Abstract
Adiponectin activates AMP-activated protein kinase (AMPK) in adipocytes, but the underlying mechanism remains unclear. Here we tested the hypothesis that AMP, generated in activating fatty acids to their CoA derivatives, catalyzed by acyl-CoA synthetases, is involved in AMPK activation by adiponectin. Moreover, in adipocytes, insulin affects the subcellular localization of acyl-CoA synthetase FATP1. Thus, we also tested whether insulin activates AMPK in these cells and, if so, whether it activates through a similar mechanism. We examined these hypotheses by measuring the AMP/ATP ratio and AMPK activation on adiponectin and insulin stimulation and after knocking down acyl-CoA synthetases in adipocytes. We show that adiponectin activation of AMPK is accompanied by an ∼2-fold increase in the cellular AMP/ATP ratio. Moreover, FATP1 and Acsl1, the 2 major acyl-CoA synthetase isoforms in adipocytes, are essential for AMPK activation by adiponectin. We also show that after 40 min. insulin activated AMPK in adipocytes, which was coupled with a 5-fold increase in the cellular AMP/ATP ratio. Knockdown studies show that FATP1 and Acsl1 are required for these processes, as well as for stimulation of long-chain fatty acid uptake by adiponection and insulin. These studies demonstrate that a change in cellular energy state is associated with AMPK activation by both adiponectin and insulin, which requires the activity of FATP1 and Acsl1.—Liu, Q., Gauthier, M.-L., Sun, L., Ruderman, N., Lodish, H. Activation of AMP-activated protein kinase signaling pathway by adiponectin and insulin in mouse adipocytes: requirement of acyl-CoA synthetases FATP1 and Acsl1 and association with an elevation in AMP/ATP ratio.
Keywords: acyl-CoA synthetases, adipokine
Adipose cells store lipids as triglycerides and also secrete several important hormones and paracrine mediators collectively termed adipokines; these include adiponectin, resistin, leptin, and TNF-α. Adiponectin is a protein hormone that has positive metabolic effects, including enhancing fatty-acid oxidation and glucose utilization in muscle and adipose tissue as well as inhibiting gluconeogenesis in liver (1–3). These beneficial effects are closely associated with activation of AMPK (1, 4) in muscle and liver and activation of nuclear factor-κB in muscle cells (5). Distinct signaling properties of adiponectin can be ascribed to its different oligomeric forms; serum adiponectin exists as trimers, hexamers, and high-molecular-weight (HMW) species, mainly 18 mers (5, 6). Hexameric and HMW forms activate NF-κB in undifferentiated or differentiated C2C12 cells (5) and improve insulin sensitivity in liver (7), whereas the trimeric form activates AMPK in muscle and adipose tissues (1, 3, 4). Adiponectin circulates at a high level in serum (7.9 to 11.7 μg/ml) (8), and levels of adiponectin are significantly decreased in obese or type II diabetic people and mice (9).
AMPK is a key energy sensor that maintains cellular energy homeostasis; it controls the balance between ATP consumption and ATP production. AMPK is activated by a reduced intracellular energy state, reflected as a high AMP/ATP ratio in the cytosol. Through its actions it brings the decreased level of intracellular ATP back to normal and reduces 5′-AMP levels (10). AMPK, when activated by adiponectin, phosphorylates and inactivates acyl-CoA carboxylase (ACC), the enzyme that catalyzes the formation of malonyl CoA. Malonyl CoA is a substrate for fatty-acid biosynthesis, and it inhibits fatty acid β-oxidation (11). Thus, AMPK activation by adiponectin results in inhibition of fatty acid and triacylglycerol synthesis and stimulation of fatty acid β-oxidation (12). Although in general, AMPK stimulates catabolic pathways and inhibits anabolic pathways, studies have demonstrated a direct interaction between AMPK and anabolic insulin signaling; AMPK phosphorylates the insulin receptor substrate-1 (IRS-1) at Ser789, which correlates with a 65% increase in insulin-stimulated IRS-1 associated phosphatidylinositol 3-kinase (PI3K) activity in C2C12 myotubes (13).
Three adiponectin receptors termed AdipoR1, AdipoR2, and T-Cadherin have been cloned (14, 15), but we do not know how or whether these or other adiponectin receptors mediate the adiponectin signal. AMPK activity is modulated by altered energy states of cells, and its activation requires the presence of 2 conditions: an increase in the cellular 5′-AMP/ATP ratio and phosphorylation on the α-subunit at Thr172 by one or more upstream AMPK kinases (AMPKK) such as LKB1 and Ca2+-calmodulin-dependent protein kinase (16–18). One hypothesis is that direct interaction of an adaptor protein APPL1 with AdipoR1 or R2 induces LKB1 translocation and anchorage in the cytosol, which subsequently phosphorylates AMPK α at Thr172 (19).
Notably, previous studies have shown that in muscle cells trimeric adiponectin causes a rapid and ∼2-fold increase in 5′-AMP level (1), which suggests the presence of uncharacterized pathways connecting adiponectin receptors with AMPK by producing 5′-AMP. Acyl-CoA synthetases catalyze the first step of fatty-acid metabolism (20): fatty acid + coenzyme A + ATP → fatty acyl-coenzyme A + 5′-AMP + 2Pi, and activation of free fatty acids to the CoA derivatives by these enzymes is one important metabolic process that generates 5′-AMP. We hypothesized that acyl-CoA synthetases are signal transduction proteins downstream of adiponectin receptors. Specifically, we hypothesize that adiponectin receptor activation is coupled to activation of acyl-CoA synthetase activity. This would lead to an increase in import of fatty acids from the medium (21, 22), and the resultant 5′-AMP formed during attachment of fatty acids to CoA would then activate AMPK. Previous reports indeed have shown that the AMP released during this process activated AMPK (23, 24).
Consistent with being a potential adiponectin coreceptor, acyl-CoA synthetases are integral membrane proteins localized to the plasma and other intracellular membranes (25), with functionally crucial domains extending to both sides of the membrane (26). Five isoforms of long-chain acyl-CoA synthetases (Acsl, Acsl3–6) and 6 isoforms of fatty acid transport proteins (FATP1–6) have been cloned in mammals. All these proteins possess long-chain- or very long-chain-acyl-CoA synthetase activity (27–29) and overexpression of Acsl1 or FATPs 1–5 lead to increases in fatty-acid incorporation (30–32). Interestingly, insulin induces expression of and translocation of FATP1 to the plasma membrane in adipocytes and skeletal muscle cells (33–35); an increase in fatty acid influx into the cells is observed in parallel with this translocation. In this work we determined a role for acyl-CoA synthetases in adiponectin trimer-induced AMPK activation and showed that a similar mechanism is involved in insulin activation of AMPK.
MATERIALS AND METHODS
Materials
Insulin solution from bovine pancreas, fatty acid-free bovine serum albumin (BSA), dexamethasone, isobutylmethylxanthine, collagenase, rosiglitazone, and nonradiolabeled fatty acids were purchased from Sigma-Aldrich (St. Louis, MO, USA). [1-14C] Oleic acid was from GE Healthcare (Little Chalfont, UK). Polyclonal phospho-specific antibodies to AMPK α (Thr172), ACC (Ser79), Akt (Ser473), and IRS-1 (Ser789) and anti-AMPK α, anti-ACC, and anti-IRS-1 antibodies were from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
3T3-L1 fibroblasts obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) were grown in DMEM supplemented with 10% (v/v) bovine serum, 2 mM l-glutamine, and 1% antibiotic (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in 5% CO2. These cells were subcultivated before reaching confluence. Fibroblasts were induced to differentiate 2 d after reaching confluence by addition of DMEM containing 10% (v/v) FBS, 5 μg/ml insulin, 0.5 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine. After 48 h, the medium was replaced with fresh DMEM containing 10% FBS and 5 μg/ml insulin and incubated for another 48 h. Adipocytes were re-fed with growth media every 48 h and used for experiments 8–10 d postdifferentiation.
Primary preadipocytes were fractionated and cultured as described previously with few modifications (36). Briefly, subcutaneous adipose tissues harvested from 3- to 4-wk-old mice were minced into small pieces, digested with collagenase, and centrifuged at 500–700 g. The resulting preadipocytes were cultured in DMEM containing 10% New Born Calf Serum (Invitrogen, Carlsbad, CA, USA) to confluence. Adipogenesis was induced by treating confluent cells with medium containing 10% FBS, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine, 5 μg/ml insulin, and 1 μM rosiglitazone. At 2 d after induction, cells were switched to DMEM containing 10% FBS and 5 μg/ml insulin. After 48 h, medium was replaced with DMEM containing 10% FBS. At d 6, cells were fully differentiated.
Human embryonic kidney (HEK) cells obtained from ATCC were grown in DMEM containing 10% FBS, 2 mM l-glutamine and 1% antibiotic at 37°C in 5% CO2 and used for transiently overexpressing adiponectin C22A.
Overexpression and purification of adiponectin trimer (C22A)
The mouse adiponectin trimer mutant C22A cDNA (residue 1–247) cloned in pcDNA3.1 was a gift from Dr. Tsu-Shuen Tsao (University of Arizona, Tucson, AX, USA; refs. 5, 15). In brief, the Flag epitope (DYKDDDDK) was cloned in between the signal sequence cleavage site and the N-terminal variable region, and an alanine was introduced by site-directed mutagenesis to replace the cysteine at position 22. To overexpress the recombinant protein, plasmids were transiently transfected into HEK cells using lipofectamine 2000, and recombinant protein was expressed in Opti-MEMI reduced serum medium containing 0.1g/L vitamin C. The conditioned medium was collected every 48 h and then subjected to affinity purification using anti-Flag M2 affinity gel (Sigma-Aldrich), according to the manufacturer’s instructions. The purified adiponectin C22A was eluted in 1× PBS (pH 7.4) supplemented with 1 mM CaCl2 and 1 mM MgSO4.
Measurement of oleic acid uptake
At 1 h before the experiment, the growth medium was removed from 3T3-L1 adipocytes grown in 12-well plates (Corning, Corning, NY, USA), and 1 ml of preincubation medium (DMEM/12 mM glucose/4 mM glutamine/25 mM HEPES with 100 μM FFA-free BSA and 300 μM nonradioactive oleic acid) was added. At the start of the experiment, [1-14C] oleic acid (100 μCi/ml; 1 Ci=37 GBq) was added to reach a final concentration of 1.7 μM, and cells were incubated with PBS, 40 μg/ml adiponectin trimer, or 50 ng/ml insulin for 0–60 min. Six replicates were performed for each treatment. After 60 min, medium was aspirated, and the cells were washed twice with iced-cold PBS, followed by trypsinization. The detached cells were transferred to a scintillation tube containing 4 ml Ecoscint H (National Diagnostics, Atlanta, GA, USA), and the amount of 14C radioactivity was determined by liquid scintillation counting.
Nucleotide studies
Cellular levels of ATP, ADP, and AMP were measured spectrophotometrically, as described previously (23, 37). In brief, adipocytes were treated with adiponectin C22A (40 μg/ml) for 5 min, or with insulin (50 ng/ml) for 60 min. After the incubation period, the cells were washed once with cold PBS, and proteins were precipitated with 1% trichloroacetic acid (TCA). The acid was then removed from the aqueous phase of the TCA extracts with repeated washes with ether. The samples were then dried to powder and kept at −80°C until the assay.
siRNA transient transfection
3T3-L1 adipocytes (d 7–8) grown in 12-well plates were transiently transfected with 1 ml of siRNA medium (DMEM/10% FBS/2 mM l-glutamine/100 nM siRNA complexes). Briefly, 100 nM specified siRNA diluted in 100 μl DMEM was mixed with 8–10 μl of DharmaFECT3 transfection reagent (Thermo Fisher Scientific, Pittsburgh, PA, USA) and incubated for 30 min at room temperature to form siRNA complexes. Then 990 μl of growth medium was added to the siRNA complexes to form 1 ml siRNA medium. Cells were incubated with siRNA medium for 24 h followed by incubation in fresh DMEM/10% FBS for 24 h. Cells were then treated or not with adiponectin trimer or insulin as described in Western Blotting Analysis. The nontargeting, FATP1- and Acsl1-specific siRNAs are combinations of 4 siRNAs (Dharmacon siGENOME ON-TARGETplus SMARTpools; Thermo Fisher Scientific), which were designed to efficiently decrease off-target effects. The nontargeting siRNA sequences were 5′-UGGUUUACAUGUCGACUAA-3′; 5′-UGGUUUACAUGUUGUGUGA-3′; 5′-UGGUUUACAUGUUUUCUGA-3′; and 5′-UGGUUUACAUGUUUUCCUA-3′. The FATP1 siRNA sequences were 5′-UGACGGUGGUACUGCGCAA-3′; 5′-CCUCUCAGGUGACGUGCUA-3′; 5′-GGGCUUUGCACCAGGCGAU-3′ and 5′-GGGCAUGGAUGAUCGGCUG-3′. The Acsl1 siRNA sequences were 5′-GGGAUUCAGGUGUCAAAUA-3′; 5′-GAGCUGACGCCAUCACCUA-3′; 5′-GAAGAUUAUCGACAGGAAA-3′ and 5′-ACCCGAAGAUCUUGCGAUA-3′.
Examination of gene knockdown efficiencies
Total RNA from transfected 3T3-L1 adipocytes (24 h after transfection) was isolated using RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) and kept at −80°C until further assays. A real-time PCR was performed on an ABI Prism 7900HT Sequence Detector (Applied Biosystems, Foster City, CA, USA) from 1 μg total RNA from adipocytes, as described previously (38). GAPDH was used as the internal control. Data were analyzed by the relative quantification (ΔΔCt) method.
Western blot analysis
Primary adipocytes or 3T3-L1 adipocytes, serum-starved for 1 or 3 h in DMEM, were stimulated with adiponectin trimer or insulin at the specified concentration. Each treatment was performed in triplicate. Western blotting was performed as described previously (39). Immunoreactive bands on autoradiography films were scanned and quantified using Gene Snap and GeneTools software (SynGene, Frederick, MD, USA). The AMPK activity was represented as the ratio of phospho-AMPK α over total AMPK α (P-AMPK/AMPK). The fold of AMPK activation was obtained by comparing to the control groups (PBS-treated) whose P-AMPK/AMPK ratio was set to 1. A same method was used to analyze the phosphorylation of ACC or IRS-1.
Statistical analysis
Data are expressed as means ± se. Statistical analysis was undertaken using unpaired, single-tailed Student’s t test and the Mann–Whitney U test to compare the 2 groups. Differences between groups were considered statistically significant when P < 0.05.
RESULTS
Adiponectin trimer increases intracellular AMP/ATP ratio in 3T3-L1 adipocytes
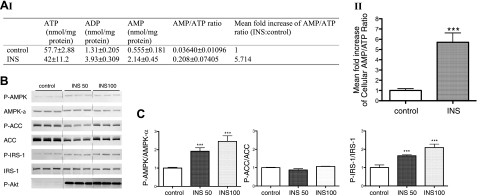
Previous studies have shown that adiponectin causes an ∼2-fold increase in 5′-AMP level in muscle cells (1). Consistent with this observation, we show here that adiponectin trimer also causes an increase in the intracellular 5′-AMP/ATP ratio in 3T3-L1 adipocytes. In these studies 3T3-L1 adipocytes were treated with 40 μg/ml adiponectin trimer or control (PBS) for 5 min and the cellular levels of ATP, ADP, and AMP were measured spectrophotometrically as described previously (23, 40–42). As shown in Fig. 1A, adiponectin treatment caused an increase of ∼2 fold in the cellular AMP/ATP ratio compared to that of control (PBS). We also tested whether the procedures involved in the assays led to partial degradation of ATP. Using an ATP standard, we demonstrated a 95% or better recovery of the ATP by this method (data not shown).
Figure 1.
A) Adiponectin trimer (C22A) treatment increases the cellular AMP/ATP ratio in 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were subjected to a 3-h preincubation period in serum-free DMEM containing 100 μM fatty-acid free BSA and then treated with control (PBS) or adiponectin C22A (40 μg/ml) for 5 min. Cellular AMP/ATP ratio was measured as described in Materials and Methods. I) Summary of cellular adenine nucleotide levels. Results are expressed as means ± sd (n=3–4). Data were obtained in 2 independent experiments. II) Mean fold increase of AMP/ATP ratio. B) Effect of adiponectin trimer (C22A) on oleic acid uptake into 3T3-L1 adipocytes. Uptake of [14C] oleic acid bound to albumin (1:3 M ratio) into control (PBS) or adiponectin trimer (40 μg/ml) treated 3T3-L1 adipocytes was measured over 60 min. Oleic acid uptake stimulated by 50 ng/ml insulin served as a positive control. Quantitative analysis of 6 wells of 3T3-L1 adipocytes grown in 12-well plates is shown; values represent means ± se (n=6). ***P < 0.05.
Adiponectin trimer significantly promotes the uptake of oleic acid into 3T3-L1 adipocytes
Acyl-CoA synthetases stimulate cellular influx of LCFA. Figure 1B shows that addition of 40 μg/ml trimeric adiponectin stimulates uptake of a typical free fatty acid, oleic acid, into 3T3-L1 adipocytes; the 2.5–3 fold increase in uptake was similar to that induced by 50 ng/ml insulin. An enhanced rate of fatty acid influx following addition of adiponectin trimer was also observed in C2C12 myotubes (Supplemental Fig. 1). These observations support our hypothesis that acyl-CoA synthetases are involved in activation of AMPK by trimeric adiponectin. Here we did not need to correct the transport rate for carry-over of extracellular water by using a nonpermeate marker because the nonspecific level of incorporated [14C] oleate should be the same between the hormone-treated and control groups.
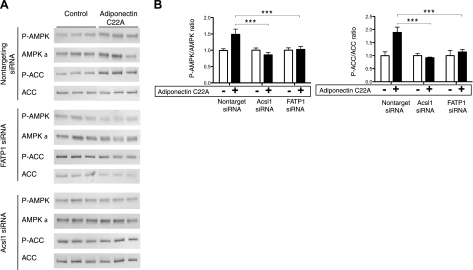
Knockdown of FATP1 or Acsl1 gene in 3T3-L1 adipocytes represses AMPK activation by adiponectin trimer
Figure 2 shows that Acsl1 and FATP1, the 2 major isoforms of acyl-CoA synthetase expressing in adipose tissue (43, 44), are required for adiponectin-induced AMPK activation in 3T3-L1 adipocytes. Nontargeting siRNA, Acsl1 siRNA, and FATP1 siRNA were transfected transiently into d 7 3T3-L1 adipocytes. The mRNAs were extracted after 24 h and analyzed for gene expression level by real-time PCR. The mRNAs of both FATP1 and Acsl1 genes were down-regulated to >70% as compared to the control cells (Supplemental Fig. 2). At 2 d after transfection, the transfected cells were first serum-starved for 2–3 h and then incubated with 40 μg/ml adiponectin trimer for 5 min. In nontargeting siRNA-transfected cells, we observed an expected AMPK activation of ∼1.5-fold (P-AMPK/AMPK ratio) by adiponectin treatment compared to those treated with control (PBS) (Fig. 2). AMPK activation by adiponectin was significantly reduced in Acsl1 and FATP1 siRNA-transfected cells (Fig. 2). Although we did not perform a kinase assay for AMPK, the extent of AMPK phosphorylation at Thr172 strongly reflects its activity (45). Phospho-ACC (Ser79, the specific downstream target of AMPK), was also examined since its level reflects AMPK activity; the approximately 2-fold increase in phosphorylation at this site after adiponectin treatment of nontargeting siRNA-transfected cells was significantly reduced in both Acsl1 and FATP1 siRNA-transfected cells (Fig. 2). This confirms the role of both FATP1 and Acsl1 in AMPK activation by adiponectin.
Figure 2.
FATP1 and Acsl1 are required in AMPK activation by adiponectin in 3T3-L1 adipocytes. A) Western blot analysis of cell extracts from d 9 3T3-L1 adipocytes expressing FATP1 siRNA, Acsl1 siRNA, or nontargeting sequence siRNA as a control. As detailed in Fig. 1, cells were treated with 40 μg/ml adiponectin for 5 min. Western blot analyses were performed on cleared cell lysates by using antibodies specific for phospho-AMPK-α (Thr172), phospho-ACC (Ser79), total ACC, and total AMPK α. Blot is representative of 1 of 3 separate experiments analyzed in B. B) AMPK or ACC activity was represented as the ratio of phospho-AMPK α to total AMPK α (P-AMPK/AMPK) or phospho-ACC to total ACC (P-ACC/ACC), respectively. Fold of AMPK or ACC phosphorylation was obtained by normalizing to the control groups (PBS) whose P-AMPK/AMPK or P-ACC/ACC ratio was set to 1. Error bars = means ± se. Nine replicates from 3 independent experiments were statistically analyzed using the Mann–Whitney U test. ***P < 0.05.
Insulin treatment results in an increased intracellular AMP/ATP ratio and AMPK activation in 3T3-L1 adipocytes
As shown above, Acsl1 and FATP1 play key roles in adiponectin induced-AMPK activation in adipocytes. Intriguingly, insulin induces plasma-membrane translocation of FATP1 in 3T3-L1 adipocytes, accompanied by an increase in LCFA influx (33). We show here that, in parallel with these processes, insulin caused an increase in the intracellular 5′-AMP/ATP ratio and in AMPK activity. In these studies 3T3-L1 adipocytes were treated with 50 (8.72 nM) or 100 ng/ml (17.44 nM) insulin or control (PBS) for 60 min. As shown in Fig. 3A, insulin treatment caused a remarkable 5–6 fold increase in the cellular AMP/ATP ratio compared to that of control cells. Consistent with this change in the 5′-AMP/ATP ratio, insulin treatments effectively activated AMPK (Fig. 3B, C). Surprisingly, AMPK activation by insulin does not change phosphorylation of ACC at Ser79, as it does following adiponectin signaling. Instead, increased phosphorylation of an upstream component of insulin signaling, IRS-1, at Ser789 occurs (Fig. 3B, C). Presumably, as in C2C12 myotubes, this is catalyzed by the activated AMPK; phosphorylation at this site has been shown to enhance PI3K activity (46). As an expected control for insulin action, insulin increased phosphorylation of Ser473 in Akt (Fig. 3B).
>Figure 3.
A) Insulin treatment increases the cellular AMP/ATP ratio in 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were preincubated for 4 h in serum-free DMEM containing 100 μM fatty-acid free BSA and then treated with or without insulin (INS) (50 ng/ml) for 60 min. I) Summary of cellular adenine nucleotide levels. Results are expressed as means ± sd (n=4–6). Data were obtained in 2 independent experiments. II) Mean fold increase of the AMP/ATP. B) Insulin treatment activates AMPK in 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were treated with or without insulin (50 and 100 ng/ml) for 60 min. Western blot analyses were performed on cleared cell lysates using antibodies specific for phospho-AMPK-α (Thr172), phospho-ACC (Ser79), phospho-IRS-1 (Ser789), phospho-Akt (Ser473), total ACC, total IRS-1, and total AMPK α. Approximate 2-fold activation of AMPK does not phosphorylate ACC at Ser79. Instead, it promotes the phosphorylation of IRS-1 at Ser789. Blots are spots of interest cut from a blot shown in Supplemental Fig. 3A. C) Quantitative analysis of Western blot in B, using unpaired Student's t test. Values represent means ± se. ***P < 0.05.
Similar to adiponectin, insulin requires the activity of FATP1 and Acsl1 for AMPK activation in 3T3-L1 adipocytes
Figure 4 shows that, similar to adiponectin, insulin also requires Acsl1 and FATP1 to activate AMPK in 3T3-L1 adipocytes. 3T3-L1 adipocytes transiently transfected with synthetic FATP1 siRNA, Acsl1 siRNA or nontargeting siRNAs (Supplemental Fig. 2) were treated with 50 ng/ml insulin or control (PBS) for 60 min. In nontargeting siRNA-transfected cells, insulin caused a 1.5–2 fold increase in AMPK activity (Fig. 4). Similar to that observed in the adiponectin study, AMPK activation by insulin was markedly repressed in Acsl1 and FATP1 siRNA-transfected cells (Fig. 4). To confirm this result, we examined phospho-Ser-789 of IRS-1, a downstream target of AMPK activation by insulin in these cells. The 1.5–2 fold phosphorylation at this site in nontargeting siRNA-transfected cells was also significantly reduced in Acsl1 and FATP1 siRNA-transfected cells (Fig. 4). Taken together, these data indicate that the activities of both Acsl1 and FATP1 are required for transmitting the signals of adiponectin and insulin to AMPK.
Figure 4.
FATP1 and Acsl1 are required in AMPK activation by insulin in 3T3-L1 adipocytes. A) Western blot analysis of cell extracts from 3T3-L1 adipocytes expressing FATP1 siRNA, Acsl1 siRNA or a nontargeting siRNA, which were treated with 50 ng/ml insulin or control (PBS). A triplicate of each treatment is shown. Blot is representative of 1 of 2 separate experiments analyzed in B. Blots presented here are spots of interest cut from a blot shown in Supplemental Fig. 3B. B) AMPK or IRS-1 activity was represented as the ratio of phospho-AMPK α to total AMPK α (P-AMPK/AMPK) or phospho-IRS-1 to total IRS-1 (P-IRS-1/IRS-1), respectively. Fold of AMPK or IRS-1 activation was obtained by normalizing to the control groups (PBS), whose P-AMPK/AMPK or P-IRS-1/IRS-1 ratio was set to 1. Error bars = means ± se. Six replicates from 2 independent experiments were statistically analyzed using the Mann–Whitney U test. ***P < 0.05.
AMPK activation by adiponectin and insulin occurs at different times of stimulation
Although the same acyl-CoA synthetase isoforms are implicated in AMPK activation by adiponectin and insulin, we show in Fig. 5 that the dynamics of this process by the 2 hormones are different. In this study 3T3-L1 adipocytes were treated with 10 μg/ml adiponectin trimer or 50 ng/ml insulin for 0–60 min, and AMPK activation was analyzed. Adiponectin-induced AMPK activation started immediately, reached a peak at 5 min, and then decayed, returning to the basal level within 60 min (Fig. 5A, B), similar to that reported previously (1). In contrast, insulin-induced AMPK activation was not observed until 40 min, and maximal activation occurred at 60 min within the timeframe we tested (Fig. 5C, D). To examine whether AMPK activation by insulin is restricted to 3T3-L1 cells, or is seen in primary adipocytes as well, primary adipocytes were treated with 50 ng/ml insulin for 0–60 min. Figure 5E, F shows that AMPK activation was also induced by insulin in primary adipocytes. Although a small activation started at 20 min, the maximal activation reached at 60 min, following a pattern similar to that in 3T3-L1 cells.
>Figure 5.
Time course of AMPK activation by adiponectin (A, B) or insulin (C, D; E, F) in 3T3-L1 adipocytes (A–D) or primary adipocytes (E, F). Day 9 3T3-L1 or primary adipocytes were treated with adiponectin trimer (10 μg/ml) or insulin (50 ng/ml) for 0–60 min. A significant increase of AMPK phosphorylation was observed in adiponectin-stimulated cells at 5 min (A, B) and in insulin-stimulated cells only after 40 min (C, D). Primary adipocytes treated with insulin (50 ng/ml) for 0–60 min (E, F) show a similar pattern of AMPK activation to that in 3T3-L1 cells. Western blot analyses were performed on cleared cell lysates by using antibodies specific for phospho-AMPK-α (Thr172) and total AMPK α (A, C, E) and quantitative analysis was performed using unpaired Student's t test (B, D, F). Values represent means ± se. ***P < 0.05.
Kinetics of oleic acid incorporation into adipocytes stimulated by adiponectin are different from that of insulin
That AMPK activation by adiponectin and insulin occurs at various times suggests that the kinetics of this process by the 2 hormones is different. To study whether AMPK activation correlates with adiponectin or insulin-mediated increase in long-chain fatty acid (LCFA) uptake, we measured the uptake of [14C] oleic acid into control (PBS), adiponectin (40 μg/ml), or insulin (50 ng/ml)-treated 3T3-L1 adipocytes for up to 60 min. In adiponectin trimer-treated adipocytes, the uptake was rapidly enhanced within the first 20 min (average 2- to 3-fold), during the same time period as adiponectin-stimulated AMPK activation (Fig. 6A). A plateau in oleate incorporation was reached soon after 20 min, with an average increase of 2-fold compared to cells treated with PBS. In contrast, uptake by insulin occurred with almost linear kinetics for 60 min, with an ∼3-fold increase at the end of 60 min (Fig. 6A). Thus, AMPK activation by 2 hormones occurs with kinetics similar to the stimulation of fatty acid uptake.
Figure 6.
Stimulation of fatty acid uptake into adipocytes by insulin and adiponectin trimer occurs with different kinetics, and both require Acsl1 and FATP1. A) Time-dependent stimulation by adiponectin trimer and insulin on oleic acid uptake into adipocytes. 3T3-L1 adipocytes were treated with PBS (●), adiponectin trimer (■, 40 μg/ml), or insulin (▲, 50 ng/ml) in the presence of [14C] oleic acid for 0–60 min at 37°C, followed by determination of the amount of radioactivity incorporated. B) Effects of Acsl1 siRNA (II), FATP1 siRNA (III), or nontargeting siRNA (I) on oleic acid uptake stimulated by adiponectin trimer or insulin. Experiments were performed on knockdown cells as in A; each data point represents quantitative analysis of 6 wells of 3T3-L1 adipocytes grown in 12-well plates. Values represent means ± se (n=6).
Effects of Acsl1 or FATP1 gene knockdown on adiponectin or insulin-induced LCFA uptake
To confirm the roles of FATP1 or Acsl1 in adiponectin or insulin-mediated LCFA uptake into adipocytes, d 9 3T3-L1 adipocytes transiently transfected with nontargeting siRNA, Acsl1 siRNA, or FATP1 siRNA were incubated with [14C] oleic acid, in the presence of 40 μg/ml adiponectin trimer or 50 ng/ml insulin for 0–60 min, and then assayed for fatty acid transport into the cells. Adiponectin trimer and insulin markedly enhanced fatty acid uptake in adipocytes transfected with nontargeting siRNA, similar to results in Fig. 6A (Fig. 6BI). However, this effect diminished markedly in adipocytes transfected with either Acsl1 siRNA (Fig. 6BII) or FATP siRNA (Fig. 6BIII). This demonstrates that Acsl1 and FATP1 are essential for adiponectin trimer or insulin-mediated LCFA uptake.
DISCUSSION
Our principal conclusion is that, in adipocytes, both adiponectin and insulin activate AMPK, albeit with different kinetics. Activation is accompanied, and presumably caused by, an increase in the cellular AMP/ATP ratio and requires FATP1 and Acsl1, the 2 major acyl-CoA synthetase isoforms in adipocytes. In adipocytes adiponectin activation of AMPK peaks at 5 min and is accompanied by an ∼2-fold increase in the cellular AMP/ATP ratio, whereas insulin-activated AMPK only after ∼40 min treatment and was coupled with an ∼5-fold increase in the cellular AMP/ATP ratio. Previously, we showed that in adipocytes insulin stimulated translocation of FATP1 to the plasma membrane and in parallel stimulated an increase in LCFA uptake. Here we showed that, consistent with findings that acyl-CoA synthetases stimulate fatty acid transport, adiponectin stimulated LCFA uptake in adipocytes, which follows a pattern similar to that of AMPK activation over the first 60 min. Our finding that in 3T3-L1 adipocytes both Acsl1 and FATP1 are essential for maximal activation of AMPK, as well as LCFA uptake by adiponectin trimer, supports our hypothesis that adiponectin receptor activation is somehow coupled to activation of acyl-CoA synthetases, which catalyze a reaction consuming significant amounts of cellular ATP and producing AMP. The up-regulated acyl-CoA synthetase activity leads to an increase in import of fatty acids, and the resultant 5′-AMP would then activate AMPK. Since in adipocytes free fatty acids are formed by hydrolysis of stored triglycerides, the fatty acids used in acyl-CoA activation could come either from the external medium or from the cytosol. Our work provides an explanation for previous findings of an increase in the 5′-AMP on adiponectin stimulation (1). In addition, our finding that insulin-induced FATP1 translocation to the plasma membrane is accompanied by an increase in LCFA transport into the cells, an increase in the 5′-AMP/ATP ratio, and in activation of AMPK suggests that these processes are presumably mediated through a similar mechanism involving FATP1 and Acsl1.
Activity of FATP1 and Acsl1 is regulated by hormonal signals
An efficient clearance of plasma free fatty acid requires the transport of free fatty acid across the plasma and mitochondrial membranes of many different cells, processes mediated predominantly via protein-requiring mechanisms (31, 47). One group of proteins crucially involved is acyl-CoA synthetases, including Acsls 1 and 3–6 and fatty acid transport proteins FATP1–6. Here we show that the activity of Acsl1 and FATP1—the principal acyl-CoA synthetases in adipocytes—are required for insulin and adiponectin signaling in adipocytes, and the presumed increased activity of these acyl-CoA synthetases results in formation of 5′ AMP and thus in AMPK activation. Adiponectin and insulin function as positive regulators of this pathway; both target acyl-CoA synthetases lead to increased fatty-acid influx as well as AMPK activation. Interestingly, resistin, a protein hormone involved in developing obesity-related diseases, appears to be a negative regulator of this pathway. Resistin significantly reduces expression of FATP1 and also phosphorylation of AMPK and ACC in skeletal muscles (48), which explains the reduction in fatty-acid oxidation in response to resistin. Opposite to that of adiponectin, resistin levels increase with obesity (49). Thus, acyl-CoA synthetase-AMPK represents a common target of several metabolically important hormones and its dysregulation caused by imbalanced hormonal levels during obesity would contribute to the pathogenesis of obesity-related diseases.
The roles of FATPs in fatty acid metabolism are complex and are defined by their tissue and organelle distributions and activities. The long-chain fatty acid transporters FAT/CD36 and FATPs that facilitate the transport of long-chain fatty acid are often coexpressed, with a ubiquitous presence of FAT/CD36 (35) and a tissue-specific expression of FATPs (50). The tissue-specific expression of FATPs, together with the hormonal regulatory features of these proteins, reflects the need for fatty acid as a regulated source of energy in metabolically active tissues such as fat and skeletal muscles. Moreover, FATP1 binds to the outer mitochondrial membrane in muscle. Collaborating with CPT1 and fatty acid translocase/CD36, FATP1 transports fatty acid into mitochondria and directly channels free fatty acids into the oxidative metabolism pathway (51, 52).
Mechanisms of activation of Acsl1 and FATP1 by insulin or adiponectin trimer
The binding of insulin and adiponectin trimer to their receptors, through unknown mechanisms and pathways, activates FATP1 and Acsl1. Possible mechanisms of activation include plasma membrane translocation (33), post-translational modifications, or both. Our findings that AMPK activation by adiponectin and insulin occur at different times after stimulation suggest that the activation mechanisms by the 2 hormonal signals are likely different. AMPK activation by insulin occurs only after 40 min, implying that it is a secondary effect of insulin signaling. The long time required for activation is consistent with a process involving plasma membrane translocation of acyl-CoA synthetases, since the maximal influx of fatty acid in 3T3-L1 adipocytes induced by insulin treatment occurs only after 30 min (33), the same time when activation of AMPK begins. In contrast, AMPK activation occurs rapidly following adiponectin stimulation, supporting our hypothesis that adiponectin receptor activation is coupled directly to activation of fatty acyl-CoA synthetase activity, possibly through post-translational modifications. Exactly how adiponectin or insulin-receptor binding activates FATP1 and Acsl1 is under further investigation.
Both Acsl1 and FATP1 are required for AMPK activation by adiponectin trimer and insulin
Knocking down either the Acsl1 or FATP1 genes in adipocytes repressed LCFA uptake as well as AMPK activation by adiponectin trimer and by insulin. We hypothesize that Acsl1 and FATP1 cooperatively mediate the adiponectin or the insulin signal. Acsl1 and FATP1 are the 2 major isoforms of acyl-CoA synthetases in skeletal muscle and adipose tissue (33, 43). These 2 proteins form a physical complex at the plasma membrane in mouse 3T3-L1 adipocytes, and they are proposed to work coordinately to facilitate fatty-acid influx (53), in a process termed vectorial acylation. The proper functioning of one acyl-CoA synthetase would require the presence of the other. Our siRNA knockdown data supports this hypothesis, since both are required for fatty acid uptake and activation of AMPK by insulin and adiponectin. Previous studies showed that insulin induces FATP1 translocation from the Golgi apparatus to the plasma membrane (33). It will be interesting to examine whether insulin triggers a similar membrane localization of Acsl1.
AMPK activation by insulin in adipocytes is not contradictory to the anabolic effects of insulin
In contrast to our findings, many studies have shown suppression rather than activation of AMPK by insulin. Thus, following insulin treatment of liver, heart, and skeletal muscle cells, there is activation of ACC (54–56). This finding is consistent with the anabolic effects of insulin on fatty acid metabolism in these tissues, which include increased fatty acid and TAG synthesis as well as decreased fatty acid oxidation. However, Folmes et al. (57) showed that the ability of insulin to inhibit AMPK activity in cardiac muscle is lost in the presence of high concentration of fatty acids, a circumstance similar to that in adipocytes where FFAs are constantly generated by lipolysis and imported from the media. More importantly, we show that AMPK activation by insulin does not lead to phosphorylation (and thus inactivation) of ACC at Ser79, presumably resulting from insulin-induced dephosphorylation at this site (46). Here we show that in adipocytes AMPK indeed phosphorylates IRS-1 at Ser789, enhancing insulin-stimulated PI3K activation. Thus AMPK activation in adipocytes is not contradictory to the anabolic effects of insulin.
We propose the following working hypothesis of AMPK activation by adiponectin and insulin in adipocytes. After insulin binds to its receptor on the plasma membrane, an unknown signal is transmitted intracellularly to the Acsl1/FATP1 complex in the Golgi apparatus, inducing plasma membrane translocation of the complex. This Acsl1/FATP1 complex subsequently transports LCFA into the cells as well as esterifies cytosolic LCFA to the CoA derivative. This process generates 5′ AMP and results in an increased AMP/ATP ratio. The AMP binds to AMPK heterotrimer in the cytosol and exposes the Thr172 residue on the α subunit to the AMPK kinase LKB1 for phosphorylation. AMPK activated by insulin does not result in phosphorylation or inactivation of ACC, presumably because of Akt-mediated dephosphorylation at Ser79 (46). Instead, AMPK phosphorylates IRS-1 at Ser789 and enhances PI3K signaling.
Binding of adiponectin to an as yet unidentified receptor results in rapid (5 min.) activation of the plasma membrane Acsl1/FATP1 complex, resulting in generation of 5′AMP and activation of AMPK and then phosphorylation and inactivation acyl-CoA carboxylase (ACC), which subsequently leads to an increased fatty acid β oxidation in mitochondrion. Thus activation of AMPK by insulin and adiponectin are both mediated through the Acsl1/FATP1 complex, but the detailed mechanisms by which the hormones activate the Acsl1/FATP1 complex are likely distinct.
Supplementary Material
Acknowledgments
The authors thank Dr. Tsu-Shuen Tsao (University of Arizona, Tucson, AZ, USA) for providing the adiponectin C22A expression vector, Dr. Kelly Wong and Dr. Yutong Sun for insightful discussions and critical reading of the manuscript, and Dr. Julia Xu for technical assistance for nucleotide studies.
This work was supported by a mentor-based fellowship from the American Diabetes Association, award 7-04-MN-27; National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases grant 2R01 DK0467618-20 (to H.F.L.); and NIH/National Heart, Lung, and Blood Institute grant P01 HL 066105 (to M.K.).
REFERENCES
- 1.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., Eto K., Akanuma Y., Froguel P., Foufelle F., Ferre P., Carling D., Kimura S., Nagai R., Kahn B. B., Kadowaki T. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288– 1295 [DOI] [PubMed] [Google Scholar]
- 2.Combs T. P., Berg A. H., Obici S., Scherer P. E., Rossetti L. (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 108, 1875– 1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X., Motoshima H., Mahadev K., Stalker T. J., Scalia R., Goldstein B. J. (2003) Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 52, 1355– 1363 [DOI] [PubMed] [Google Scholar]
- 4.Tomas E., Tsao T. S., Saha A. K., Murrey H. E., Zhang Cc C., Itani S. I., Lodish H. F., Ruderman N. B. (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl. Acad. Sci. U. S. A. 99, 16309– 16313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao T. S., Murrey H. E., Hug C., Lee D. H., Lodish H. F. (2002) Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J. Biol. Chem. 277, 29359– 29362 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki S., Wilson-Kubalek E. M., Wert D., Tsao T. S., Lee D. H. (2007) The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 581, 809– 814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pajvani U. B., Hawkins M., Combs T. P., Rajala M. W., Doebber T., Berger J. P., Wagner J. A., Wu M., Knopps A., Xiang A. H., Utzschneider K. M., Kahn S. E., Olefsky J. M., Buchanan T. A., Scherer P. E. (2004) Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 279, 12152– 12162 [DOI] [PubMed] [Google Scholar]
- 8.Hotta K., Funahashi T., Arita Y., Takahashi M., Matsuda M., Okamoto Y., Iwahashi H., Kuriyama H., Ouchi N., Maeda K., Nishida M., Kihara S., Sakai N., Nakajima T., Hasegawa K., Muraguchi M., Ohmoto Y., Nakamura T., Yamashita S., Hanafusa T., Matsuzawa Y. (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 20, 1595– 1599 [DOI] [PubMed] [Google Scholar]
- 9.Hara K., Boutin P., Mori Y., Tobe K., Dina C., Yasuda K., Yamauchi T., Otabe S., Okada T., Eto K., Kadowaki H., Hagura R., Akanuma Y., Yazaki Y., Nagai R., Taniyama M., Matsubara K., Yoda M., Nakano Y., Tomita M., Kimura S., Ito C., Froguel P., Kadowaki T. (2002) Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes 51, 536– 540 [DOI] [PubMed] [Google Scholar]
- 10.Hardie D. G., Carling D., Carlson M. (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67, 821– 855 [DOI] [PubMed] [Google Scholar]
- 11.McGarry J. D., Brown N. F. (1997) The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 244, 1– 14 [DOI] [PubMed] [Google Scholar]
- 12.Hardie D. G. (2003) Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144, 5179– 5183 [DOI] [PubMed] [Google Scholar]
- 13.Jakobsen S. N., Hardie D. G., Morrice N., Tornqvist H. E. (2001) 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J. Biol. Chem. 276, 46912– 46916 [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., Murakami K., Ohteki T., Uchida S., Takekawa S., Waki H., Tsuno N. H., Shibata Y., Terauchi Y., Froguel P., Tobe K., Koyasu S., Taira K., Kitamura T., Shimizu T., Nagai R., Kadowaki T. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762– 769 [DOI] [PubMed] [Google Scholar]
- 15.Hug C., Wang J., Ahmad N. S., Bogan J. S., Tsao T. S., Lodish H. F. (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. U. S. A. 101, 10308– 10313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060– 29066 [DOI] [PubMed] [Google Scholar]
- 17.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13, 2004– 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. (2003) Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao X., Kikani C. K., Riojas R. A., Langlais P., Wang L., Ramos F. J., Fang Q., Christ-Roberts C. Y., Hong J. Y., Kim R. Y., Liu F., Dong L. Q. (2006) APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 8, 516– 523 [DOI] [PubMed] [Google Scholar]
- 20.Watkins P. A. (1997) Fatty acid activation. Prog. Lipid. Res. 36, 55– 83 [DOI] [PubMed] [Google Scholar]
- 21.Black P. N., DiRusso C. C. (2007) Vectorial acylation: linking fatty acid transport and activation to metabolic trafficking. Novartis Found. Symp. 286, 127– 138; discussion 138–141, 162–163, 196–203 [DOI] [PubMed] [Google Scholar]
- 22.Tong F., Black P. N., Coleman R. A., DiRusso C. C. (2006) Fatty acid transport by vectorial acylation in mammals: roles played by different isoforms of rat long-chain acyl-CoA synthetases. Arch. Biochem. Biophys. 447, 46– 52 [DOI] [PubMed] [Google Scholar]
- 23.Gauthier M. S., Miyoshi H., Souza S. C., Cacicedo J. M., Saha A. K., Greenberg A. S., Ruderman N. B. (2008) AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 283, 16514– 16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi T., Osatomi K., Yamashita H., Kabashima T., Uyeda K. (2002) Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J. Biol. Chem. 277, 3829– 3835 [DOI] [PubMed] [Google Scholar]
- 25.Gargiulo C. E., Stuhlsatz-Krouper S. M., Schaffer J. E. (1999) Localization of adipocyte long-chain fatty acyl-CoA synthetase at the plasma membrane. J. Lipid Res. 40, 881– 892 [PubMed] [Google Scholar]
- 26.Hesler C. B., Olymbios C., Haldar D. (1990) Transverse-plane topography of long-chain acyl-CoA synthetase in the mitochondrial outer membrane. J. Biol. Chem. 265, 6600– 6605 [PubMed] [Google Scholar]
- 27.Herrmann T., Buchkremer F., Gosch I., Hall A. M., Bernlohr D. A., Stremmel W. (2001) Mouse fatty acid transport protein 4 (FATP4): characterization of the gene and functional assessment as a very long chain acyl-CoA synthetase. Gene 270, 31– 40 [DOI] [PubMed] [Google Scholar]
- 28.Hall A. M., Smith A. J., Bernlohr D. A. (2003) Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J. Biol. Chem. 278, 43008– 43013 [DOI] [PubMed] [Google Scholar]
- 29.Hall A. M., Wiczer B. M., Herrmann T., Stremmel W., Bernlohr D. A. (2005) Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J. Biol. Chem. 280, 11948– 11954 [DOI] [PubMed] [Google Scholar]
- 30.Li L. O., Mashek D. G., An J., Doughman S. D., Newgard C. B., Coleman R. A. (2006) Overexpression of rat long chain acyl-coa synthetase 1 alters fatty acid metabolism in rat primary hepatocytes. J. Biol. Chem. 281, 37246– 37255 [DOI] [PubMed] [Google Scholar]
- 31.Schaffer J. E., Lodish H. F. (1994) Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 79, 427– 436 [DOI] [PubMed] [Google Scholar]
- 32.Gimeno R. E., Hirsch D. J., Punreddy S., Sun Y., Ortegon A. M., Wu H., Daniels T., Stricker-Krongrad A., Lodish H. F., Stahl A. (2003) Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J. Biol. Chem. 278, 49512– 49516 [DOI] [PubMed] [Google Scholar]
- 33.Stahl A., Evans J. G., Pattel S., Hirsch D., Lodish H. F. (2002) Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev. Cell. 2, 477– 488 [DOI] [PubMed] [Google Scholar]
- 34.Wu Q., Ortegon A. M., Tsang B., Doege H., Feingold K. R., Stahl A. (2006) FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol. Cell. Biol. 26, 3455– 3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonen A., Chabowski A., Luiken J. J., Glatz J. F. (2007) Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda) 22, 15– 29 [DOI] [PubMed] [Google Scholar]
- 36.Rodbell M. (1964) Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 239, 375– 380 [PubMed] [Google Scholar]
- 37.Passoneau J. V., Lowry O. H. (1993) Enzymatic Analysis, pp157–159, Humana Press, Totowa, NJ, USA [Google Scholar]
- 38.Rao P. K., Kumar R. M., Farkhondeh M., Baskerville S., Lodish H. F. (2006) Myogenic factors that regulate expression of muscle-specific microRNAs. Proc. Natl. Acad. Sci. U. S. A. 103, 8721– 8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong K. A., Lodish H. F. (2006) A revised model for AMP-activated protein kinase structure: The alpha-subunit binds to both the beta- and gamma-subunits although there is no direct binding between the beta- and gamma-subunits. J. Biol. Chem. 281, 36434– 36442 [DOI] [PubMed] [Google Scholar]
- 40.Koh H. J., Hirshman M. F., He H., Li Y., Manabe Y., Balschi J. A., Goodyear L. J. (2007) Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem. J. 403, 473– 481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly M., Gauthier M. S., Saha A. K., Ruderman N. B. (2009) Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes 58, 1953– 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiczer B. M., Lobo S., Machen G. L., Graves L. M., Bernlohr D. A. (2009) FATP1 mediates fatty acid-induced activation of AMPK in 3T3–L1 adipocytes. Biochem. Biophys. Res. Commun. 387, 234– 238 [DOI] [PubMed] [Google Scholar]
- 43.Mashek D. G., Li L. O., Coleman R. A. (2006) Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J. Lipid Res. 47, 2004– 2010 [DOI] [PubMed] [Google Scholar]
- 44.Stahl A., Gimeno R. E., Tartaglia L. A., Lodish H. F. (2001) Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol. Metab. 12, 266– 273 [DOI] [PubMed] [Google Scholar]
- 45.Hardie D. G. (2004) The AMP-activated protein kinase pathway–new players upstream and downstream. J. Cell Sci. 117, 5479– 5487 [DOI] [PubMed] [Google Scholar]
- 46.Berggreen C., Gormand A., Omar B., Degerman E., Goransson O. (2009) Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am. J. Physiol. Endocrinol. Metab. 296, E635– 646 [DOI] [PubMed] [Google Scholar]
- 47.Kampf J. P., Kleinfeld A. M. (2007) Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda) 22, 7– 14 [DOI] [PubMed] [Google Scholar]
- 48.Palanivel R., Sweeney G. (2005) Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett. 579, 5049– 5054 [DOI] [PubMed] [Google Scholar]
- 49.Rajala M. W., Qi Y., Patel H. R., Takahashi N., Banerjee R., Pajvani U. B., Sinha M. K., Gingerich R. L., Scherer P. E., Ahima R. S. (2004) Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes 53, 1671– 1679 [DOI] [PubMed] [Google Scholar]
- 50.Hirsch D., Stahl A., Lodish H. F. (1998) A family of fatty acid transporters conserved from mycobacterium to man. Proc. Natl. Acad. Sci. U. S. A. 95, 8625– 8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sebastian D., Guitart M., Garcia-Martinez C., Mauvezin C., Orellana-Gavalda J. M., Serra D., Gomez-Foix A. M., Hegardt F. G., Asins G. (2009) Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells. J. Lipid Res. 50, 1789– 1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell S. E., Tandon N. N., Woldegiorgis G., Luiken J. J., Glatz J. F., Bonen A. (2004) A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J. Biol. Chem. 279, 36235– 36241 [DOI] [PubMed] [Google Scholar]
- 53.Richards M. R., Harp J. D., Ory D. S., Schaffer J. E. (2006) Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J. Lipid Res. 47, 665– 672 [DOI] [PubMed] [Google Scholar]
- 54.Witters L. A., Kemp B. E. (1992) Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J. Biol. Chem. 267, 2864– 2867 [PubMed] [Google Scholar]
- 55.Gamble J., Lopaschuk G. D. (1997) Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism 46, 1270– 1274 [DOI] [PubMed] [Google Scholar]
- 56.Winder W. W., Holmes B. F. (2000) Insulin stimulation of glucose uptake fails to decrease palmitate oxidation in muscle if AMPK is activated. J. Appl. Physiol. 89, 2430– 2437 [DOI] [PubMed] [Google Scholar]
- 57.Folmes C. D., Clanachan A. S., Lopaschuk G. D. (2006) Fatty acids attenuate insulin regulation of 5′-AMP-activated protein kinase and insulin cardioprotection after ischemia. Circ. Res. 99, 61– 68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.