Abstract
Despite support for receptor of activated NF-κB ligand (RANKL) as a mediator of mammary progesterone action, the extent to which this cytokine can functionally contribute to established progesterone-induced mammary morphogenetic responses in the absence of other presumptive effectors is still unclear. To address this uncertainty, we developed an innovative bigenic system for the doxycycline-inducible expression of RANKL in the mammary epithelium of the progesterone receptor knockout (PRKO) mouse. In response to acute doxycycline exposure, RANKL is specifically expressed in the estrogen receptor α (ER) positive/progesterone receptor negative (ER+/PR−) cell type in the PRKO mammary epithelium, a cell type that is equivalent to the ER+/PR+ cell type in the wild-type (WT) mammary epithelium. Notably, the ER+/PR+ mammary cell normally expresses RANKL in the WT mammary epithelium during pregnancy. In this PRKO bigenic system, acute doxycycline-induced expression of RANKL results in ordered mammary ductal side branching and alveologenesis, morphological changes that normally occur in the parous WT mouse. This mammary epithelial expansion is accompanied by significant RANKL-induced luminal epithelial proliferation, which is driven, in part, by indirect induction of cyclin D1. Collectively, our findings support the conclusion that RANKL represents a critical mediator of mammary PR action and that restricted expression of this effector to the ER+/PR+ mammary cell-type is necessary for a spatially ordered morphogenetic response to progesterone.—Mukherjee, A., Soyal, S. M., Li, J., Ying, Y., He, B., DeMayo, F. J., Lydon, J. P. Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression.
Keywords: progesterone receptor knockout, cell-type specific expression, doxycycline, induction-deinduction, minichromosome maintenance protein
Rodent mammary epithelial cells that are double positive for progesterone receptor (PR) and estrogen receptor α (ER) expression have been shown to represent a separate cell type from a subgroup of epithelial cells, which undergo proliferation in response to progesterone and/or estradiol exposure (1, 2), suggesting a paracrine mechanism underpins mammary steroid hormone action in this species (3). Notably, this segregation pattern for steroid receptor expression and steroid hormone-induced cellular proliferation has also been detected in human mammary tissue and cells (4–6), implicating an evolutionary conserved regulatory mechanism by which cell-type-restricted steroid hormone signaling is necessary for spatially ordered mammary epithelial expansion and morphogenesis. Indeed, dysregulation of the strict regional patterning for mammary PR expression has been shown to account, in part, for disorganized and precocious ductal side-branching and alveologenesis (7–10). Together, these findings suggest that the normal spatiotemporal expression of mammary PR (and its downstream molecular targets) requires tight control to avoid unscheduled proliferative changes that could enable hyperplasia and perhaps neoplastic progression to occur over time (8).
Although significant advances have been attained in understanding the cellular principles by which PR projects its proliferative influence within the mammary epithelium, the molecular mechanisms that underlie this mode of action are only now being addressed with the identification of a growing number of molecular targets ascribed as downstream effectors of mammary PR action (11–16). Of these presumptive targets, compelling data suggest that receptor of activated nuclear factor κ ligand (RANKL), a cytokine member of the tumor necrosis factor (TNF) superfamily (17, 18), may act [via its cell membrane signaling receptor (RANK)] as a key mediator of mammary PR function (12, 15). As a type II transmembrane protein (or as a soluble ectodomain), RANKL has been shown to be essential for a myriad of physiological processes that include osteoclast differentiation, dendritic cell survival, T-cell communication, and lymph node organogenesis, as well as tumor cell migration and metastasis (17, 19). In the case of the murine mammary gland, absence of the RANKL signaling axis was shown to result in a significant attenuation in the proliferative response of the mammary epithelium during pregnancy resulting in impairments in mammary ductal and alveolar expansion (12). Moreover, the observation that mammary RANKL transcription can be acutely induced by progesterone alone in the WT virgin (but not in the PRKO) (13) and that mammary RANKL expression is only induced during early-mid pregnancy (20–22) and exclusively in PR-positive cells (15, 20) strongly implicates RANKL as a mammary PR effector.
Despite the above support, however, we are currently unclear as to the degree to which RANKL—independent of other presumptive signaling effectors of mammary PR action—is involved in progesterone-induced mammary morphogenesis. This uncertainty is further confounded by the finding that prolactin (another critical hormone in parity-induced mammary proliferation and differentiation) has also been shown to induce mammary RANKL expression (22).
Therefore, to address this issue, we have developed a state-of-the-art bigenic mouse system in which RANKL expression is selectively targeted in the PRKO mouse to a mammary cell type (ER+/PR−) that is equivalent to the ER+/PR+ cell type in the WT mammary epithelium, a cell-type that normally expresses RANKL during early pregnancy (15, 20). The unparalleled precision by which RANKL is targeted with this genetic approach essentially recapitulates in the PRKO mammary epithelium the normal spatial restrictions imposed on RANKL expression in the mammary gland of the parous WT mouse. With this bigenic system, our studies underscore the individual importance of mammary RANKL as a mediator of stereotypical morphogenetic responses to progesterone exposure and that cell type-restricted expression of this cytokine is mandatory to ensure normal elaboration of these responses in a spatially ordered fashion throughout the mammary gland.
MATERIALS AND METHODS
Generation of the TetO-RANKL transgenic mouse
To generate the pTETO-RANKL transgene construct, a 2.3-kb cDNA fragment encoding full-length murine RANKL (obtained from BlueHeron Biotechnology, Bothell, WA, USA) was cloned into ClaI and SpeI restriction sites located within the pTETSplice cloning vector (Gibco BRL, Rockville, MD, USA). This cloning step positions the RANKL cDNA downstream of the cytomegalovirus (CMV) minimal promoter with seven juxtaposed tet operator sites and upstream of the simian virus 40 (SV40) splicing/polyadenylation signal cassette (23). Prior to microinjection into pronuclei of single-cell (FVB/N) embryos, the TETO-RANKL transgene was purified from vector sequences. Transgenic F0 founders [and their progeny (F1 and later)] were identified by screening tail biopsy genomic DNA for the presence of the transgene by Southern blot analysis. For Southern blot analysis, ClaI/SpeI digested genomic DNA was transferred to nitrocellulose before hybridization with a radioactively labeled internal BamH1 RANKL cDNA fragment (669 bp). To evaluate relative doxycycline responsiveness by quantitative real-time PCR of mammary tissue RNA, all five TetO-RANKL transgenic lines (F1 generation) were crossed with PRrtTA/rtTA effector male mice (24) to generate PRrtTA/+/TetO-RANKL bigenic progeny. On the basis of the levels of doxycycline-induced mammary RANKL transcription, 2 of the 5 TetO-RANKL transgenic lines (2562 and 2589) were subsequently utilized to generate the final PRrtTA/rtTA/TetO-RANKL bigenic model for studies described herein. Generation and characterization of the PRrtTA/+ and PRrtTA/rtTA knock-in models, as well as the MTB effector and the TZA responder transgenic mouse, has been previously reported (23, 24). Briefly, MTB effector mice carry a transgene, in which the mouse mammary tumor virus long-terminal repeat (MMTV-LTR) drives the expression of the reverse tetracycline-dependent transactivator (rtTA) mostly to the mammary epithelium. The TZA responder mouse harbors the TetO-LacZ transgene consisting of tet-operator sequences 5′ to the lacZ reporter gene. In the MTB/TZA bigenic reporter mouse, therefore, lacZ expression is targeted to the mammary epithelium only in response to doxycycline exposure (23). Creation of the MTB/TetO-RANKL bigenic model followed a similar strategy for the previously described MTB/TZA bigenic model (23). For timed pregnant mice, the morning (8:00 AM) of detecting a vaginal plug was considered d 1 of pregnancy. Mice used in these studies were housed in a temperature-controlled (22±2°C) room with a 12-h light-dark recurrent photocycle. If not treated with doxycycline (see below), mice were fed rodent chow meal (Purina Mills Inc., St. Louis, MO, USA) and fresh water daily. Animal care was approved by the Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine and was in accordance with the procedures and practices described in the Guide for Care and Use of Laboratory Animals (National Institutes of Health publication 85-23).
Doxycycline administration
To induce responder transgene expression in the bigenic model within a specified time period, adult (9 wk old) mice (along with control monogenic siblings) were provided ad libitum chow meal fortified with doxycycline at 6g/kg (BioServ, St. Louis, MO, USA), as well as water (supplied in light-protected bottles) containing doxycycline at 2 mg/ml (BD Clontech, San Diego, CA, USA) (24). To ameliorate taste aversion, doxycycline-treated water was supplemented with 5% sucrose (Fisher Scientific, Pittsburgh, PA, USA) and changed every 3 or 4 d to maintain induction potency. For the doxycycline induction-deinduction experiments described in Fig. 8, mice were first treated with dietary doxycycline for 28 d (induction). Before doxycycline withdrawal from food and water (deinduction), the right inguinal mammary gland from each mouse was biopsied for whole-mount and immunohistochemical analysis. Following 3 d of doxycycline withdrawal, biopsied mice were sacrificed and remaining mammary tissue taken for analysis. To confirm that the surgical biopsy procedure did not contribute to subsequent changes in mammary morphology, an additional set of control mice (n=4) were treated with doxycycline both before and after mammary tissue biopsy. As expected, mammary tissue morphology was not altered by the surgical procedure (data not shown).
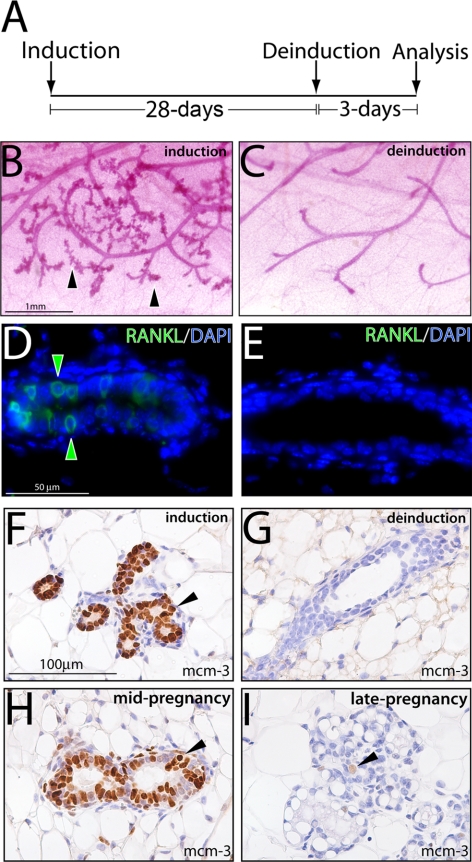
Figure 8.
Mammary ductal side-branching and alveolar development requires continued RANKL expression. A) Schematic of the 28-d doxycycline-induction and 3-d deinduction protocol applied to adult PRrtTA/rtTA/TetO-RANKL bigenic mice prior to mammary gland whole-mount and immunohistochemical analysis. B) Typical whole mount of a mammary gland biopsied from the PRrtTA/rtTA/TetO-RANKL mouse following 28 d of doxycycline treatment. Note the presence of ductal side-branches and alveoli (arrowheads). C) Typical whole mount of the remaining mammary gland from the PRrtTA/rtTA/TetO-RANKL mouse administered doxycycline for 28 d followed by 3 d without doxycycline in the food and water (deinduction). Note the precipitous loss of ductal side-branching and alveolar budding following doxycycline withdrawal. D) Immunofluorescence detection of mammary RANKL (green arrowheads; merged with DAPI) following 28 d of doxycycline induction. E) Absence of RANKL expression following 3 d of doxycycline withdrawal. F) Significant mcm-3 expression in the mammary epithelium following 28 d of RANKL induction in the PRrtTA/rtTA/TetO-RANKL bigenic (arrowhead). G) Absence of mammary epithelial mcm-3 expression following RANKL deinduction in the PRrtTA/rtTA/TetO-RANKL bigenic. H, I) Representative immunostaining for mcm-3 expression in the mammary epithelium of the midpregnant (d 12) (H) and late-pregnant (d 18) WT mouse I.
Whole-mount staining and immunohistochemical analysis
Whole mounts (and sections thereof) of the inguinal mammary gland (gland 4) were stained with carmine-red or for β-gal activity (X-gal staining), as described previously (24). The following primary antibodies were used for immunohistochemical and/or immunofluorescence staining: chicken polyclonal anti-β-galactosidase (Ab9361; Abcam, Cambridge, MA, USA); mouse monoclonal anti-human ERα (M7047) and rabbit polyclonal anti-human PR (A0098) from Dako (Carpinteria, CA, USA); biotinylated mouse monoclonal anti-5-bromo-2-deoxyuridine (BrdU) (BD550803; BD Biosciences, San Jose, CA, USA); mouse monoclonal anti-BrdU (RPN20; Amersham Biosciences, Piscataway, NJ, USA); goat polyclonal anti-mouse RANKL (TRANCE; AF462; R&D Systems, Minneapolis, MN, USA); rabbit monoclonal anti-human cyclin D1 (RB-9041-P0; Lab Vision Corp., Fremont, CA, USA); and goat polyclonal anti-human MCM3 (N-19; sc-9850; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
To simultaneously detect β-gal and ER by dual immunofluoresence, the following AlexaFluor-conjugated secondary antibody combinations were used: AlexaFluor 594 goat anti-chicken (A-11042) and AlexaFluor 488 goat anti-mouse (A-11001). AlexaFluor 488 goat anti-rabbit (A-11008) and AlexaFluor 594 goat anti-mouse (A-11005) were used to detect PR and ER, respectively. RANKL and ER were detected using AlexaFluor 488 donkey anti-goat (A-11055) and AlexaFluor 546 donkey anti-mouse (A-10036), respectively. AlexaFluor 488 donkey anti-goat (A-11055) and AlexaFluor 546 donkey anti-mouse (A-10036) were used to detect RANKL and BrdU, respectively. Secondary antibodies: AlexaFluor 488 donkey anti-goat (A-11055) and AlexaFluor 594 goat anti-rabbit (A-11012) were used to detect RANKL and cyclin D1, respectively. AlexaFluor conjugated secondary antibodies were obtained from Invitrogen Corp. (Carlsbad, CA, USA). Apoptotic cells were immunofluorescently detected by TUNEL assay using the In-Situ Cell Death Detection Kit (cat. 11684795910; Roche Applied Sciences, Mannheim, Germany). Slides were mounted with Vectashield mounting medium with 4′, 6′-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA, USA).
Immunohistochemical detection of ER and MCM3 using the immunoperoxidase method was followed according to Fernandez-Valdivia et al. (20). For BrdU immunohistochemical and immunofluoresence detection, mice received an intraperitoneal injection of BrdU (Amersham Biosciences) at a dose of 1 mg BrdU/20g body weight 2 h prior sacrifice. Immunohistochemical detection of cellular BrdU incorporation was accomplished following established methods (20). In general, the percentage of immunopositive mammary epithelial cells was calculated using methods previously described (20). Briefly, the number of immunopositive cells was counted from a total of 1000 luminal epithelial cells/tissue section/mouse. Three separate sections per mouse were counted and 5 mice/genotype and/or treatment were analyzed. The se was calculated, and a 2-tailed version of the Student's t test was used to assess statistical significance (P≤0.05).
Molecular analysis
Real-time reverse transcriptase PCR
Briefly, total RNA was extracted from mammary tissue using the TRIzol reagent (Invitrogen), and 1 μg of total RNA was reverse-transcribed to cDNA template using the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (11752-050; Invitrogen). Expression levels of RANKL were assessed in TaqMan Universal PCR Master Mix using an ABI Prism 7700 Sequence Detector System (PE Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. The probes for RANKL (Mm00441908_m1) and 18S rRNA (4319413E; an internal control) were obtained from PE Applied Biosystems. All experiments were performed in triplicate using 3 independent RNA preparations in each case; transcript levels were normalized to 18S rRNA with the ABI rRNA control reagents. Again, values of P < 0.05 were considered statistically significant, and values < 0.01 highly significant; results are presented as mean ± se.
Western immunoblot analysis
Protein isolates were prepared from highly enriched mammary epithelial cell fractions and Western immunoblot performed according to Fernandez-Valdivia et al. (20). Prior to transfer to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA), protein (40 μg) was resolved by electrophoresis on a 4–15% gradient sodium dodecylsulfate-polyacrylamide gel. The following primary antibodies (at 1:1000 dilution) were used: goat polyclonal anti-mouse RANKL (AF462; R&D Systems); goat anti-human β-actin (sc-1616; Santa Cruz Biotechnology). Following incubation with appropriate secondary antibodies, immunoreactive bands were observed using an enhanced chemiluminescence substrate detection kit (Pierce Biotechnology, Rockford, IL, USA).
RESULTS
Doxycycline-induced β-galactosidase activity in the mammary epithelium of the PRrtTA/rtTA/TZA bigenic mouse is confined to luminal cells positive for ER expression
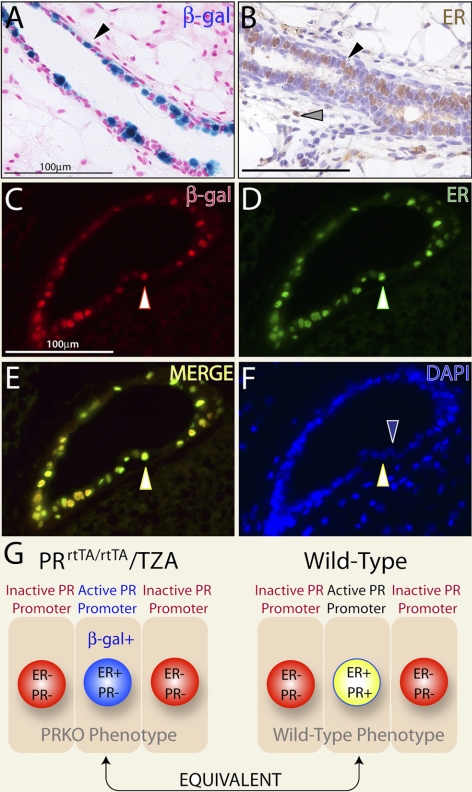
Using established gene-targeting methods, we recently targeted the reverse tetracycline-dependent transactivator (rtTA) downstream of the murine PR promoter (24). Such a targeting strategy is designed to enable the endogenous PR promoter to control the spatiotemporal expression of the rtTA in a manner similar to endogenous PR protein. Mice were subsequently generated in which one (PRrtTA/+) or both (PRrtTA/rtTA) PR alleles carried the rtTA insertion (24). Of relevance here, the PRrtTA/+ and PRrtTA/rtTA knock-in models were shown to phenocopy the WT and PRKO mouse, respectively. Crossed with the TZA reporter mouse (23), which harbors the TetO-LacZ responder transgene, both PRrtTA/+/TZA and PRrtTA/rtTA/TZA bigenic mice were shown to exhibit doxycycline-induced β-galactosidase activity specifically in progesterone responsive target tissues (including the mammary gland). For both bigenic mice, β-galactosidase activity was not detected in the absence of doxycycline. Notably, in the case of the PRrtTA/+/TZA mammary epithelium, we also demonstrated that PR expression and doxycycline-induced β-galactosidase activity colocalized to the same cell type (24), indicating doxycycline-induced β-galactosidase activity in PRrtTA/+/TZA mammary epithelium is restricted to the PR+ cell type (β-gal+/PR+). In Fig. 1, we show that the adult PRrtTA/rtTA/TZA mammary epithelium displays a similar punctate spatial pattern for cells positive for doxycycline-induced β-galactosidase activity and that these cells also score positive for ER expression (β-gal+/ER+; Fig. 1A–F). Because of the PRrtTA/rtTA (or PRKO) genotype, however, these cells are negative for PR expression (β-gal+/ER+/PR−; data not shown). Despite the absence of PR expression, these results show that the endogenous PR promoter is still active in the PRrtTA/rtTA (or PRKO) mammary epithelium and is restricted to ER+ luminal epithelial cells. Since we (Supplemental Fig. 1) and others (25) have shown that the majority of luminal cells that express ER are also positive for PR expression in the mammary epithelium of the adult WT mouse (ER+/PR+), our results suggest that the subset of mammary epithelial cells, which scores β-gal+/ER+/PR− in the PRrtTA/rtTA/TZA mammary epithelium (Fig. 1E) is equivalent to the subset that registers ER+/PR+ in the WT mammary epithelium (Fig. 1G and Supplemental Fig. 1). With this in mind, we reasoned that the PRrtTA/rtTA effector mouse could be used to conditionally target the expression (from a responder transgene) any gene of interest (i.e., RANKL) specifically to the ER+/PR− cell type within the PRrtTA/rtTA (or PRKO) mammary epithelium. Such a genetic strategy would faithfully recapitulate (at the cellular level) the spatial expression pattern of RANKL in the PRKO mammary epithelium that normally occurs in the mammary epithelium of the WT mouse in response to hormone exposure during pregnancy.
Figure 1.
Progesterone receptor promoter activity is restricted to ER+ cells in the PRKO mammary epithelium. A) Adult PRrtTA/rtTA/TZA bigenic females were arbitrarily treated with doxycycline for 1 mo. Doxycycline-induced β-gal activity in a longitudinal section of a PRrtTA/rtTA/TZA mammary duct is shown (black arrowhead). X-gal staining is punctate and restricted to the luminal epithelium. B) Immunohistochemical detection of ER expression in a serial section of the same tissue. Note the nonuniform expression pattern for ER in the luminal epithelial compartment (black arrowhead); a small subset of cells is also positive for ER expression in the stromal compartment (gray arrowhead). C, D) Immunofluorescence detection of β-gal (red) and ER (green) in the same mammary section from the doxycycline-treated PRrtTA/rtTA/TZA female, respectively. E) Merge of panels C and D. White arrowheads in panels C–E highlight a representative luminal epithelial cell positive for both β-gal activity and ER expression. F) Same section stained for DAPI. Blue arrowhead indicates a luminal cell negative for β-gal and ER positivity juxtaposed to a luminal cell positive for β-gal/ER immunostaining (white arrowhead). Scale bars = 100 μgmm. G) Schematic representation of the various luminal epithelial cell types in terms of ER, PR, and/or β-gal positivity in the mammary gland of the PRrtTA/rtTA/TZA bigenic and WT mouse.
Conditional induction of mammary RANKL expression in the PRrtTA/rtTA/TetO-RANKL bigenic mouse
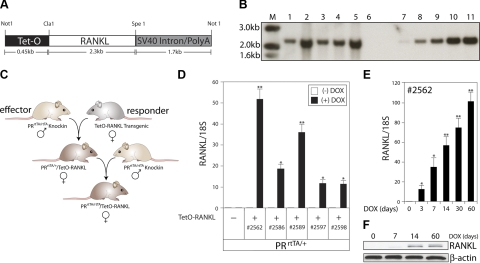
To conditionally target RANKL expression to ER+/PR− cells in the mammary epithelium of the PRrtTA/rtTA effector mouse, we generated a number of TetO-RANKL responder transgenic mouse lines (Fig. 2). Using standard transgenic approaches, we created 5 individual TetO-RANKL transgenic mouse lines (2562, 2586, 2589, 2597, and 2598), which transferred the TetO-RANKL responder transgene through the germline (Fig. 2A, B). By Southern blot analysis, all 5 transgenic lines harbored multiple copies of the TetO-RANKL transgene; in particular, lines 2586 and 2598 carried >30 copies of the TetO-RANKL transgene (Fig. 2B). To generate the PRrtTA/+/TetO-RANKL and ultimately the PRrtTA/rtTA/TetO-RANKL bigenic models, each of the 5 TetO-RANKL transgenic lines was crossed with a PRrtTA/rtTA effector male (Fig. 2C). To initially assess doxycycline-induced transgene-derived RANKL transcription in the mammary epithelium, adult PRrtTA/+/TetO-RANKL bigenic mice were treated with doxycycline for 1 mo (an arbitrary chosen time period) (Fig. 2D). While quantitative real-time PCR did not detect mammary RANKL transcripts in the absence of dietary doxycycline, induction of mammary RANKL transcription is clearly evident in the presence of doxycycline in the PRrtTA/+/TetO-RANKL bigenics derived from all 5 responder transgenics (Fig. 2D). As expected, doxycycline-induced mammary RANKL transcription was not detected either in absence of doxycycline or the TetO-RANKL transgene (also, mammary RANKL transcription was not detected in the doxycycline-treated TetO-RANKL monogenic control; data not shown). Because lines 2562 and 2589 consistently displayed the highest level of doxycycline-induced mammary RANKL transcription in the PRrtTA/+/TetO-RANKL bigenic mice, these lines were chosen to backcross further to generate the PRrtTA/rtTA/TetO-RANKL bigenic mice. For studies described herein, only data derived from the PRrtTA/rtTA/TetO-RANKL (2562) bigenic model are presented, which are also representative of the PRrtTA/rtTA/TetO-RANKL (2589) model. It is important to note that only the PRrtTA/rtTA/TetO-RANKL bigenic model (rather than the PRrtTA/+/TetO-RANKL version) is the focus of the studies described here. Using quantitative real-time PCR analysis, we monitored doxycycline-induced mammary RANKL transcription over time in the PRrtTA/rtTA/TetO-RANKL bigenic mice (Fig. 2E). While RANKL transcript levels were detected as early as 3 d of doxycycline intake, transgene-derived RANKL transcription attained its highest levels by 1–2 mo of doxycycline stimulation (further increases in transcript levels were not observed beyond this time point; data not shown). Western blot analysis confirmed the PCR results in that RANKL protein expression levels increased during the 2-mo period of dietary doxycycline intake (Fig. 2F). Interestingly, we observed similar induction kinetics with the PRrtTA/rtTA/TZA bigenic reporter (24). For studies described here, the 2-mo period was the longest time period during which mice were administered doxycycline.
Figure 2.
Generation of the PRrtTA/rtTA/TetO-RANKL bigenic mouse. A) Schematic of the TetO-RANKL transgene (ClaI and SpeI releases the full-length RANKL cDNA). B) Southern blot result that identifies the TetO-RANKL transgenic founder lines. Genomic DNA was digested with ClaI and SpeI and probed with a radioactively labeled RANKL cDNA subfragment (see Materials and Methods). Lanes 1–5 show the expected 2.3-kb hybridizing band in all 5 founder lines carrying the TetO-RANKL transgene. Because of 2 intron sequences in the endogenous murine RANKL gene, a hybridizing band of 2.3 kb is not observed in WT genomic DNA (lane 6). Lanes 7–11 represent WT genomic DNA spiked with ∼1, 5, 10, 20, and 30 copies of the full-length RANKL cDNA, respectively. C) Breeding scheme to generate the PRrtTA/+/TetO-RANKL and ultimately the PRrtTA/rtTA/TetO-RANKL mouse. D) Relative transcript levels (as measured by quantitative RT-PCR) of mammary RANKL in adult monogenic PRrtTA/+ and bigenic PRrtTA/+/TetO-RANKL mice in the absence (−) or presence (+) of doxycycline for 1 mo. Columns and error bars represent means ± se (n=5). *P < 0.05, **P < 0.001 vs. diet without doxycycline. E) Time course for doxycycline-induction of mammary RANKL transcription in the adult PRrtTA/rtTA/TetO-RANKL female Columns and error bars represent means ± se (n=5). *P < 0.05, **P < 0.001 vs. d 0 of doxycycline treatment. F) Representative Western immunoblot of doxycycline-induced mammary RANKL protein (∼45 kDa) expression in the PRrtTA/rtTA/TetO-RANKL with time. β-Actin (∼42 kDa) is a loading control.
RANKL triggers mammary ductal-side branching and alveolar budding in the absence of PR expression
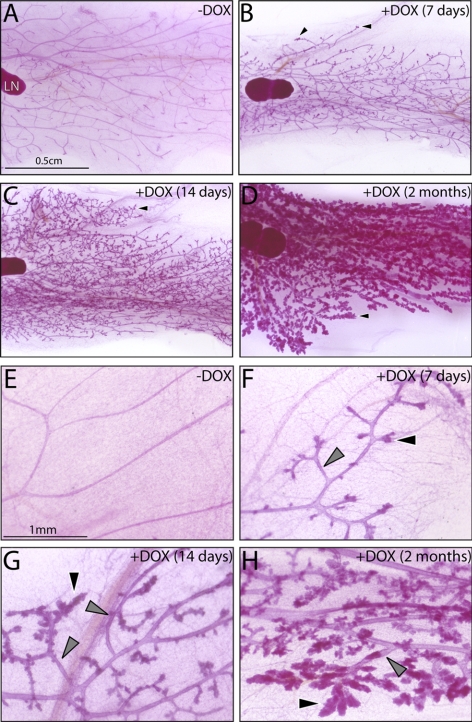
Remarkably, induction of RANKL expression in the mammary gland of the PRrtTA/rtTA/TetO-RANKL bigenic mice elicited significant branching morphogenesis and alveologenesis in a spatially ordered manner in the absence of PR expression (Fig. 3). Nascent ductal side-branching and incipient alveolar budding was clearly evident throughout the PRrtTA/rtTA/TetO-RANKL mammary gland following a week of doxycycline intake (Fig. 3A, B). This result is significant in that these morphological changes fail to occur in the hormone-treated PRKO mammary gland (26). In addition to an extensive branched ductal network, lobuloalveolar development is clearly evident with continued doxycycline intake (Fig. 3C–H). However, these lobuloalveolar structures fail to fully expand to infill the interductal spaces as occurs with late pregnancy (27). While histological examination of hematoxylin and eosin (H&E)-stained sections of these tissues confirmed the dramatic change in mammary morphology following prolonged doxycycline exposure, evidence of alveolar differentiation (i.e., cellular vacuolization or alveolar luminal secretions) was not observed (Supplemental Fig. 3). Interestingly, the relatively ordered mammary ductal side-branching and alveolar development in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mice markedly contrasts with the disorganized ductal and alveolar architecture that occurs in the similarly treated MTB/TetO-RANKL bitransgenic mice (Fig. 4). The MMTV-LTR in the MTB mouse indiscriminately targets RANKL expression within the mammary epithelial compartment of the bitransgenic. Apart from different levels of RANKL transcription (data not shown), these results suggest that RANKL spatial expression requires tight control in order to avoid aberrant regional expansion of the epithelial compartment. This conclusion is further supported by recent observations made with transgenic and viral transduction approaches that globally target RANKL expression to the mammary epithelium (20, 21, 28).
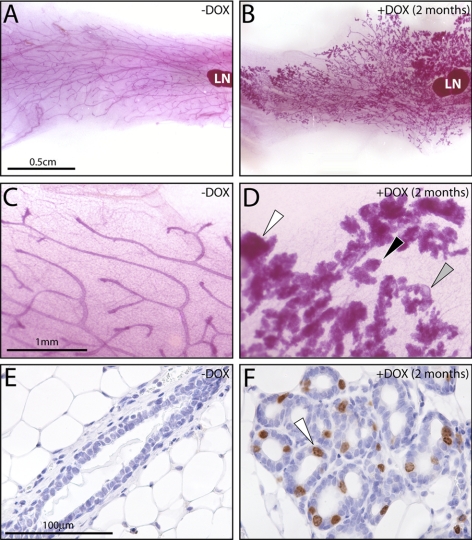
Figure 3.
Doxycycline-induced RANKL expression elicits mammary ductal side-branching and alveolar development in the adult PRrtTA/rtTA/TetO-RANKL bigenic mouse. A) Mammary gland whole mount from an adult PRrtTA/rtTA/TetO-RANKL bigenic mouse that did not receive doxycycline. LN, lymph node (a structural reference point in the inguinal gland). B–D) Representative whole mounts of inguinal mammary glands from PRrtTA/rtTA/TetO-RANKL mouse treated with doxycycline for the time-periods indicated. B) Note the presence of nascent alveolar buds (arrowhead) following only 1 wk of doxycycline administration. C, D) With increased time of doxycycline intake, ductal side-branching and expansion of the alveolar compartment become more pronounced (arrowhead). E–H) Higher-magnification images of subfields shown in A–D, respectively. Again, note the clear evidence of ordered ductal side-branching (gray arrowhead) and lobuloalveolar development (black arrowhead) that occurs with continued doxycycline intake. Scale bars = 0.5 cm (A–D); 1 mm (E–H).
Figure 4.
Doxycycline-induced RANKL expression in the mammary gland of the MTB/TetO-RANKL bigenic mouse induces a disorganized morphogenetic response. A, B) Whole mounts of the mammary gland from the MTB/TetO-RANKL bigenic mouse following intake of standard chow (A) or chow (and water) supplemented with doxycycline for 2 mo (B). C, D) Higher-magnification images of a subfield shown in A, B, respectively. Note the spatially disorganized ductal branching and alveologenesis in response to indiscriminate targeting of RANKL to the mammary epithelium. D) Clear evidence of hyperplasia (white arrowhead), alveolar budding (black arrowhead), and dilated or cystic ducts (gray arrowhead) are observed with doxycycline-induced RANKL expression. E, F) BrdU immunoreactivity in the mammary epithelium of the MTB/TetO-RANKL bigenic mouse after 2 mo on a standard or doxycycline-supplemented diet, respectively. E) In the absence of doxycycline, BrdU-positive cells are not detected in a transverse section of a typical duct. F) With doxycycline-induced RANKL expression, numerous BrdU-positive cells are detected in a transverse section of a lobule of alveoli (white arrowhead). Data are representative of 5 MTB/TetO-RANKL mice/treatment group.
Mammary RANKL expression in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL mouse is spatially nonuniform
Immunofluorescence analysis reveals that RANKL-positive cells are distributed in a nonuniform or punctate pattern throughout the mammary epithelium of the doxycycline-stimulated PRrtTA/rtTA/TetO-RANKL bigenic mice (Fig. 5). The nonuniform pattern of expression is to be expected if transgene-derived RANKL expression is correctly targeted to the ER+/PR− subset of cells, which is scattered in a punctate pattern throughout the PRrtTA/rtTA/TetO-RANKL mammary epithelium (Fig. 1). The nonuniform spatial patterning for RANKL expression is observed in both the alveolar and ductal epithelial compartments of the mammary gland of the PRrtTA/rtTA/TetO-RANKL mouse (Fig. 5A–F and Supplemental Fig. 5). This regional distribution pattern for RANKL expression is also observed in the mammary epithelium of the d 12 pregnant WT mouse (and ref. 20) (Fig. 5G–I). In contrast, a more uniform patterning for mammary RANKL expression is observed in the similarly treated MTB/TetO-RANKL bitransgenic due to indiscriminate targeting of RANKL to the mammary epithelium (Fig. 5J–L).
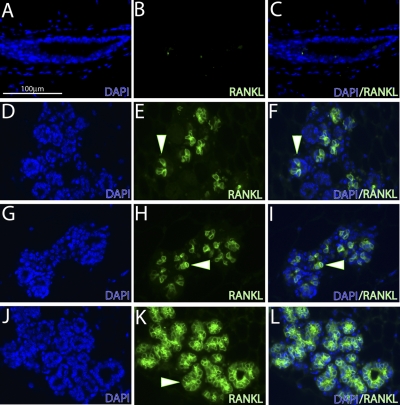
Figure 5.
Mammary RANKL expression in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic and parous wild-type mouse share a similar spatial pattern. A, B) Immunofluorescence staining for DAPI (A) and RANKL (B) in a mammary tissue section derived from the PRrtTA/rtTA/TetO-RANKL mouse without doxycycline intake. As expected, RANKL is not detected in the PRrtTA/rtTA/TetO-RANKL mammary gland in the absence of doxycycline administration. C) Merge of panels A and B. D, E) Immunofluorescence staining for DAPI (D) and RANKL (E) in a mammary section derived from the PRrtTA/rtTA/TetO-RANKL bigenic mouse administered doxycycline for 2 mo. Note the punctate spatial pattern for RANKL expression in the alveolar compartment (white arrowhead). F) Merge of panels D and E. G, H) Immunofluorescence staining for DAPI (G) and RANKL (H) in a mammary gland section from a midpregnant (d 12) wild-type mouse. Note a similar punctate staining pattern for RANKL expression in the mammary alveolar compartment of the parous mouse (compare E with H). J, K) Immunofluorescence staining for DAPI (J) and RANKL (K) expressionin the mammary gland of the similarly treated adult MTB/TetO-RANKL bitransgenic mouse. Unlike the RANKL expression pattern observed in the mammary gland of the PRrtTA/rtTA/TetO-RANKL bigenic mouse (treated with doxycycline) and of the parous WT mouse, RANKL expression is indiscriminately targeted to most mammary alveolar cells of MTB/TetO-RANKL bitransgenic. L) Merge of panels J and K. Scale bar = 100 μm.
Transgene-derived RANKL expression is targeted to mammary ER+/PR− epithelial cells in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mouse
Using dual immunofluorescence staining for ER and RANKL, we showed that transgene-derived RANKL expression is targeted specifically to the ER+/PR− cell type in the mammary epithelium of the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mice (Fig. 6). This result demonstrates that, by exploiting the endogenous PR promoter in the PRKO mammary epithelium (Fig. 1), we have successfully targeted RANKL expression to the ER+/PR− cell type, a subset of cells that we believe is equivalent to luminal epithelial cells that exhibit ER+/PR+ positivity in the mammary gland of WT mouse. High-power magnification images clearly show absence of RANKL expression (green) in stromal, basal, and ER−/PR− luminal cells (Fig. 6A–D). Lower-power magnification images confirm that ER and RANKL colocalize to the same cell type in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL mammary gland in a pattern similar to that observed in the mammary gland of the d 12 pregnant WT mouse (Fig. 6E, F).
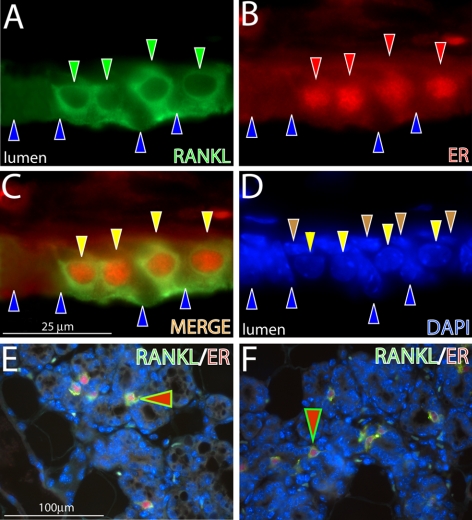
Figure 6.
Doxycycline-induced RANKL expression in the PRrtTA/rtTA/TetO-RANKL mammary epithelium is restricted to luminal cells positive for ER expression. A) Mammary epithelium of the adult PRrtTA/rtTA/TetO-RANKL bigenic mouse (treated with doxycycline for 2 mo) stained for RANKL immunofluorescence (green). Note the presence of 4 cells that are positive for cytoplasmic RANKL expression (green arrowheads) that are interspersed with cells negative for RANKL expression (blue arrowheads). B) Same section stained for ER expression (red arrowheads). C) Merge of panels A and B. Note that RANKL and ER expression colocalize to the same cell type (yellow arrowheads). D) Same section stained for DAPI to reveal all nuclei within this field. Note that basal cells are negative for both RANKL and ER expression (brown arrowhead). E, F) Lower-magnification images of RANKL/ER expression in the alveolar compartment of the mammary gland from the doxycycline treated PRrtTA/rtTA/TetO-RANKL bigenic and WT parous mouse (d 12 postcoitum), respectively. Note the colocalization of RANKL and ER in a subset of cells (red arrowhead). Scale bars = 25 μm (A–D); 100 μm (E, F).
Mammary RANKL elicits epithelial proliferation in the absence of PR expression
A key cellular hallmark of the PRKO mammary defect is an inability of the PRKO mammary epithelium to launch a proliferative response when coadministered estradiol and progesterone (26). Immunohistochemistry clearly shows that transgene-derived RANKL alone can significantly increase the number of luminal cells scoring positive for BrdU incorporation in the mammary epithelium of the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mice (Fig. 7A–D). Despite the absence of PR expression, these results indicate that the PRKO mammary epithelium retains the ability to proliferate in response to RANKL signaling. Interestingly, dual immunofluorescence for RANKL and BrdU in the mammary epithelium of the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mice shows a clear segregation of RANKL+ cells from BrdU+ cells (Fig. 7E–H), suggesting that RANKL elicits mammary epithelial proliferation through an indirect mechanism. This segregation pattern is also observed between RANKL+ and cyclin D1+ mammary epithelial cells (cyclin D1 has been shown to be a target of both mammary RANKL and PR; refs. 29, 30). Notably, this cellular segregation pattern has similarly been detected in the WT mammary gland during pregnancy or when exposed to estradiol and progesterone (15, 20). However, there is not a direct correlation between the number of cyclin D+ and RANKL+ cells (Fig. 7I), suggesting that RANKL also induces other (as yet unidentified) signaling pathways to achieve full mammary epithelial expansion.
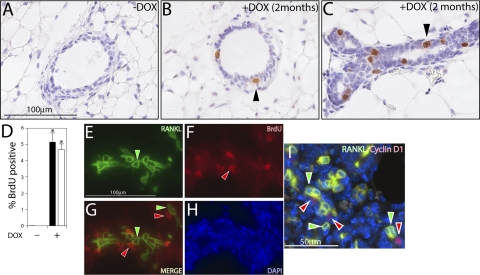
Figure 7.
RANKL elicits luminal epithelial proliferation in the mammary gland of the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mouse. A, B) Transverse sections of a mammary duct stained for BrdU incorporation from the PRrtTA/rtTA/TetO-RANKL bigenic mouse in the absence or presence of doxycycline in the diet, respectively. Cells positive for BrdU incorporation are not detected in the absence of doxycycline-induced RANKL expression (A). However, a subset of cells positive for BrdU incorporation is clearly observed with doxycycline-induced RANKL expression (B; arrowhead). C) Longitudinal section of a mammary duct from the doxycycline-treated PRrtTA/rtTA/TetO-RANKL bigenic mouse showing numerous cells positive for BrdU incorporation (arrowhead). D) Histogram of the percentage of luminal epithelial cells that are positive for BrdU in the ductal (solid column) and alveolar (open column) epithelial compartments in the absence (−) or presence (+) of doxycycline (DOX) in the diet. Error bars = se. *P < 0.05 vs. (−) doxycycline. E) Mammary section stained for RANKL immunofluorescence from the PRrtTA/rtTA/TetO-RANKL mouse administered doxycycline for 2 mo. Note the presence of numerous RANKL-positive cells in the field (green arrowhead). F) Cells in the same field positive for BrdU incorporation (red arrowhead). G) Merge of panels E and F. Note that most RANKL-positive cells (green arrowheads) are segregated from BrdU-positive cells (red arrowheads). H) DAPI stain of the same section to reveal all nuclei in the field. I) Combined dual immunofluorescence detection of RANKL (green) and cyclin D1 (red) in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL mammary gland. Note that RANKL-positive cells (green arrowhead) are segregated from cyclin D1-positive cells (red arrowhead). Scale bars = 100 μm (A–C, E–H); 50 μm (I).
Mammary ductal side-branching and alveologenesis requires continued RANKL expression
We next asked whether continued RANKL expression is necessary for maintaining mammary ductal branching and alveolar expansion or whether downstream secondary pathways are sufficient to maintain these morphological changes following the removal of RANKL expression. This question was prompted because mammary RANKL expression is induced only during the first half of the pregnancy time period in the WT mouse (20–22), indicating alternate pathways (either downstream or parallel) replace RANKL to advance mammary development to the end of pregnancy. To address this question, we employed a doxycycline induction/deinduction protocol as outlined in Fig. 8A (and detailed in Materials and Methods). Following 28 d of doxycycline intake, the biopsied mammary gland of the PRrtTA/rtTA/TetO-RANKL bigenic mouse exhibits obvious mammary ductal side-branching and alveologenesis (Fig. 8B). However, following 3 d of deinduction (or 3 d without doxycycline in the diet), there is a clear absence of these morphological changes in the remaining contralateral inguinal gland (Fig. 8C). With loss of RANKL expression (deinduction), the mammary gland essentially reverts to a simple ductal architecture, which typifies the PRKO mammary phenotype. Immunofluoresence analysis confirmed the induction of mammary RANKL with doxycycline (Fig. 8D) and its absence following 3 d without dietary doxycycline (Fig. 8E). Deinduction of RANKL expression is also coincident with an increase in mammary epithelial cell death as measured by TUNEL assay (Supplemental Fig. 8), suggesting programmed cell death may be one mechanism by which the mammary gland reverts to the PRKO morphology following deinduction. Although the full spectrum of RANKL effectors have yet to be identified in the murine mammary gland, we have recently shown that minichromosome maintenance 3 (mcm-3) protein expression is markedly stimulated with RANKL induction in the doxycycline-treated PRrtTA/rtTA/TetO-RANKL mammary epithelium (Fig. 8F). Recent studies have shown that mcm-3 is induced by progesterone in the human mammary epithelial cell (5, 6), whereas progesterone down-regulates mcm-3 expression in the uterine epithelium (31). Following RANKL deinduction (Fig. 8G), mcm-3 expression is no longer detected, underscoring a possible effector role for mcm-3 in mammary RANKL signaling. Interestingly, the highest levels of mammary mcm-3 in the WT mouse are induced during early/mid pregnancy, a time period during which RANKL is also normally induced (Fig. 8H). With pregnancy progression, however, mammary expression levels for mcm3 significantly attenuate in the WT mouse (Fig. 8I); low levels of mcm3 are also detected in the mammary gland during lactation, involution, and the virgin state (data not shown).
DISCUSSION
Finding that mammary RANKL expression can be induced by progesterone specifically in the PR-positive cell type provides compelling support for a possible mediator role for RANKL in mammary progesterone action. To functionally test this proposal in vivo, we have described the use of an innovative bigenic mouse system in which RANKL expression is conditionally targeted to a PRKO mammary cell type that is equivalent to a cell type that normally coexpresses PR and RANKL in the WT mammary epithelium during pregnancy. By virtue of its ability to faithfully recapitulate in the PRKO the spatial restrictions imposed on mammary RANKL expression observed in the mammary gland of the parous mouse, this powerful targeting system affords clear advantages over other approaches (i.e., conventional transgenesis or retroviral infection; refs. 20, 21, 28), which indiscriminately target gene expression within the mammary epithelial compartment. In recent transplant studies of Beleut et al. (28), RANKL was indiscriminately targeted to the PRKO mammary epithelium, and its function was assessed in the context of the complex milieu of pregnancy hormones. To recapitulate the normal spatial expression pattern of mammary RANKL, our studies selectively targeted RANKL to cell types in the PRKO mammary gland that would normally express RANKL in the WT mammary gland. Another important distinction is that our studies evaluated the functional contribution of RANKL alone in the PRKO mammary gland without the confounding effects of pregnancy hormones.
With RANKL induction, spatially ordered ductal side-branching and alveolar development was achieved throughout the PRKO bigenic mammary ductal system. This result indicates that the absence of PR does not preclude the development of its cell of origin (ER+ luminal cell) in the PRKO mammary epithelium or of nearby ductal and alveolar progenitors that retain the capability to receive and transduce the RANKL output signal. Indeed, recent studies suggest that RANKL may be a critical link between PR+ luminal cells and mammary stem/progenitor cells (32, 33). The ordered ductal arborization and alveolar development attained following RANKL induction is in striking contrast to the spatially disorganized ductal branching and alveologenesis observed in the MTB/TetO-RANKL bitransgenic model, which occurs due to indiscriminate mammary RANKL targeting. These observations support the proposal that the potent proliferative effects of RANKL signaling require to be regionally confined to the ER+/PR+ mammary cell type in the WT to ensure an ordered morphogenetic response during pregnancy. Not surprisingly, mammary epithelial proliferation accounts for much of the morphological changes that are observed in the PRKO bigenic mice following RANKL induction. As similarly observed in the WT mammary epithelium (15, 20), mammary cells that proliferate in response to RANKL induction are segregated from cells positive for RANKL expression in the PRKO bigenic mice. In the case of the WT, such a segregation pattern has suggested a paracrine mechanism may underlie RANKL as a mediator of mammary PR action (3, 15, 20). However, it is important to note that our findings here do not formally confirm (or refute) this supposition. On the basis of our current data, a number of signaling scenarios are possible: 1) mammary RANKL may directly engage the RANK receptor located on a juxtaposed stem and/or bipotent progenitor cell to execute signaling programs required for ductal and alveolar development (a paracrine mechanism, as suggested recently; refs. 32, 33); 2) the RANKL signal may be received by an intermediate RANK+ cell (nonprogenitor), which, in turn, relays the initial signal to a neighboring bipotent progenitor cell; and/or 3) mammary RANKL may act via its processed ectodomain to engage RANK on the same cell (autocrine) or on nearby cells (juxtacrine) to trigger signaling cascades that, in turn, act at a distance. Much of the current uncertainty also lies with the apparent uniform expression pattern for mammary RANK receptor (20–22). Obviously, determining the regulatory mechanisms by which the appropriate RANK+ responder cell is selected will significantly advance our current understanding of mammary RANKL signaling at the cellular level.
Although the full spectrum of downstream effector pathways of mammary RANKL has yet to be identified, we demonstrate that cyclin D1 is induced by RANKL in the PRKO mammary epithelium. Notably, cyclin D1 has been shown not only to be a molecular target of mammary RANKL signaling (via activation of NF-κB; ref. 29) but also for mammary PR (30), suggesting that the P4→PR→RANKL→RANK→cyclin D1 pathway may represent one of many critical signaling arms of PR-mediated mammary proliferation. Interestingly, other candidate effectors ascribed to murine mammary PR-mediated proliferation [i.e., wnt-4 (11), msx-2 (16), amphiregulin (13), calcitonin (14), and Id4 (13)] were not induced by RANKL (data not shown), indicating that these factors may comprise effector pathways that operate parallel to the RANKL signaling axis (cyclin D1, however, may represent a convergence point for both RANKL and wnt-4 signaling). Moreover, significant changes in CAAT/enhancer binding protein β (C/EBPβ) expression (34) and Id2 nuclear translocation (35) were not observed in the PRKO bigenic mammary epithelium; both proteins have been implicated as murine mammary targets of RANKL signaling. Therefore, identifying the key RANKL effectors that are sufficient to trigger the complex morphological changes observed in the PRKO bigenic mammary gland is an urgent priority.
Apart from murine mammary targets, recent investigations using primary human breast epithelial cells have revealed that mcm-3 is induced by progesterone (5). Mammalian mcm-3 comprises a family of 6 evolutionary conserved members (mcm-2 to mcm-7) which are indispensable for DNA replication licensing, an obligate first step toward replication of the genome and cellular proliferation (36). As part of a prereplicative complex, mcm-3 protein ensures that DNA replication occurs only once per cell division cycle. Notably, mcm-3 expression represents a marker not only for actively proliferating cells but also for cells that possess proliferative potential (37). Depending on changes in signaling context, however, these cells may either reenter the active cell division cycle or progress to terminal differentiation in the future. As a molecular indicator of cells with proliferative potential, as well as of active cell division, mcm-3 is now favored as a more accurate biomarker (compared to Ki67) for high-grade disease (38).
Recent proteomic studies revealed that the murine mammary epithelium expresses the highest levels of mcm-3 during the midpregnancy period (39), a developmental stage in which the mammary epithelium is undergoing its highest level of proliferation in response to the hormones of pregnancy. Our observation that initiation of the RANKL signaling axis alone can induce mcm-3 in the mammary luminal epithelial compartment of the PRKO suggests that RANKL may represent an important mediator of progesterone-induced mammary mcm-3 expression during midpregnancy. Therefore, we propose that mcm-3 induced by this signaling environment reprograms much of the mammary epithelium of the midpregnant mouse to a developmental state in which cells can either further proliferate in the short-term or differentiate (when exposed to a differentiation signal such as prolactin) as pregnancy progresses to term. Obviously to test this proposal, future in vitro and/or in vivo studies will need to evaluate the functional importance of mcm-3 in RANKL induced mammary epithelial proliferation. Also, revealing the intermediate signaling pathways that mediate RANKL induction of mammary mcm-3, as well as the hormonal cues that trigger attenuation of mcm-3 expression with pregnancy progression constitute important future goals. Moreover, determining whether deregulation of mcm-3 expression is linked with the involvement of PR and/or RANKL in aberrant growth effects in the mammary gland will also be an important priority. Indeed, deregulation of the prereplicative complex has been linked to early-stage neoplastic progression (40).
In summary, we have generated an innovative PRKO bigenic system in which RANKL expression is conditionally targeted to a mammary cell type that is equivalent to a specific subset of cells that normally coexpresses PR and RANKL in the mammary epithelium of the pregnant WT mouse. With this bigenic system, we demonstrate that conditional induction of RANKL elicits ordered ductal side-branching and alveolar development in the PRKO mammary gland. Induction of RANKL programs the PRKO mammary epithelium to a proliferative state, which fails to progress to terminal differentiation presumably due to the absence of prolactin signaling. However, the observation that installation of the RANKL signaling axis in the PRKO mammary epithelium (in the absence of further endocrine stimulation) is sufficient to elaborate the signature morphological changes that occur with progesterone exposure attests to the prominent effector role of RANKL. Future research objectives will use this bigenic system to define the purported involvement of RANKL in progesterone-induced mammary tumor promotion (41), as well as to target other presumptive mediators of mammary PR action.
Supplementary Material
Acknowledgments
The authors thank Jie Han for her technical expertise, and the Darwin Transgenic Core Facility, Baylor College of Medicine, for its DNA microinjection services. The authors also thank Dr. Daniel Medina (Baylor College of Medicine) for his expertise in mammary histopathology. The authors are grateful to Dr. Lewis A. Chodosh (Department of Cancer Biology, Abramson Family Cancer Research Institute, University of Pennsylvania School of Medicine, Philadelphia, PA, USA) for providing the p-Tet-Splice cloning vector, as well as for the transgenic MTB and TZA reporter mouse.
This research was funded by U.S. National Institutes of Health grant CA077530 (to J.P.L.).
Note strong immunoreactivity for mammary mcm-3 in the luminal epithelial compartment of the midpregnant mouse as compared to low levels of mammary mcm-3 expression in the late-pregnant mouse (arrowhead). Scale bars = 1 mm (B, C); 50 μm (D, E); 100 μm (F–I).
REFERENCES
- 1..Russo J., Ao X., Grill C., Russo I. H. (1999) Pattern of distribution of cells positive for estrogen receptor-α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res. Treatment. 53, 217– 227 [DOI] [PubMed] [Google Scholar]
- 2..Seagroves T. N., Lydon J. P., Hovey R. C., Vonderhaar B. K., Rosen J. M. (2000) C/EBPβ(CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol. Endocrinol. 14, 359– 368 [DOI] [PubMed] [Google Scholar]
- 3..Rosen J. M. (2003) Hormone receptor patterning plays a critical role in normal lobuloalveolar development and breast cancer progression. Breast Dis. 18, 3– 9 [DOI] [PubMed] [Google Scholar]
- 4..Clarke R. B., Howell A., Potten C. S., Anderson E. (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 57, 4987– 4991 [PubMed] [Google Scholar]
- 5..Graham J. D., Mote P. A., Salagame U., van Dijk J. H., Balleine R. L., Huschtscha L. I., Reddel R. R., Clarke C. L. (2009) DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 150, 3318– 3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6..Lydon J. P., Edwards D. P. (2009) Finally! A model for progesterone receptor action in normal human breast. Endocrinology 150, 2988– 2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7..Grimm S. L., Seagroves T. N., Kabotyanski E. B., Hovey R. C., Vonderhaar B. K., Lydon J. P., Miyoshi K., Hennighausen L., Ormandy C. J., Lee A. V., Stull M. A., Wood T. L., Rosen J. M. (2002) Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development. Mol. Endocrinol. 16, 2675– 2691 [DOI] [PubMed] [Google Scholar]
- 8..Poole A. J., Li Y., Kim Y., Lin S. C., Lee W. H., Lee E. Y. (2006) Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 314, 1467– 1470 [DOI] [PubMed] [Google Scholar]
- 9..Shyamala G., Yang X., Cardiff R. D., Dale E. (2000) Impact of progesterone receptor on cell-fate decisions during mammary gland development. Proc. Natl. Acad. Sci. U. S. A. 97, 3044– 3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10..Shyamala G., Yang X., Silberstein G., Barcellos-Hoff M. H., Dale E. (1998) Transgenic mice carrying an imbalance in the native-ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc. Natl. Acad. Sci. U. S. A. 95, 696– 701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11..Brisken C., Heineman A., Chavarria T., Elenbaas B., Tan J., Dey S. K., McMahon J. A., McMahon A. P., Weinberg R. A. (2000) Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 14, 650– 654 [PMC free article] [PubMed] [Google Scholar]
- 12..Fata J. E., Kong Y. Y., Li J., Sasaki T., Irie-Sasaki J., Moorehead R. A., Elliott R., Scully S., Voura E. B., Lacey D. L., Boyle W. J., Khokha R., Penninger J. M. (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 103, 41– 50 [DOI] [PubMed] [Google Scholar]
- 13..Fernandez-Valdivia R., Mukherjee A., Creighton C. J., Buser A. C., Demayo F. J., Edwards D. P., Lydon J. P. (2008) Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 149, 6236– 6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14..Ismail P. M., DeMayo F. J., Amato P., Lydon J. P. (2004) Progesterone induction of calcitonin expression in the murine mammary gland. J. Endocrinol. 180, 287– 295 [DOI] [PubMed] [Google Scholar]
- 15..Mulac-Jericevic B., Lydon J. P., DeMayo F. J., Conneely O. M. (2003) Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. U. S. A. 100, 9744– 9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16..Satoh K., Hovey R. C., Malewski T., Warri A., Goldhar A. S., Ginsburg E., Saito K., Lydon J. P., Vonderhaar B. K. (2007) Progesterone enhances branching morphogenesis in the mouse mammary gland by increased expression of Msx2. Oncogene. 26, 7526– 7534 [DOI] [PubMed] [Google Scholar]
- 17..Theill L. E., Boyle W. J., Penninger J. M. (2002) RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20, 795– 823 [DOI] [PubMed] [Google Scholar]
- 18..Walsh M. C., Choi Y. (2003) Biology of the TRANCE axis. Cytokine Growth Factor Rev. 14, 251– 263 [DOI] [PubMed] [Google Scholar]
- 19..Jones D. H., Nakashima T., Sanchez O. H., Kozieradzki I., Komarova S. V., Sarosi I., Morony S., Rubin E., Sarao R., Hojilla C. V., Komnenovic V., Kong Y. Y., Schreiber M., Dixon S. J., Sims S. M., Khokha R., Wada T., Penninger J. M. (2006) Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692– 696 [DOI] [PubMed] [Google Scholar]
- 20..Fernandez-Valdivia R., Mukherjee A., Ying Y., Li J., Paquet M., DeMayo F. J., Lydon J. P. (2009) The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev. Biol. 328, 127– 139 [DOI] [PubMed] [Google Scholar]
- 21..Gonzalez-Suarez E., Branstetter D., Armstrong A., Dinh H., Blumberg H., Dougall W. C. (2007) RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol. Cell. Biol. 27, 1442– 1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22..Srivastava S., Matsuda M., Hou Z., Bailey J. P., Kitazawa R., Herbst M. P., Horseman N. D. (2003) Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem. 278, 46171– 46178 [DOI] [PubMed] [Google Scholar]
- 23..Gunther E. J., Belka G. K., Wertheim G. B., Wang J., Hartman J. L., Boxer R. B., Chodosh L. A. (2002) A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 16, 283– 292 [DOI] [PubMed] [Google Scholar]
- 24..Mukherjee A., Soyal S. M., Fernandez-Valdivia R., DeMayo F. J., Lydon J. P. (2007) Targeting reverse tetracycline-dependent transactivator to murine mammary epithelial cells that express the progesterone receptor. Genesis. 45, 639– 646 [DOI] [PubMed] [Google Scholar]
- 25..Aupperlee M. D., Haslam S. Z. (2007) Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology 148, 2290– 2300 [DOI] [PubMed] [Google Scholar]
- 26..Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Jr., Shyamala G., Conneely O. M., O'Malley B. W. (1995) Mice lacking progesterone receptors exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266– 2278 [DOI] [PubMed] [Google Scholar]
- 27..Anderson S. M., Rudolph M. C., McManaman J. L., Neville M. C. (2007) Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis!. Breast Cancer Res. 9, 204– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28..Beleut M., Rajaram R. D., Caikovski M., Ayyanan A., Germano D., Y. C., Schneider P., Brisken C. (2010) Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. U. S. A. 107, 2989– 2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29..Cao Y., Bonizzi G., Seagroves T. N., Greten F. R., Johnson R., Schmidt E. V., Karin M. (2001) IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 107, 763– 775 [DOI] [PubMed] [Google Scholar]
- 30..Said T. K., Conneely O. M., Medina D., O'Malley B. W., Lydon J. P. (1997) Progesterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell, in vivo. Endocrinology 138, 3933– 3939 [DOI] [PubMed] [Google Scholar]
- 31..Pan H., Deng Y., Pollard J. W. (2006) Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc. Natl. Acad. Sci. U. S. A. 103, 14021– 14026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32..Asselin-Labat M. L., Vaillant F., Sheridan J. M., Pal B., Wu D., Simpson E. R., Yasuda H., Smyth G. K., Martin T. J., Lindeman G. J., Visvader J. E. (2010) Control of mammary stem cell function by steroid hormone signalling. Nature. 465, 798– 802 [DOI] [PubMed] [Google Scholar]
- 33..Joshi P. A., Jackson H. W., Beristain A. G., Di Grappa M. A., Mote P., Clarke C., Stingl J., Waterhouse P. D., Khokha R. (2010) Progesterone induces adult mammary stem cell expansion. Nature 465, 803– 807 [DOI] [PubMed] [Google Scholar]
- 34..Kim H. J., Yoon M. J., Lee J., Penninger J. M., Kong Y. Y. (2002) Osteoprotegerin ligand induces beta-casein gene expression through the transcription factor CCAAT/enhancer-binding protein beta. J. Biol. Chem. 277, 5339– 5344 [DOI] [PubMed] [Google Scholar]
- 35..Kim N. S., Kim H. J., Koo B. K., Kwon M. C., Kim Y. W., Cho Y., Yokota Y., Penninger J. M., Kong Y. Y. (2006) Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell. Biol. 26, 1002– 1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36..Madine M. A., Khoo C. Y., Mills A. D., Laskey R. A. (1995) MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature 375, 421– 424 [DOI] [PubMed] [Google Scholar]
- 37..Endl E., Kausch I., Baack M., Knippers R., Gerdes J., Scholzen T. (2001) The expression of Ki-67, MCM3, and p27 defines distinct subsets of proliferating, resting, and differentiated cells. J. Pathol. 195, 457– 462 [DOI] [PubMed] [Google Scholar]
- 38..Soling A., Sackewitz M., Volkmar M., Schaarschmidt D., Jacob R., Holzhausen H. J., Rainov N. G. (2005) Minichromosome maintenance protein 3 elicits a cancer-restricted immune response in patients with brain malignancies and is a strong independent predictor of survival in patients with anaplastic astrocytoma. Clin. Cancer Res. 11, 249– 258 [PubMed] [Google Scholar]
- 39..Davies C. R., Morris J. S., Griffiths M. R., Page M. J., Pitt A., Stein T., Gusterson B. A. (2006) Proteomic analysis of the mouse mammary gland is a powerful tool to identify novel proteins that are differentially expressed during mammary development. Proteomics. 6, 5694– 5704 [DOI] [PubMed] [Google Scholar]
- 40..Ha S. A., Shin S. M., Namkoong H., Lee H., Cho G. W., Hur S. Y., Kim T. E., Kim J. W. (2004) Cancer-associated expression of minichromosome maintenance 3 gene in several human cancers and its involvement in tumorigenesis. Clin. Cancer Res. 10, 8386– 8395 [DOI] [PubMed] [Google Scholar]
- 41..Branstetter D., Jacob A., Gonzalez-Suarez E., Erwert R., Chaisson-Blake M., Dougall W. C. (2008) RANKL inhibition decreases the incidence of mammary adenocarcinomas in wild type (WT) and MMTV-RANK transgenic mice. San Antonio Breast Cancer Symposium, Abstract 4167; San Antonio, TX, USA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.