reported that progression of EAE was suppressed in C57Bl/6 × 129 F1 mice lacking MIF (32). Because the 129 genetic background has been thought to be a confounder in EAE susceptibility studies, we repeatedly backcrossed MIF−/− C57Bl/6 × 129 mice onto the C57Bl/6 background. Following EAE induction with MOG35–55 peptide, we found that those C57Bl/6 mice lacking MIF had significantly fewer severe clinical signs of disease, during both the acute and chronic phases of EAE (Fig. 1A). Knockout animals had a reduced incidence of EAE, cumulative disease index, and peak clinical score. However, we observed no difference in the day of onset (Table 1).
Figure 1.
Genetic deletion of MIF is protective against EAE. MIF-deficient (■) and wild-type (○) male C57Bl/6 mice were immunized with MOG35–55 and adjuvant on d 0. Clinical scores for each animal were monitored daily, and means were reported for each group. A) MIF-knockout mice were protected from EAE relative to wild-type controls. B–E) H&E staining of spinal cord (B, C) and brain sections (D, E) showed less perivascular infiltration in MIF−/− mice (C, E) vs. wild-type animals (B, D). F, G) Luxol fast blue with silver contrast showed less axonal severing in knockout mice (G) vs. wild-type (F). H) Quantification is representative of 4 sections of the brain and spinal cord (B–E). Results are representative of 3 separate experiments. *P < 0.05.
Table 1.
MIF-knockout mice have reduced incidence and severity of EAE
| Genotype | Incidence | Onset | CDI | Peak score |
|---|---|---|---|---|
| C57Bl/6 | 15/18 (83%) | 15.9±4.6 | 24.8±18.1 | 2.2±1.3 |
| MIF−/− | 7/13 (54%)** | 14.6±2.3 | 6.4±8.1** | 0.9±1.0** |
Values are means ±sd. Day of onset was calculated as the first day of a clinical score ≥1 for each animal and averaged within each group. Cumulative disease index (CDI) was based on the sum of clinical scores over the entire observation period for each animal and averaged within each group. Peak score was measured over the duration of disease per animal and averaged.
P < 0.01.
We explored whether MIF−/− mice were protected from EAE due to an immunosuppressive environment. Despite dramatic differences in clinical scores, we observed no significant differences in levels of IL-10, IL-17, or IFN-γ produced by lymph node or spleen cells isolated from knockout animals (Table 2). This suggested that MIF was not required for TH1-mediated immunity, and the absence of MIF did not substantially shift the immune system to a protective TH2 environment. Cells isolated from both lymphoid organs proliferated equally well to MOG35–55 in vitro (Table 2). Furthermore, there were no differences in the populations of B lymphocytes isolated from the lymph nodes and no net shift in the population of CD11b- or CD11c-positive antigen-presenting cells (Table 2). This was observed in naive knockout and wild-type animals as well (data not shown), suggesting that knockout animals do not exhibit an immunological deficit. There was a larger population of CD4+ CD25+ lymphocytes in knockout mice, and these mice had a higher expression of Foxp3, a transcription factor expressed in regulatory cells (Table 2). We also noted enhanced mononuclear cell infiltration in the brains and spinal cords of wild-type mice vs. MIF−/− (Fig. 1B–E, H). MIF-knockout mice had significantly less inflammation and infiltration. Also, we noted that MIF-knockout mice had less axonal severing as measured by staining with luxol fast blue with silver stain contrast (Fig. 1F, G). These data led us to postulate that 2 mechanisms could be responsible for the reduced EAE in MIF-knockout mice: an enhanced population of protective regulatory T lymphocytes, or reduced CNS migration of pathogenic T cells. We pursued a small-molecule inhibitor of MIF to test whether these factors could be reproduced in a therapeutic setting.
Table 2.
MIF-knockout mice have functionally similar peripheral immune systems
| Parameter | C57Bl/6 | MIF−/− |
|---|---|---|
| Proliferation (CPM) | ||
| MOG35–55 | 2.3 × 104±0.6 | 2.2 × 104±0.7 |
| Anti-CD3 | 5.0 × 104±0.6 | 4.4 × 104±0.3* |
| Cytokines (ng/ml) | ||
| IL-10 | 1.8±1.9 | 9.2±8.9 |
| IFN-γ | 16.4±17.5 | 7.8±3.1 |
| IL-17 | 1.4±0.7 | 1.1±0.2 |
| Leukocytes (%) | ||
| CD4 | 22.3±2.0 | 21.3±2.5 |
| CD8 | 18.2±0.5 | 19.5±0.9 |
| CD19 | 53.2±4.2 | 51.0±0.8 |
| CD11c | 1.4±0.2 | 1.45±0.5 |
| CD11b | 8.8±0.4 | 9.4±0.7 |
| CD4/CD25 | 4.2±0.2 | 5.0±0.4* |
| Foxp3 | 3.8±0.2 | 4.6±0.3* |
P < 0.05.
A small-molecule inhibitor of MIF reduces ongoing inflammatory disease
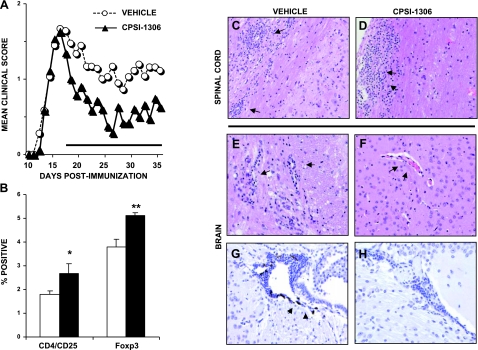
We evaluated the ability of a small-molecule inhibitor of MIF to slow the progression of EAE. We utilized 2 structurally related isoxazolines, CPSI-1306 and CPSI-2705, which have been previously described to inhibit the tautomerase activity of MIF (33). We immunized wild-type C57Bl/6 mice with MOG35–55 and monitored them daily for clinical scores. At 17 d postimmunization, we randomized animals into 2 groups, one receiving 1 mg/kg CPSI-1306 orally and the other receiving a vehicle control. Mice were fed daily for 21 d. Mice receiving inhibitor showed an immediate and significant improvement in clinical disease score (Fig. 2A). Treated mice had a lower cumulative disease index during the treatment period than those mice receiving vehicle (12.9±14.9 in inhibitor-treated mice vs. 22.5±18.6) and a lower mean clinical score (0.6±0.7 vs. 1.1±1.0, P<0.05). Prior to drug administration, the peak score was similar between groups (2.0±1.2 vs. 2.1±1.2).
Figure 2.
A small-molecule inhibitor of MIF is therapeutic in a C57Bl/6 model of EAE. Male C57Bl/6 mice were induced for EAE and monitored for clinical signs. At 17 d postinduction, mice in the inhibitor-treated group (▴) were fed a daily dose of MIF inhibitor. Vehicle control mice (○) were fed a dose of 15% DMSO in 0.5% methylcellulose. Mean clinical scores within each group are reported in text. A) Mice fed inhibitor had reduced severity of disease immediately following administration relative to vehicle controls. B) Splenocytes collected 10 d following inhibitor administration had a larger population of regulatory T lymphocytes. C, D) There was no difference in infiltration by H&E stain at the end of treatment period in the spinal cord. E, F) Inhibitor-treated mice had significantly less infiltration in the brain. G, H) F4/80+ stained macrophages were not identified in inhibitor-treated mice.
Following the end of treatment, we evaluated the inflammatory environment of mice treated with the MIF inhibitor. As in the knockout mice following EAE, there was no significant shift in inflammatory or antiinflammatory cytokines, including IL-10, IL-17, and IFN-γ, as a result of inhibitor treatment (Table 3). Also as in the knockout mice, there were no differences in proliferation to MOG35–55 peptide or anti-CD3 (Table 3). As we observed in knockout mice, cytometric analysis of splenocytes isolated after treatment with inhibitor showed an expanded population of CD4+ CD25+ lymphocytes (Fig. 2B). The expression of FoxP3 in inhibitor-treated mice was also higher, suggesting a larger regulatory population of T lymphocytes.
Table 3.
MIF inhibitor-treated mice had a larger population of regulatory T cells
| Parameter | Vehicle | CPSI-1306 |
|---|---|---|
| Proliferation (CPM) | ||
| MOG35–55 | 3.2 × 104±0.3 | 2.5 × 104±1.2 |
| Anti-CD3 | 2.2 × 104±0.2 | 2.4 × 104±0.3 |
| Cytokines (ng/ml) | ||
| IL-10 | ND | ND |
| IFN-γ | 12.2±6.9 | 5.9±4.4 |
| IL-17 | 5.0±2.8 | 3.2±2.2 |
| Leukocytes (%) | ||
| CD4 | 24.4±1.0 | 26.8±0.7** |
| CD8 | 17.5±0.6 | 19.8±0.9* |
| CD19 | 50.8±2.5 | 45.0±0.8* |
| CD11c | 1.6±0.4 | 1.8±0.7 |
| CD11b | 3.4±1.0 | 4.7±2.7 |
| CD4/CD25 | 1.8±0.1 | 2.7±0.4* |
| Foxp3 | 3.8±0.3 | 5.1±0.1** |
ND, not detected.
P < 0.05;
P < 0.01.
An inhibitor of MIF reduces relapses and lengthens remissions in SJL mice
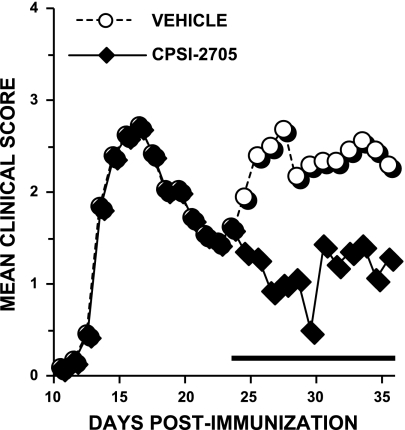
We next evaluated whether an inhibitor of MIF could suppress disease in a relapsing-remitting model of MS. Using female SJL mice, we began oral administration of a second MIF inhibitor, CPSI-2705, during remission following acute disease. Mice receiving inhibitor remained in remission and were significantly protected relative to vehicle-treated animals (Fig. 3). Furthermore, the cumulative disease index was lower for those mice receiving inhibitor compared to control animals (13.9±14.5 in inhibitor-treated mice vs. 28.3±10.9, P<0.05) following drug administration.
Figure 3.
An MIF inhibitor prevents relapses of EAE in SJL mice. Female SJL mice were immunized with PLP 139–151 and monitored for clinical signs. Following the first relapse of disease, at d 24, mice in the inhibitor-treated group (♦) were fed a daily dose of MIF inhibitor. Vehicle control mice (○) were fed a dose of 15% DMSO in 0.5% methylcellulose. Mean clinical scores within each group are reported in text. Inhibitor-treated animals failed to have relapses of disease.
Administration of an inhibitor of MIF prevents mononuclear infiltration into the CNS
We evaluated whether an inhibitor of MIF could prevent infiltration of leukocytes into the CNS. We previously reported that MIF−/− C57Bl/6 × 129 of mice had reduced CNS infiltration following EAE (32) and showed similar results in MIF−/− C57Bl/6 mice (Fig. 1B, C). After 21 d of inhibitor administration, we removed spinal cord and brain sections from mice and evaluated them for inflammatory infiltrates. Although we found no differences in infiltration in the spinal cord, there was significantly less mononuclear infiltration in the brain of inhibitor-treated mice (Fig. 2C–F). We also looked specifically at macrophages and their ability to infiltrate the CNS by labeling sections of brain and spinal cord with antibodies recognizing F4/80, a marker specific for macrophages. We found little or no infiltration of macrophages in the spinal cord (data not shown), but significant infiltration in the brains of vehicle-treated mice (Fig. 2G). There was no measurable infiltration of macrophages in the brains of inhibitor-treated mice (Fig. 2H).
MIF-deficient mice have reduced expression of VCAM-1 but not ICAM-1
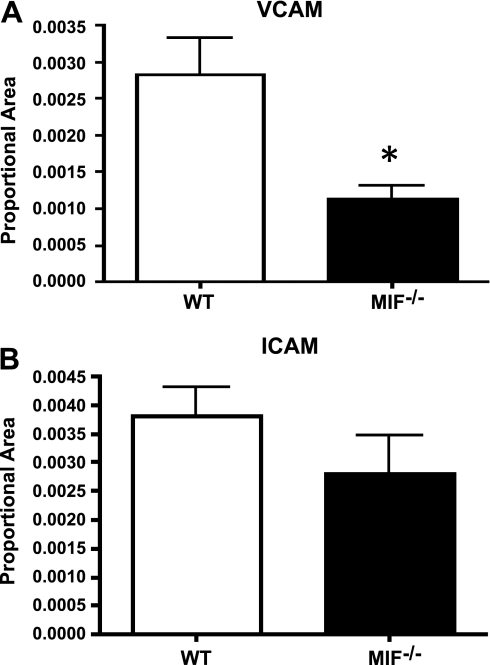
Following reduced migration of leukocytes into the CNS in both MIF-knockout and MIF inhibitor-treated mice with EAE, we explored the expression of adhesion molecules on the blood-brain barrier. Using immunofluorescence, we found MIF-knockout mice had reduced expression of the adhesion molecule VCAM-1 on CD34-labeled blood vessels within the brain (Fig. 4A). We found no difference in the expression of ICAM-1 between MIF-knockout and wild-type mice (Fig. 4B).
Figure 4.
Absence of MIF reduces expression of VCAM-1 but not ICAM-1. CNS tissue was collected on d 10 postimmunization from wild-type (WT) and MIF−/− C57Bl/6 mice. Immunofluorescent staining was used to quantify the expression of VCAM-1 (A) and ICAM-1 (B) in spinal cord sections. Proportional area was quantified using MCID elite 6.0. Data are representative of 10 random fields averaged over 4 mice/group. Each animal had 3 sections representing different levels of the neuroaxis. Expression of VCAM-1 and ICAM-1 on endothelial cells was confirmed by colabeling with CD31. *P < 0.05.
DISCUSSION
MIF has been shown to be expressed in a number of different cell types and its presence linked to a variety of inflammatory diseases, including atherosclerosis, diabetes, rheumatoid arthritis, and cancer. MIF is elevated in the cerebrospinal fluid of MS patients experiencing a relapse of symptoms. The role of MIF was first linked to EAE through the use of anti-MIF antibodies, in which administration of these antibodies inhibited the progression of disease in SJL mice (34). In this report we extend earlier findings in the F1 C57Bl/6 × 129 MIF−/− mice (32) and show therapeutic potential of a small-molecule inhibitor of MIF.
Following backcrossing of the C57Bl/6 × 129 mice onto the C57Bl/6 strain, we immunized mice with MOG35–55 peptide. Corroborating earlier reports from our lab, we found that mice lacking MIF had reduced progression of disease, with most mice fully recovering (Fig. 1). The results of cytokine and flow cytometric analysis showed no differences in the responses of knockout or wild-type mice following EAE. We examined levels of 2 known inflammatory cytokines, IFN-γ and IL-17, in lymphocytes derived from draining lymph nodes and the spleen and found no significant differences in protein levels. However, there was a significant reduction in disease severity and fewer CNS inflammatory infiltrates in knockout mice (Fig. 1A–C). This supports earlier evidence that showed that MIF was necessary for the progression of EAE but not required for initial activation of autoreactive lymphocytes. Thus, we tested recently developed small-molecule inhibitors of MIF for their ability to modulate inflammatory responses. Specifically, we explored whether such an inhibitor could slow progression of EAE and halt migration of inflammatory cells into the CNS.
As we have shown, small-molecule inhibitors of MIF are therapeutic in 2 models of EAE. C57Bl/6 mice exhibit an acute period of disease followed by chronic progression. When given during acute EAE, CPSI-1306 reduced the severity of disease and delayed progression. The SJL model mimics the relapsing-remitting course of MS observed in most patients. Current therapies available reduce the severity of relapses but few lengthen the time between relapses. These data suggest that an inhibitor of MIF could slow progression of both remitting-relapsing and progressive forms of MS.
This study showed that small-molecule inhibitors of MIF could prevent migration of inflammatory cells into the CNS after induction of EAE. Inflammation was significantly reduced in the brains of inhibitor-treated animals. However, there was no difference in inflammation in the spinal cords. This important difference is likely due to the timing of inhibitor treatment. EAE is characterized by ascending paralysis, by which inflammation begins in the distal spinal cord and spreads superiorly through the course of disease (35). We began administration of an MIF inhibitor at d 17 postimmunization, when leukocyte migration was already present in the spinal cord, but before we would predict to find inflammation in the brain. We propose that inhibitors of MIF do not reverse previous leukocyte infiltration but slow infiltration of subsequent inflammatory cells into the brain. In our current model, we cannot differentiate new vs. existing inflammation in the CNS. However, our data support earlier published results that show MIF is critical for the progression of inflammatory disease, but unnecessary for the induction of EAE. Future studies could further define our understanding of the timing of MIF expression during inflammation and the mechanism in which MIF mediates migration.
We explored whether the absence of MIF in knockout mice changed the expression of MIF receptors, including CD74, CXCR2, and CXCR4, and found no differences in the expression of these surface molecules and chemokine receptors (data not shown). We then searched for other mechanisms in which MIF could control leukocyte trafficking. Denkinger et al. (34) proposed that MIF mediates migration through expression of adhesion molecules along the blood-brain barrier. Those researchers showed that following administration of an anti-MIF antibody, expression of the adhesion molecule VCAM-1 was reduced. VCAM-1, and others in its class, including ICAM-1, are important mediators of adhesion in the blood-brain barrier and have been shown to be important in the pathogenesis of MS (36, 37). We sought to measure the expression of VCAM-1 and ICAM-1 in MIF-knockout mice. We report here that MIF-deficient mice have reduced expression of VCAM-1 and comparable levels of ICAM-1 within the CNS. We predict similar changes following administration of an MIF inhibitor, the results of which would slow leukocyte migration into the brain and spinal cord. This is supported by data in which we show fewer F4/80+ macrophages in the perivascular space of mice treated with CPSI-1306, compared to significant infiltration in vehicle treated animals. Ongoing or previous migration is not reversed in the spinal cord, but MIF inhibitor does prevent subsequent new infiltration into the CNS.
The early improvement in clinical scores within 2–3 d suggests that MIF also reduces ongoing inflammation. Down-regulation of adhesion molecules does not alone explain the immediate response to the MIF inhibitors. We noted an expanded population of regulatory T lymphocytes in both knockout and inhibitor-treated mice. Although we observed a modest increase in the percentage of lymphocytes expressing FoxP3, previous studies have shown as few as 106 CD4+CD25+ lymphocytes can have a profound effect on inflammatory disease (38). In our model we predict even a small change in the population of these regulatory cells can alter the inflammatory environment. We propose the expression of MIF reduces the population of these regulatory cells, which allows inflammation in the context of EAE in both the brain and spinal cord. Further studies are needed to determine whether, in the absence of MIF, regulatory T cells selectively traffic to the CNS and by what mechanism MIF may alter the population of regulatory cells.
We have shown that small-molecule inhibitors of MIF, which reduce the biological activity of MIF, are therapeutic in a mouse model of MS and prevent the migration of inflammatory cells into the CNS. Initial data suggest that MIF is required during a critical step of cell migration and may influence the population of regulatory lymphocytes. We believe that these inhibitors could have therapeutic potential in MS patients if given early in the course of disease. Other groups have recently reported the therapeutic efficacy of MIF inhibitors in an animal model for noninsulin-dependent diabetes (39). This would suggest MIF modulates inflammation through a number of antigen-specific mechanisms, possibly in addition to the ones proposed here. Furthermore, because of their oral availability and low dosing, they could be a novel therapeutic option for patients with MS and other inflammatory diseases.
Acknowledgments
The authors thank additional members of the C.C.W. laboratory for their support and scientific discussion, including Kristen Smith, Jessica Williams, Amy Lovett-Racke, and Michael Racke.
This project was supported by U.S. National Institutes of Health grant AI 064320 and National MS Society grant RG3272.
REFERENCES
- 1. Noseworthy J. H., Lucchinetti C., Rodriguez M., Weinshenker B. G. (2000) Multiple sclerosis. N. Engl. J. Med. 343, 938– 952 [DOI] [PubMed] [Google Scholar]
- 2. Trapp B. D., Peterson J., Ransohoff R. M., Rudick R., Mork S., Bo L. (1998) Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278– 285 [DOI] [PubMed] [Google Scholar]
- 3. Chou Y. K., Bourdette D. N., Offner H., Whitham R., Wang R. Y., Hashim G. A., Vandenbark A. A. (1992) Frequency of T cells specific for myelin basic protein and myelin proteolipid protein in blood and cerebrospinal fluid in multiple sclerosis. J. Neuroimmunol. 38, 105– 113 [DOI] [PubMed] [Google Scholar]
- 4. Wingerchuk D. M., Lucchinetti C. F., Noseworthy J. H. (2001) Multiple sclerosis: current pathophysiological concepts. Lab. Invest. 81, 263– 281 [DOI] [PubMed] [Google Scholar]
- 5. Lundmark F., Duvefelt K., Iacobaeus E., Kockum I., Wallstrom E., Khademi M., Oturai A., Ryder L. P., Saarela J., Harbo H. F., Celius E. G., Salter H., Olsson T., Hillert J. (2007) Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat. Genet. 39, 1108– 1113 [DOI] [PubMed] [Google Scholar]
- 6. Gregory S. G., Schmidt S., Seth P., Oksenberg J. R., Hart J., Prokop A., Caillier S. J., Ban M., Goris A., Barcellos L. F., Lincoln R., McCauley J. L., Sawcer S. J., Compston D. A., Dubois B., Hauser S. L., Garcia-Blanco M. A., Pericak-Vance M. A., Haines J. L. (2007) Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 39, 1083– 1091 [DOI] [PubMed] [Google Scholar]
- 7. Hafler D. A., Compston A., Sawcer S., Lander E. S., Daly M. J., De Jager P. L., de Bakker P. I., Gabriel S. B., Mirel D. B., Ivinson A. J., Pericak-Vance M. A., Gregory S. G., Rioux J. D., McCauley J. L., Haines J. L., Barcellos L. F., Cree B., Oksenberg J. R., Hauser S. L. (2007) Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 357, 851– 862 [DOI] [PubMed] [Google Scholar]
- 8. McFarland H. F., Martin R. (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 8, 913– 919 [DOI] [PubMed] [Google Scholar]
- 9. Racke M. K. (2008) The role of B cells in multiple sclerosis: rationale for B-cell-targeted therapies. Curr. Opin. Neurol. 21(Suppl. 1), S9– S18 [DOI] [PubMed] [Google Scholar]
- 10. Peterson J. W., Trapp B. D. (2005) Neuropathobiology of multiple sclerosis. Neurol. Clin. 23, 107– 129, vi–vii [DOI] [PubMed] [Google Scholar]
- 11. Niino M., Ogata A., Kikuchi S., Tashiro K., Nishihira J. (2000) Macrophage migration inhibitory factor in the cerebrospinal fluid of patients with conventional and optic-spinal forms of multiple sclerosis and neuro-Behcet's disease. J. Neurol. Sci. 179, 127– 131 [DOI] [PubMed] [Google Scholar]
- 12. David J. R. (1966) Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. U. S. A. 56, 72– 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Popa C., van Lieshout A. W., Roelofs M. F., Geurts-Moespot A., van Riel P. L., Calandra T., Sweep F. C., Radstake T. R. (2006) MIF production by dendritic cells is differentially regulated by Toll-like receptors and increased during rheumatoid arthritis. Cytokine 36, 51– 56 [DOI] [PubMed] [Google Scholar]
- 14. Zernecke A., Bernhagen J., Weber C. (2008) Macrophage migration inhibitory factor in cardiovascular disease. Circulation 117, 1594– 1602 [DOI] [PubMed] [Google Scholar]
- 15. Meyer-Siegler K. L., Vera P. L., Iczkowski K. A., Bifulco C., Lee A., Gregersen P. K., Leng L., Bucala R. (2007) Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun. 8, 646– 652 [DOI] [PubMed] [Google Scholar]
- 16. Bozza M., Satoskar A. R., Lin G., Lu B., Humbles A. A., Gerard C., David J. R. (1999) Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189, 341– 346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Jong Y. P., Abadia-Molina A. C., Satoskar A. R., Clarke K., Rietdijk S. T., Faubion W. A., Mizoguchi E., Metz C. N., Alsahli M., ten Hove T., Keates A. C., Lubetsky J. B., Farrell R. J., Michetti P., van Deventer S. J., Lolis E., David J. R., Bhan A. K., Terhorst C. (2001) Development of chronic colitis is dependent on the cytokine MIF. Nat. Immunol. 2, 1061– 1066 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Sosa M., Rosas L. E., David J. R., Bojalil R., Satoskar A. R., Terrazas L. I. (2003) Macrophage migration inhibitory factor plays a critical role in mediating protection against the helminth parasite Taenia crassiceps. Infect. Immun. 71, 1247– 1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Satoskar A. R., Bozza M., Rodriguez Sosa M., Lin G., David J. R. (2001) Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect. Immun. 69, 906– 911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flores M., Saavedra R., Bautista R., Viedma R., Tenorio E. P., Leng L., Sanchez Y., Juarez I., Satoskar A. A., Shenoy A. S., Terrazas L. I., Bucala R., Barbi J., Satoskar A. R., Rodriguez-Sosa M. (2008) Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii. FASEB J. 22, 3661– 3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleemann R., Bucala R. (2010) Macrophage migration inhibitory factor: critical role in obesity, insulin resistance, and associated comorbidities. Mediators Inflamm. 2010, 610479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosengren E., Bucala R., Aman P., Jacobsson L., Odh G., Metz C. N., Rorsman H. (1996) The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol. Med. 2, 143– 149 [PMC free article] [PubMed] [Google Scholar]
- 23. Weiser W. Y., Temple P. A., Witek-Giannotti J. S., Remold H. G., Clark S. C., David J. R. (1989) Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc. Natl. Acad. Sci. U. S. A. 86, 7522– 7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleemann R., Kapurniotu A., Frank R. W., Gessner A., Mischke R., Flieger O., Juttner S., Brunner H., Bernhagen J. (1998) Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J. Mol. Biol. 280, 85– 102 [DOI] [PubMed] [Google Scholar]
- 25. Matsunaga J., Sinha D., Pannell L., Santis C., Solano F., Wistow G. J., Hearing V. J. (1999) Enzyme activity of macrophage migration inhibitory factor toward oxidized catecholamines. J. Biol. Chem. 274, 3268– 3271 [DOI] [PubMed] [Google Scholar]
- 26. Bernhagen J., Krohn R., Lue H., Gregory J. L., Zernecke A., Koenen R. R., Dewor M., Georgiev I., Schober A., Leng L., Kooistra T., Fingerle-Rowson G., Ghezzi P., Kleemann R., McColl S. R., Bucala R., Hickey M. J., Weber C. (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587– 596 [DOI] [PubMed] [Google Scholar]
- 27. Schober A., Bernhagen J., Weber C. (2008) Chemokine-like functions of MIF in atherosclerosis. J. Mol. Med. 86, 761– 770 [DOI] [PubMed] [Google Scholar]
- 28. Miller A. (1997) Current and investigational therapies used to alter the course of disease in multiple sclerosis. South. Med. J. 90, 367– 375 [DOI] [PubMed] [Google Scholar]
- 29. Polman C. H., O'Connor P. W., Havrdova E., Hutchinson M., Kappos L., Miller D. H., Phillips J. T., Lublin F. D., Giovannoni G., Wajgt A., Toal M., Lynn F., Panzara M. A., Sandrock A. W. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899– 910 [DOI] [PubMed] [Google Scholar]
- 30. Yousry T. A., Major E. O., Ryschkewitsch C., Fahle G., Fischer S., Hou J., Curfman B., Miszkiel K., Mueller-Lenke N., Sanchez E., Barkhof F., Radue E. W., Jager H. R., Clifford D. B. (2006) Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 354, 924– 933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fitzgerald D. C., Ciric B., Touil T., Harle H., Grammatikopolou J., Das Sarma J., Gran B., Zhang G. X., Rostami A. (2007) Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 179, 3268– 3275 [DOI] [PubMed] [Google Scholar]
- 32. Powell N. D., Papenfuss T. L., McClain M. A., Gienapp I. E., Shawler T. M., Satoskar A. R., Whitacre C. C. (2005) Cutting edge: macrophage migration inhibitory factor is necessary for progression of experimental autoimmune encephalomyelitis. J. Immunol. 175, 5611– 5614 [DOI] [PubMed] [Google Scholar]
- 33. Aroca P., Solano F., Garcia-Borron J. C., Lozano J. A. (1991) Specificity of dopachrome tautomerase and inhibition by carboxylated indoles: considerations on the enzyme active site. Biochem. J. 277(Pt. 2), 393– 397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Denkinger C. M., Denkinger M., Kort J. J., Metz C., Forsthuber T. G. (2003) In vivo blockade of macrophage migration inhibitory factor ameliorates acute experimental autoimmune encephalomyelitis by impairing the homing of encephalitogenic T cells to the central nervous system. J. Immunol. 170, 1274– 1282 [DOI] [PubMed] [Google Scholar]
- 35. Stromnes I. M., Goverman J. M. (2006) Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 1, 1810– 1819 [DOI] [PubMed] [Google Scholar]
- 36. Engelhardt B. (2006) Regulation of immune cell entry into the central nervous system. Results Probl. Cell Differ. 43, 259– 280 [DOI] [PubMed] [Google Scholar]
- 37. Bo L., Peterson J. W., Mork S., Hoffman P. A., Gallatin W. M., Ransohoff R. M., Trapp B. D. (1996) Distribution of immunoglobulin superfamily members ICAM-1, -2, -3, and the beta 2 integrin LFA-1 in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 55, 1060– 1072 [PubMed] [Google Scholar]
- 38. Mottet C., Uhlig H. H., Powrie F. (2003) Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170, 3939– 3943 [DOI] [PubMed] [Google Scholar]
- 39. Sanchez-Zamora Y., Terrazas L. I., Vilches-Flores A., Leal E., Juarez I., Whitacre C., Kithcart A., Pruitt J., Sielecki T., Satoskar A. R., Rodriguez-Sosa M. (2010) Macrophage migration inhibitory factor is a therapeutic target in treatment of non-insulin-dependent diabetes mellitus. FASEB J. 24, 2583– 2590 [DOI] [PubMed] [Google Scholar]