Abstract
Aberrant hyperphosphorylation of neuronal cytoskeletal proteins is one of the major pathological hallmarks of neurodegenerative disorders such as Alzheimer disease (AD), amyotrophic lateral sclerosis (ALS), and Parkinson's disease (PD). Human NF-M/H display a large number of multiple KSP repeats in the carboxy-terminal tail domain, which are phosphorylation sites of proline-directed serine/threonine (pSer/Thr-Pro, KS/T-P) kinases. The phosphorylation sites of NF-M/H have not been characterized in AD brain. Here, we use quantitative phosphoproteomic methodology, isobaric tag for relative and absolute quantitation (iTRAQ), for the characterization of NF-M/H phosphorylation sites in AD brain. We identified 13 hyperphosphorylated sites of NF-M; 9 Lys-Ser-Pro (KSP) sites; 2 variant motifs, Glu-Ser-Pro (ESP) Ser-736 and Leu-Ser-Pro (LSP) Ser-837; and 2 non-S/T-P motifs, Ser-783 and Ser-788. All the Ser/Thr residues are phosphorylated at significantly greater abundance in AD brain compared with control brain. Ten hyperphosphorylated KSP sites have been identified on the C-terminal tail domain of NF-H, with greater abundance of phosphorylation in AD brain compared with control brain. Our data provide the direct evidence that NF-M/H are hyperphosphorylated in AD compared with control brain and suggest the role of both proline-directed and non-proline-directed protein kinases in AD. This study represents the first comprehensive iTRAQ analyses and quantification of phosphorylation sites of human NF-M and NF-H from AD brain and suggests that aberrant hyperphosphorylation of neuronal intermediate filament proteins is involved in AD.—Rudrabhatla, P., Grant, P., Jaffe, H., Strong, M. J., Pant, H. C. Quantitative phosphoproteomic analysis of neuronal intermediate filament proteins (NF-M/H) in Alzheimer's disease by iTRAQ.
Keywords: neurofilament-M/H, phosphorylation sites, iTRAQ, MS/MS mass spectrometry, TiO2, proline-directed kinases
Neurofilaments (NFs) are the most abundant proteins of the nervous system. Abnormal NF perikaryal aggregates are pathological hallmarks of many neurodegenerative diseases including neurofibrillary tangles (NFTs) of Alzheimer disease (AD; refs. 1–3), Lewy bodies of Parkinson's disease (PD; refs. 4, 5), and spinal cord inclusions of amyotrophic lateral sclerosis (ALS; refs. 6–8). In normal neurons, NFs are phosphorylated in the axonal compartment. However, in AD, PD, and ALS, NF proteins are aberrantly hyperphosphorylated in the cell bodies. The high-molecular-weight neurofilament protein (NF-H) is among the highly phosphorylated proteins in brain (9). Human NF-H has 43/44 KSP repeats and human NF-M has 13 KSP repeats in the carboxy-terminal domain. In mature myelinated axons, most of the phosphorylation of NF-M/H occurs in the carboxy-terminal tail domain of proline-directed Ser/Thr repeats (10–12). Phosphorylation of the tail domain is reported to affect interaction between NF-M/H and microtubules (13–15), determine axonal caliber (16, 17), protect neurofilament proteins (NFPs) from proteolysis (18, 19), and contribute to calcium buffering in the axonal compartment (20). Phosphorylation also regulates the distribution of neurofilaments between stationary and mobile phases in the axon (21). In AD, the phosphorylation of NF-M/H is deregulated and occurs in the cell body compartment (1–3). The precise mechanism for this compartment-specific phosphorylation is not well understood.
Increase in NF phosphorylation in the cell body results in the blockage of axonal transport. The role of NFs has been studied in a transgenic mouse. Transgenic mice that overexpress neurofilament proteins (NFTPs) exhibit extensive NF protein accumulations in motor neurons (22). In a cellular model, defects in the topographic cytoskeletal protein (NF-M/H) phosphorylation in neurons may lead to blockage of axonal transport and neuronal cell death (23). Because of their highly phosphorylated state, quantitative characterization of NF-M/H phosphorylation sites by mass spectrometry certainly represents a challenge. Identification of phosphorylated motifs in NF-M/H can provide direct information on the endogenous kinases that phosphorylate in vivo and may also provide insight into the disease pathology.
Identification of phosphorylated and unphosphorylated epitopes in human NF-M/H have been reported utilizing specific monoclonal antibodies (24). However, these methods do not precisely determine the number of phosphorylated residues in a given protein nor do they distinguish between the phosphorylation of specific S/T residues in KSP or KTP repeats or in other motifs. The recent introduction of isobaric (equal mass) peptide tags for relative and absolute quantification (iTRAQ) of peptides in different samples is a major breakthrough in quantitative proteomics (25, 26). The iTRAQ (4-plex) method is based on the differential covalent labeling of each of 4 batches of peptides from proteolytic protein digests with 1 of the 4 different iTRAQ reagents: 114.1, 115.1, 116.1, and 117.1, all of which result in the incorporation of an isobaric tag 144.1 Da into peptide N termini and lysine residues. Since the 4 tags are isobaric, identical peptides from different batches labeled with the different tags are indistinguishable by mass. They can, however, be differentiated and quantified by collision-induced dissociation (CID), which is normally used for MS peptide sequencing, through release of a reporter ion, each of which has a different mass (114.1, 115.1, 116.1, or 117.1 Da). The advantage of iTRAQ over the other quantitative mass spectrometry methods such as isotope coded affinity tag (ICAT), stable isotope labeling with amino acids in cell culture (SILAC), and metabolic labeling is that 4 samples can be analyzed and compared, thereby reducing the amount of mass spectrometry time needed for analysis. In this study, we utilize this iTRAQ and pulsed Q dissociation (PQD) methodology to characterize and quantify phosphorylation sites of NF-M and NF-H for the first time in AD brain.
We report 13 phosphorylation sites in NF-M of carboxy-terminal domain and 10 phosphorylation sites of NF-H in AD brain. Furthermore, we show that all the phosphorylation sites are in high abundance in AD (∼4- to 8-fold higher) compared with control brain. The finding of a significant number of phosphorylation sites in NF-M and NF-H that are the proline-directed Ser/Thr (S/T-P) residues suggests that either proline-directed kinases (Cdk5, GSK3β, or MAPKs) are hyperactivated or the protein phosphatases [e.g., protein phosphatase 2A (PP2A)] are down-regulated. Furthermore, identification of non-SP phosphorylation sites in NF-M (Ser-783 and Ser-788) at a significantly higher level in AD suggests the involvement of non-proline-directed phosphorylation of NF-M/H in AD brain.
MATERIALS AND METHODS
Materials
Frozen postmortem human brains (frontal cortex) from clinically and pathologically confirmed AD patients and age-matched (62–75 yr) and postmortem date-matched normal control adult human brains (n=4; 4-different controls and 4-different AD brains) were obtained from the Harvard Brain Resource Centre (Boston, MA, USA. The brain tissue from control samples comprised non-neurological diseases, atherosclerotic cardiovascular disease (ASCVD), or multiple injuries. RT97 was kindly provided by Dr. Brian Anderton (Medical Research Council center for neurodegeneration research, Institute of Psychiatry, London, UK) and Dr. Ralf Nixon (Nathan Kline Institute, Orangeburg, NY, USA). SMI31 and MAP2 (MMS-485P) were commercially obtained from Covance (Gaithersburg, MD, USA). Sequencing-grade trypsin was obtained from Promega (Madison, WI, USA). The iTRAQ 4-plex kit was obtained from Applied Biosystems (Foster City, CA, USA).
Immunohistochemistry
Control brain sections were obtained from a 64-yr-old donor of who died of ASCVD. The AD brain sections were from a 70-yr-old adult diagnosed with AD with cognitive decline and behavior problems with aggressive outbursts. Another AD brain section was obtained from a 63-yr-old male who had been diagnosed with AD for 9 yr. Paraffin-embedded control and AD brain sections were prepared for immunostaining through xylene treatment and gradual rehydration with 95–75% ethanol. Sections were blocked (5% goat serum/0.1% Triton X-100) and then incubated with primary antibodies (RT97 and SMI31 antibodies) overnight at 4°C in blocking solution. The sections were incubated with peroxidase conjugated secondary antibodies for 1 h at room temperature. The sections were stained using 3,3′ diaminobenzidine and counterstained with methylene blue, and slides were coverslipped using Permount (Biomeda, Foster City, CA, USA). Images were captured with a ×40 objective on a Nikon Eclipse E400 microscope using SPOT software (Nikon, Tokyo, Japan).
Preparation of NF-M and NF-H from human control brain and AD brain
Frozen human autopsy brain tissue (frontal cortex) was washed thoroughly in ice-cold buffer (20 mM Tris/HCl, pH 6.8, containing 100 mM NaCl, 1 mM EDTA, and 1 mM dithiothritol), protease inhibitor cocktail (Roche, Indianapolis, IN, USA) to remove blood clots and meninges as described previously. Tissues were homogenized in the same buffer (1:5 w/v), with 1% Triton-X 100 containing complete protease inhibitor cocktail pellet (Roche), 0.1% SDS, PMSF, and leupeptin and centrifuged at 13,000 rpm (18,000 g) for 20 min at 4°C. The lysate, ∼5 μg/μl protein, was first analyzed to detect phosphorylated NF-M/H using RT97 and SMI31 by Western blot analysis.
Protein estimation
Samples from control and AD brains were estimated in triplicate using bicinchoninic acid protein assay (27).
Western blotting
Immunoblotting was performed as described previously (28). Western blotting was performed using the phospho-NF-M/H antibodies RT97 and SMI31. The equal loading of the protein samples was confirmed by immunoblotting with β-tubulin.
Quantitative phosphoproteomics (iTRAQ)
The iTRAQ method is a non-gel-based technique used to identify and quantify proteins from different sources in 1 single experiment. It uses isotope-coded covalent tags. The method is based on the covalent labeling of the N terminus and side-chain amines of peptides from protein digestions with tags of varying mass using 4-plex [control 1 (CON1), 114.1; control 2 (CON2), 115.1; AD1, 116.1; and AD2, 117.1], which can be used to label all peptides from different samples/treatments. These samples are then combined and analyzed by tandem mass spectrometry (MS/MS). A database search is then performed using the fragmentation data to identify the labeled peptides and hence the corresponding proteins. The fragmentation of the attached tag generates a low-molecular-mass reporter ion that can be used to relatively quantify the peptides and the proteins from which they originated, using software such as the freely available SEQUEST (http://fields.scripps.edu/sequest).
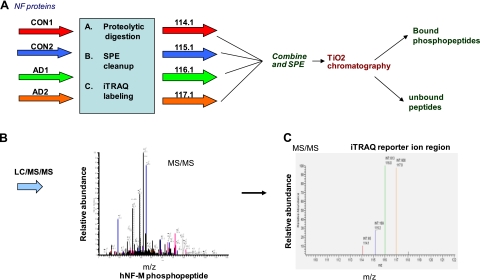
As described in the Fig. 3 flow chart, 100-μg protein samples each from 2 control and 2 AD brain lysates were subjected to reduction and alkylation, digestion with trypsin, and iTRAQ labeling in parallel. Digests were cleaned up by solid phase extraction (SPE) before iTRAQ labeling. The iTRAQ-labeled digests were combined and cleaned up by SPE before phosphopeptide enrichment by TiO2 chromatography. The iTRAQ labeled phosphopeptides were analyzed by liquid chromatography (LC)/MS/MS and identified by database searching, and relative quantification was achieved by comparison of the ion counts of the m/z 114.1, 115.1, 116.1, and 117.1 marker ions in the MS/MS PQD spectrum. The stepwise details are given below.
Figure 3.
Scheme of a multiplex reaction of 4 different samples, designated by 4 different colors. A) Four samples (2 controls, CON1 and CON2, and 2 AD, AD1 and AD2) of brain lysates (100 μg; each labeled with different colors) were subjected to proteolytic digestion by trypsin followed by SPE clean up. Digested peptides were labeled in each sample by iTRAQ reagents [control 1 (red), 114.11; control 2 (blue), 115.11; AD1 (green), 116.11; and AD2 (orange), 117.11]. All the samples were processed in parallel. Labeled samples were combined, and phosphopeptides are enriched by TiO2 chromatography and analyzed by LC/MS/MS. B) MS/MS spectra of one of the phosphopeptides. C) iTRAQ reporter ion region of the MS/MS spectrum.
Protein digestion
Protein (100 μg) from 2 controls and 2 AD was dried in the SpeedVac (ThermoSavant, Farmingham, NY, USA) and dissolved in 8 M urea/0.4 M NH4HCO3 for reduction by dithiothritol and alkylation by indole acetic acid. After dilution to 2 M urea/0.1 M NH4HCO3, tryptic digestion was performed according to a standard protocol (29) as described previously (30).
iTRAQ labeling
The 4 digests (trypsin digested samples) were cleaned up by solid phase extraction (SPE) on an Oasis 200 mg extraction column (Waters, Milford, MA, USA), and the resulting dried digests were subjected to iTRAQ labeling utilizing the 4-plex kit (Applied Biosystems) according to the manufacturers protocol and as described previously (30). Normal control sample digests, CON1 and CON2, were labeled with the 114.1 and 115.1 iTRAQ reagents, respectively, and AD sample digests, AD1 and AD2, were labeled with the 116.1 and 117.1 iTRAQ reagents, respectively. After incubation of the digests with the iTRAQ regents for 1 h, excess iTRAQ reagents were removed by addition of 20 μl of 1 M Tris (pH 8.0) to each sample.
Phosphopeptide enrichment
The 4 labeled digests were combined and cleaned up by SPE on an Oasis 500 mg extraction column (Waters), and the resulting combined dried digest was subjected to phosphopeptide enrichment by TiO2 chromatography utilizing a 1- to 200-μl TiO2 Top Tip (Glygen Corp., Columbia, MD, USA) essentially according to the method of Wu et al. (31) as described previously (30).
LC/MS/MS analysis
Samples were analyzed by LC/MS/MS on linear trap quadraplole (LTQ) XL with 2 Surveyor MS Pump plus HPLC pumps and Micro AS (Thermo Scientific, Waltham, MA, USA) and equipped with an Advance ESI (electrospray ionization) source (Michrom Bioresources Inc., Auburn, CA, USA), with instrument configuration, columns, gradient, and source conditions as described previously (30). The LTQ XL was set up to acquire a survey scan between m/z 400-1400 followed by a PQD MS/MS spectrum on each of the top 10 most abundant ions in the survey scan. Source conditions were as described previously and are listed here as follows: capillary temperature, 165°C; sheath gas flow, 2 U; spray voltage, 1.6 kV. Key optimized PQD instrument parameters (32) were as follows: CE, 35%; isolation width, 3 m/z; activation Q, 0.700; activation time, 0.100 ms; minimum signal threshold, 10,000 cts; dynamic exclusion, repeat count 2, repeat duration 30 s, exclusion duration 60 s; MS/MS target, 4.0 × e4; maximum fill time, 100 ms; 4 microscans.
PQD
The type of CID employed in these experiments is termed PQD. CID is a mechanism to fragment molecular ions in the gas phase, and the ions are accelerated by electrical potential to high kinetic energy in vacuum in the mass spectrometer and allowed to collide with neutral gas molecules (helium, nitrogen, or argon). In the collision, some of the kinetic energy is converted into internal energy that results in bond breakage and the fragmentation of the molecular ion into smaller fragments. PQD allows for the detection of low-mass ions such as aforementioned iTRAQ m/z reporter ions that are not seen in standard CID implemented on linear ion trap instruments (1/3 cutoff rule). In PQD-type CID, key ion trap instrument parameters such as resonance excitation amplitude and main RF amplitude are manipulated to effect a CID in which the resultant low-mass ions are stable in the trap and can be scanned out as observed. With the ability to trap and delete lower m/z product ions, PQD has been successfully applied to quantitate peptides utilizing iTRAQ labels.
Data analysis and iTRAQ quantification
Uninterpreted PQD MS/MS spectra were searched in the human database utilizing BioWorks 3.3.1 SP1 (Thermo Scientific) for site-specific phosphopeptide identification and iTRAQ quantification. Search parameters were set and data analysis was done as described previously (30). Search parameters were set as follows: static modifications: C = 57.0215, N-term = 144, and K = 144; differential modifications: S, T, Y = 79.9799 and M = 16. Search results were reported in descending order of the X correlation (XC) score subject to the default charge vs. XC filter: 1+ = 1.50, 2+ = 2.00, and 3+ = 2.50. Sequence assignments including the specific phosphorylated residue were based on the selection of the phosphopeptide with highest XC score concurrent with the second ranked peptide displaying a ΔCn (the difference in normalized XC score between top scoring sequence and the next highest scoring sequence) of >0.1. MS/MS spectra were manually reviewed for spectral quality, assignment of most major ions, and the usual presence of prominent neutral loss (−H3PO4) ions. The iTRAQ reporter ions at m/z 114, 115, 116, and 117 observed in the PQD spectra were quantified utilizing BioWorks 3.3.1 SP1. All iTRAQ ratio data with cts < 50 were rejected.
Statistical analysis
Data were reported as means ± se from 4 different controls and 4 different AD patient brain lysates. Student's t test was used for comparisons between control and AD brains. Statistical significance was accepted with values of P < 0.05. In Figs. 5C and 6C, the P values (P<0.001 and P<0.05) represent the fold difference of phosphorylation of NF-M and NF-H sites in AD brain compared with matched control brain.
Figure 5.
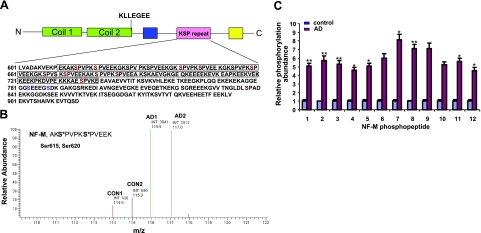
Mass spectrometric sequence coverage and hyperphosphorylated sites identified on human NF-M from AD brain. A) Schematic representation of C-terminal domain of NF-M. Phosphopeptides identified are in boldface. Phosphorylation sites identified by iTRAQ analyses corresponding to KSP motifs are shown in red. We identified 13 phosphorylation sites in NF-M, including 9 KSP sites (Ser-615, Ser-620, Ser-641, Ser-646, Ser-659, Ser-667, Ser-672, Ser-680, and Ser-685), 2 non-KSP sites (Ser-783 and Ser-788), and 2 variant SP motifs (LSP, Ser-837; and ESP, Ser-736). All the sites identified by iTRAQ analysis are significantly hyperphosphorylated in AD brain compared with control brain, as represented in Tables 1 and 2. B) As an example, the NF-M diphosphopeptide AKS*PVPKS*PVEEK demonstrated an average of 8.1-fold higher phosphorylation at both Ser-615 and Ser-620 in AD brain compared with control brain. C) Fold difference of relative phosphorylation between control (blue) and AD brains (red), obtained by geometric mean from 4 different control and AD patient brain samples. Control phosphorylation level of each phosphopeptide is normalized to 1. Phosphopeptides, listed as in Tables 1 and 2, are proline-directed and non-proline-directed, respectively. *P < 0.05, **P < 0.001 vs. matched control; Student's t test.
Figure 6.
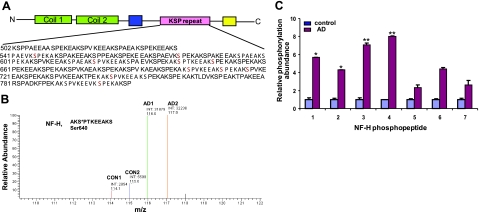
Sequence coverage and phosphorylation sites of human NF-H identified by iTRAQ analysis. A) Schematic representation of C-terminal domain of NF-H. NF-H phosphopeptides identified by iTRAQ analysis are in boldface. Hyperphosphorylated sites of NF-H corresponding to KSP repeats are shown in red. B) As an example, the NF-H phosphopeptide AKS*PTKEEAK demonstrated an average of 8.01-fold higher phosphorylation at Ser-640 in AD brain compared with control brain. C) Fold difference of relative phosphorylation between control (blue) and AD brains (red), obtained by geometric mean from 4 different control and AD patient brain samples. Control phosphorylation level of each phosphopeptide is normalized to 1. Phosphopeptides are listed as in Table 3. Statistical analyses was performed by Student's t test. *P < 0.05, **P < 0.001 vs. matched control; Student's t test.
RESULTS
Immunohistochemistry of control and AD brains with SMI31 and RT97 antibodies
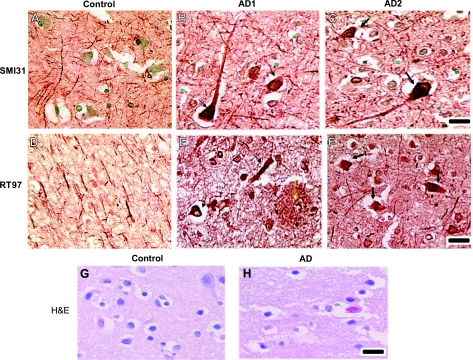
We performed immunohistochemistry of control adult brain (brain from donor that died due to another cause, ASCVD) and age-matched AD brains (details of the control and AD brain donor cases are described in Materials and Methods) with the phospho-NF antibodies SMI31 and RT97. SMI31 and RT97 mouse monoclonal antibodies detect phosphorylated epitopes of the carboxy-terminal domains of NF-M/H. Figure 1 shows the immunohistochemistry of frontal cortex brain sections with SMI31 and RT97 antibodies from control and AD brains. The frontal cortex is most vulnerable to degeneration in AD. In normal healthy adult brain, SMI31 antibody strongly decorated axons, processes, and neuropil fibers, with no staining in the cell bodies. Nuclei were counterstained with methyl green. However, in AD brain, SMI31 antibody strongly stained aggregated proline-directed Ser/Thr phosphorylated proteins in neurofibrillary tangles in the neuronal perikarya of the frontal cortex (Fig. 1B, C). RT97 antibody is specific to high-molecular-weight neurofilament (NF-H; see later, Fig. 2A), and it does not crossreact with phospho-Tau under the present experimental conditions (see later, Fig. 2A). Immunohistochemistry of the non-AD brains showed that RT97 recognizes NFPs in the axonal compartment and processes, with no staining in the cell bodies (Fig. 1D). In AD brain sections, RT97 stained NFTs in the neuronal perikarya (Fig. 1E, F), suggesting the presence of NF phosphorylation in the NFTs. Figure 1E, F shows the RT97 staining in 2 different AD brains. We have also observed the staining of RT97 antibody on extracellular senile plaques (Fig. 1E, yellow arrows). Figure 1G, H shows the hematoxylin and eosin (H&E) staining of control and AD brain sections. These findings suggest that NFTs are composed of phospho-NF accumulations and NFPs are aberrantly phosphorylated in AD neuronal perikarya.
Figure 1.
Aberrant hyperphosphorylation of NF in NFTs in AD brain. Paraffin sections of frontal cortex from control and AD brains were subjected to immunohistochemistry (IHC) as described in Materials and Methods using the mouse monoclonal phospho-NF antibodies SMI31 and RT97. A, D, G) IHC of control brain. B, E, H) IHC of first AD brain (AD1). C, F) IHC of second AD brain (AD2). A–C) SMI31 staining of control (A) and AD brains (B, C). SMI31 antibody decorated axons and neuropil fibers of neurons of frontal cortex in the control brain section (A). In sections of 2 AD brains (B, C), SMI31 antibody predominantly detected phospho-NF (black arrows) in NFTs. D) Staining of control brain with RT97 antibody. E, F) IHC of AD brain sections with RT97 antibody. RT97 strongly detected phospho-NF in NFTs. We have also detected phospho-NF staining in extracellular plaques by RT97 antibody (E). G, H) H&E staining of control (G) and AD brain sections (H). Scale bars = 50 μm.
Figure 2.
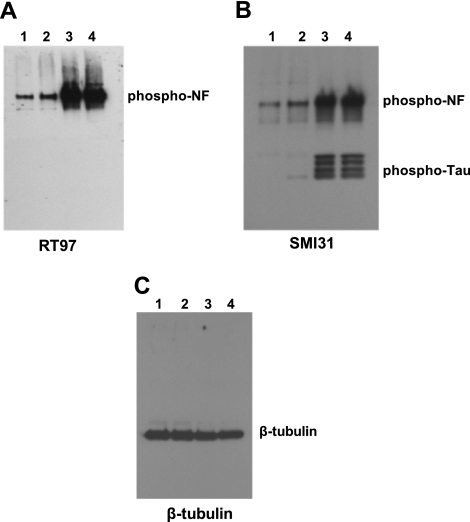
Western blot analyses of phosphorylated NF-M/H from control and AD brains. Immunoblot from 2 control (lanes 1, 2) and 2 AD brains (lanes 3, 4) using the phospho-NF-M/H antibodies RT97 (A), SMI31 (B), and β-tubulin (C). Phosphorylated NF-M/H is significantly increased in AD brain compared with control brain. RT97 antibody specifically detects phosphorylated NF-H (A), while SMI31 antibody at a higher concentration detects both phospho-NF and phospho-Tau (B). Equal loading of the protein sample was confirmed by β-tubulin antibodies (C).
NF phosphorylation is significantly increased in AD brain compared with control brain
Western blotting was performed from equal amounts of protein obtained from matched adult control and AD brain lysates. Figure 2A shows that the phospho-NF levels are significantly higher in AD brains (lanes 3, 4) compared with control brain (lanes 1 and 2) as detected by RT97 antibody. We then performed immunoblot analysis using SMI31. At lower concentrations, SMI31 antibody specifically recognizes phospho-NF, and at higher concentrations, SMI31 crossreacts with phospho-Tau. We used higher concentrations of SMI31. Figure 2B shows that the phospho-NF levels are higher in AD brain compared with control brain. We have also observed crossreactivity of SMI31 with phospho-Tau, suggesting that phospho-Tau levels are significantly higher in AD compared with matched control brain consistent with earlier observations. We have added protease inhibitors in lysis buffer to prevent degradation of the proteins by proteases. The Western blotting of the control and AD brain lysates with MAP2 antibody, which is the most sensitive to proteases, showed no degradation, suggesting that lysates were not subjected to protease degradation (data not shown). Equal loading of the protein was confirmed by Western blotting with β-tubulin (Fig. 2C). However, we cannot pinpoint specific Ser/Thr phosphorylation sites among 43/44 KSP repeats of human NF-H or 13 KSP repeats of human NF-M, which are aberrantly phosphorylated in AD. To map the exact phosphorylation sites and the abundance of NF-M and NF-H in AD compared with control brain, 100 μg of each sample was processed to characterize and quantify phosphorylation sites using iTRAQ methodology.
iTRAQ methodology employed to characterize and quantify phosphorylation sites from control and AD brain samples
The general workflow is depicted in Fig. 3. Four samples, 2 control brains (CON1 and CON2) and 2 AD brains (AD1 and AD2), were used. In a parallel set of reactions and procedures, each individual protein sample was reduced, alkylated, and enzymatically digested with trypsin. The resulting peptides from each sample digest were then labeled with 1 member reagent of the multiplex set (114.1, 115.1, 116.1, and 117.1), combined, and enriched by TiO2 chromatography for phosphopeptides that were subsequently analyzed by LC-MS/MS. The mass spectrometer was set up to acquire a survey scan MS spectrum between m/z 400 and 1400, followed by a collisionally induced CID MS/MS spectrum of the 10 most abundant peptide ions in the MS survey scan. In fact, the instrument was set up to acquire a type of CID spectrum known as PQD spectrum, which allows for the detection of low-mass ions, such as the iTRAQ reporter ions, between m/z 114 and 117, which is not the case for a normal CID spectrum on a ion trap instrument (1/3 cutoff rule). It is important to note that the peptide ions detected in the MS survey scan result from contributions from all 4 samples that were labeled with the isobaric iTRAQ tags, thus allowing for a degree of enhancement of individual peptides that may be in low abundance in any given sample. On PQD of the parent peptide ion produced in MS scan, the peptide backbone was fragmented into y and b ions (Fig. 3B), which contains the sequence information and enabled identification of the parent protein by comparing this fragmentation fingerprint to theoretical fragmentation patterns of peptides from digests of proteins in the database. In addition, iTRAQ tags on the N terminus and lysine side chain fragmented to release the 4 reporter group ions that appeared as distinct masses between m/z 114–117. The resulting PQD MS/MS spectra were batched searched against the human database utilizing the SEQUEST program, with search parameters appropriate to reduction, alkylation, potential STY phosphorylation, and iTRAQ modification as described in the Material and Methods. Results for the search of each PQD MS/MS spectrum were reported in the SEQUEST search output report in which phosphopeptide matches were listed in order of their probability of being a correct ID or with decreasing XC scores. Phosphopeptides were identified on the basis of the selection of the phosphopeptide with the highest XC score (first phosphopeptide) concurrent with the second ranked peptide displaying a ΔCn (the difference in normalized XC score between top scoring sequence and the next highest scoring sequence) of >0.1. MS/MS spectra were manually reviewed for spectral quality, assignment of most major ions, and the usual presence of prominent neutral loss (−H3PO4) ions. Relative quantification was reported by SEQUEST as a result of the comparison of the ion counts of the iTRAQ reporter ions at m/z 114–117.
iTRAQ analysis of NF-M
Identification of in vivo phosphorylation sites of NF-M
The in vivo phosphorylation sites of human NF-M have not been identified in control and AD brain. Human NF-M has 13 KSP repeats in the C-terminal domain. Using the methodology depicted in Fig. 3, we could identify 13 phosphorylation sites on carboxy-terminal region of human NF-M, which are assigned unambiguously to specific serine residues. All the sites are phosphorylated at greater abundance in AD brain compared with control brain (Table 1). The data have been validated to be statistically significant. We identified 9 phosphorylated KSP sites and 2 variant SP motifs, ESP (Ser-736) and LSP (Ser-837). In addition to these proline-directed S/T-P motifs, 2 non-SP-motif phosphorylation sites (Ser-783 and Ser-788, AGGEGGS*EEEGS*DKGAK) are significantly phosphorylated in AD brain compared with control brain, which suggests the involvement of non-proline-directed kinases such as casein kinase 1 (CK1). CK1 was shown to be up regulated in AD hippocampus compared with control brain (33). Table 1 summarizes the phosphopeptides elevated in AD brain, comprising 11 proline-directed Ser sites. Table 2 summarizes the phosphopeptides comprising 2 non-proline-directed phosphorylation sites.
Table 1.
NF-M phosphopeptides (proline-directed kinase sites) identified and quantified by iTRAQ methodology
| Phosphopeptide identified | hNF-M sequence | MH+ | z | Phosphorylation sites | Control | AD | |
|---|---|---|---|---|---|---|---|
| P1 | AKS*PVPKS*PVEEAK | 676–692 | 2202.7 | 2 | S680, S685 | 1 | 5.1 |
| P2 | GVVTNGLDLS*PADEK | 827–843 | 1882.7 | 2 | S837 | 1 | 5.7 |
| P3 | SPVPKS*PVEEK | 653–665 | 1708.61 | 2 | S659 | 1 | 5.3 |
| P4 | GKSPVPKS*PVEEK | 651–665 | 2037 | 2 | S659 | 1 | 4.58 |
| P5 | GKS*PVSKS*PVEEK | 638–652 | 2117.7 | 2 | S641, S646 | 1 | 5.09 |
| P6 | SPVPKS*PVEEAK | 678–692 | 1779.655 | 3 | S685 | 1 | 5.97 |
| P7 | AKS*PVPKS*PVEEK | 612–626 | 2131.71 | 3 | S615, S620 | 1 | 8.1 |
| P8 | GKS*PVSKS*PVEEK | 664–676 | 2107.68 | 2 | S667, S672 | 1 | 7.09 |
| P9 | AES*PVKEEAVAEVVTITK | 732–752 | 2684.08 | 3 | S736 | 1 | 7.11 |
S/T-P sites hyperphosphorylated in human (h)NF-M. NF-M protein from frontal cortex of normal control brains and AD brains was analyzed as described in Materials and Methods. Asterisk indicates phosphorylated residue. z indicates charge of precursor phosphopeptide. Last 2 columns show the iTRAQ ratios of phosphorylated residue in normal and AD brain.
Table 2.
NF-M phosphopeptides (non-proline-directed kinase sites) identified and quantified by iTRAQ methodology
| Phosphopeptide identified | hNF-M Sequence | MH+ | z | Phosphorylation sites | Control | AD | |
|---|---|---|---|---|---|---|---|
| P10 | AGGEGGS*EEEGS*DKGAK | 776–794 | 2156.60 | 3 | S783, S788 | 1 | 5.25 |
| P11 | AGGEGGSEEEGS*DKGAK | 744–792 | 2076.33 | 3 | S783 | 1 | 5.6 |
| P12 | EKAGGEGGSEEEGS*DK | 774–792 | 2077.62 | 3 | S788 | 1 | 4.54 |
{tabft}Non-proline-directed kinase sites hyperphosphorylated in hNF-M. NF-M protein from frontal cortex of normal control brains and AD brains was analyzed as described in Materials and Methods. Asterisk indicates phosphorylated residue. z indicates charge of precursor phosphopeptide. Last 2 columns show the iTRAQ ratios of phosphorylated residue in normal and AD brain.
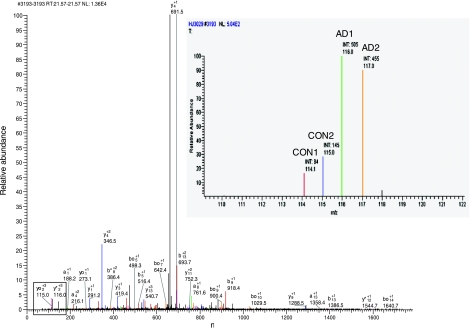
As an example, Fig. 4 shows the MS/MS PQD spectrum corresponding to 1 NF-M phosphopeptide, AGGEGGSEEEGS*DKGAK, obtained after TiO2 enrichment. MS/MS of the triply charged ion confirmed the identity of the phosphopeptide sequence of NF-M with m/z 2076. The inset in Fig. 4 displays the iTRAQ analysis, showing the abundance of Ser-783 phosphorylation in AD brain compared with control brain. Similarly, MS/MS PSD spectra were obtained and identified for all other phosphopeptides identified, listed in Tables 1 to 3.
Figure 4.
MS/MS of NF-M phosphopeptide. MS/MS PQD mass spectrum of the NF-M phosphopeptide (AGGEGGSEEEGS*DKGAK). Major ion at m/z 659 corresponds to the neutral loss of the elements of phosphoric acid (m/z, 98/3 = 32.7) from the triply charged precursor ion (m/z 2073.3/3 = 69.5). iTRAQ reporter ions are boxed. Inset: iTRAQ quantitation of the phosphopeptide from control and AD brains.
Table 3.
NF-H phosphopeptides identified and quantified by iTRAQ methodology
| Phosphopeptide identified | hNF-H Sequence | MH+ | z | Phosphorylation sites | Control | AD | |
|---|---|---|---|---|---|---|---|
| P1 | SPVKEEVKPS*PEK | 792–805 | 2012.703 | 3 | S801 | 1 | 5.7 |
| P2 | SPVKEEAKS*PEK | 701–714 | 1984.67 | 3 | S710 | 1 | 4.31 |
| P3 | AKS*PVKEEAK | 699–710 741–752 | 1742.58 | 2 | S702, S744, S716 | 1 | 7.1 |
| P4 | AKS*PTKEEAK | 637–648 | 1744.56 | 3 | S640 | 1 | 8.01 |
| P5 | SPAEAKS*PVKEEAK | 613–628 | 2126.74 | 3 | S620 | 1 | 2.31 |
| P6 | SPTKEEAKS*PEK | 639–652 | 1986.65 | 3 | S648 | 1 | 4.4 |
| P7 | SPAEVKS*PEK | 538–550 573–584 | 1583.53 | 2 | S546, S580 | 1 | 2.6 |
Phosphorylated residues in hNF-H. NF-H protein from frontal cortex of normal control brains and AD brains was analyzed as described in Materials and Methods. Asterisk indicates phosphorylated residue. z indicates charge of precursor phosphopeptide. Last 2 columns show the iTRAQ ratios of phosphorylated residue in normal and AD brain.
As all the 13 KSP repeats correspond to almost similar sequences spanning the KSP repeat domain, mass spectrometry is the only means to identify the phosphorylation of specific Ser residues. The phosphorylation sites in NF-M are identified based on minor variations of single amino acid residues. For example, if Pro is 1 residue before KSP, (AKS*PVPKS*PVEEK), Ser-615 and Ser-620 are specifically identified, or if Ser is 1 residue before KSP (GKS*PVSKS*PVEEK), Ser-641 is specifically identified. Such analyses list all the phosphopeptides of NF-M (Table 1). The phosphorylated Ser/Thr is highlighted in Table 1 with an asterisk. These residues correspond to Ser-615, Ser-620, Ser-641, Ser-646, Ser-659, Ser-667, Ser-672, Ser-680, and Ser-685 within the KSP repeats and Ser-736 on ESP and Ser-837 on LSP variant motifs. The numbers up-regulated in AD brain are displayed in Tables 1 and 2.
Figure 5A shows the sequence coverage obtained and phosphorylation sites identified in human NF-M from control and AD brain. As an example, Fig. 5B shows the quantitative iTRAQ analysis of phosphorylated sites from 2 control and 2 AD brains in the NF-M phosphopeptide AKS*PVPKS*PVEEA, which demonstrated a combined total of 8.1-fold higher phosphorylation at both Ser-615 and Ser-620 in AD brain compared with control brain.
The collision-induced fragmentation of the phosphopeptide P1 (Table 1), doubly charged ion phosphopeptide with m/z 2202.7, characterized the peptide residues of human NF-M (676–692) AKS*PVPKS*PVEEAK, with Ser-680 and Ser-685 phosphorylated. The relative abundance of the iTRAQ reagent tags at m/z 116.11 (AD1) and 117.11 (AD2) compared with 114.11 (CON1) and 115.11 (CON2), as calculated by peak height showed 5.1-fold higher phosphorylation at Ser-685 in AD compared with control brain.
Similar analyses of the phosphorylation sites of human NF-M from control and AD brain are summarized in Table 1 (proline-directed phosphorylation sites) and Table 2 (non-proline-directed phosphorylation sites). Figure 5C shows the relative phosphorylation site abundance of individual phosphopeptides of NF-M (Table 1, P1–P9; Table 2, P10–P13) in AD brain compared with control brain.
Data analysis of human NF-H from control and AD brain
Human NF-H has 43/44 KSP repeats in the tail domain (Fig. 6A). Earlier studies (34) reported from our laboratory showed that human NF-H is phosphorylated at 33 KSP sites. Here, we performed iTRAQ quantitation of human NF-H from AD brain compared with control brain and showed that 10 KSP sites are phosphorylated at a higher level in AD brain compared with control brain (Table 3). We identified 10 Ser sites in KSP motifs that are highly phosphorylated in AD brain compared with control brain. Human NF-H comprises KSPXK, KSPXXK. and KSPXXXK repeats. Our laboratory (35) has reported earlier that KSPXK sites are the targets for Cdk5, while KSPXXXK sites are targets for Erk1/2. The iTRAQ analysis of NF-H (Table 3) revealed that all the sites correspond to KSPXK sites, suggesting that Cdk5 is a major kinase involved in hyperphosphorylation of NF-H in AD. These results suggest that the hyperphosphorylation of NF-H in NFTs involves aberrant activation of Cdk5. Non-proline-directed protein kinases also play a significant role in AD brain. The role of CKI as a potential NF kinase is also of particular interest, because it has been reported that CK1δ protein is increased by 30-fold in the hippocampus of AD brain and that CK1 is tightly associated with paired helical filament (33). Figure 6A shows the sequence coverage obtained and phosphorylation sites identified in human NF-H. Figure 6B shows the quantitative iTRAQ analysis of the phosphorylated site Ser-640 from 2 control and 2 AD sample brains and that the phosphopeptide AKS*PTKEEAK demonstrates an increase of 8-fold higher phosphorylation at Ser-640 in AD brain compared with control brain.
As an example, the PQD spectrum of the triply charged ion was identified as phosphopeptide P1, SPVKEEVKPS*PEK, with m/z 2012.7, corresponding to the peptide residues of human NF-H (792–805), with Ser-801 clearly phosphorylated. The relative abundance of the iTRAQ reagent tags at 116.1 (AD1) and 117.1 (AD2) compared with 114.1 (CON1) and 115.1 (CON2), as calculated by peak height showed significantly higher phosphorylation in AD (5.7-fold) compared with control brain.
Similar analyses of the phosphopeptides of NF-H and their phosphorylation abundance are summarized in Table 3 and Fig. 6C. These results suggests that NF-P proteins are significantly phosphorylated in AD brain. Mass spectrometry also suggested that the levels of β-tubulin did not change in AD brain compared with control brain (data not shown).
DISCUSSION
AD is the most common form of degenerative dementia, characterized by neuronal degeneration and the formation of senile plaques and neurofibrillary tangles (1–3). AD is a complex and chronic disorder that involves the disruption of the neuronal network in the human brain. To our knowledge, our data form the first comprehensive report of quantitative mass spectrometric analysis of human NF-M and NF-H from control adult and AD brain. The current study aimed to extend our knowledge of specific phosphorylation sites to provide insight into the role of kinases and phosphatases in the formation of NFTs and aggregates. This approach is superior to 2-D electrophoresis-based Pro-Q Diamond staining or even 32P-labeling methods in that the dynamics of particular sites within a protein can be monitored rather than the integrated signal from all of its phosphorylation sites. This is particularly important in proteins with multiple phosphorylation sites that may not share the same regulatory network. The quantitative mass spectroscopic technique used in this study identified specific phosphorylation sites among multiple KSP repeats and non-SP sites in NF-M/H. For example, evidence of phosphorylation of non-proline-directed Ser residue suggests the involvement of non-proline-directed kinases in AD.
The iTRAQ analysis revealed primarily that specific Ser/Thr sites are phosphorylated at a higher fold in AD brain compared with control brain and that not all the neurofilament KSP sites are phosphorylated at similar levels in AD. Some of them are phosphorylated at a greater abundance compared with other sites. CA1 pyramidal cells are known for susceptibility to neurofibrillary degeneration in AD. In human brain, these neurons show an age-related perikaryal accumulation of NFs. Most of the phosphorylation sites in AD tangles are perikaryonal constituents. Normal perikarya do not contain phosphorylated neurofilament proteins. The aberrant phosphorylation in both plaques and tangles seems to be largely restricted to individual phosphorylation sites among the many sites in NFs. The aberrant phosphorylation of S/T residues in NF-M/H could also be due to the low PP2A activity in AD.
Recently, it was shown that RT-97 immunoreactivity is generated by phosphorylation at KSPXK or KSPXXXK motifs and requires flanking lysines at specific positions (36). It is sometimes questioned whether AD tangles are composed of NFs or whether their formation is due to disturbance of other proteins. RT97 antibody staining detected phospho-NF in NFTs and plaques, suggesting the presence of NF in AD tangles. Our immunohistochemistry data suggest that phospho-NF is predominantly detected in intracellular NFTs and extracellular plaques in AD. Previous studies (37) suggested that the neurofilament heavy-chain isoform NF-H SMI35 is a diagnostic marker to monitor neurodegeneration in vivo. The iTRAQ data obtained with phosphorylated NF-M from AD brain also show the higher abundance of phosphorylation of non-proline-directed Ser or Thr residues, suggesting the involvement of non-proline-directed kinases in AD brain. It has also been reported that CK1 is deregulated in AD brain. Thus, we may attribute the phosphorylation of those sites in NF-M in AD brain to CK1 and further experiments are necessary. The present study forms the first report of direct evidence of phosphorylation of individual sites of the KSP repeats and non SP phosphorylation sites of human AD brain. This study also suggests that specific antibodies could be generated to the SP and non-SP sites that are phosphorylated at a greater abundance and can be used as biomarkers in AD.
The iTRAQ analysis did not reveal the phosphorylation of any of the Ser/Thr residues of the N-terminal region of NF-M and NF-H, suggesting that the N-terminal phosphorylation does not occur in AD, as in control brain. The PKA, PKC, and CAMK are the protein kinases involved in transient phosphorylation of the N terminus of NF-M and NF-L before assembly into 10 nm filament structure. Also, we did not detect any of the Tyr sites being phosphorylated in control and AD brains. The NF-subunit stoichiometry is NF-L:NF-M:NF-H (4/5:2:1) to form a 10-nm filament structure. The present study demonstrates that the abundance and number of KSP repeat phosphorylation of NF-M are significantly higher compared with NF-H, despite the greater number of these sites in NF-H. We were able to obtain the sequence coverage for 13/18 phosphorylation sites of NF-M; however, iTRAQ analysis resulted in the aberrant phosphorylation of 10/43 phosphorylation sites of NF-H in AD brain. Based on the stoichiometry, there are 2-fold KSP repeats phosphorylated in NF-M compared with NF-H; therefore, NF-M might contribute more to aberrant phosphorylation in AD compared with NF-H.
All the phosphorylated KSP motifs of NF-H correspond to KSPXK, suggesting aberrant activation and involvement of Cdk5 phosphorylation of NF-H in AD brain. This suggests that Cdk5 inhibitors may have potential therapeutic value in inhibiting aberrant hyperphosphorylation of NF-H in AD.
Interestingly, the phospho-NF was reported to be detected in the plasma and cerebrospinal fluid (CSF) of aneurysmal subarachnoid hemorrhage patients (37), suggesting that this could be a diagnostic marker of neuropathology. Lewis et al. (38) detected phospho-NF-H in the blood of rats subjected to brain and spinal cord injuries but not in the blood of control animals. They correlated the levels of phospho-NF-H and the amount of axonal injury. The NF proteins may be released into extracellular environment following the neuronal degeneration, thus finding their way into CSF and across the blood brain barrier. This is an important observation that phospho-NF-H is detectable in the blood and CSF and can be used as a diagnostic marker in AD.
The involvement of the NF triplet in neuropathology has had a checkered history in AD research. Early studies emphasized the ultrastructural relationship between NFs and neurofibrillary pathology (39) as well as the immunolocalization of NF proteins in tangles (2, 39–41). Nukina and co-workers (42, 43) have indicated that the immunolabeling of neurofibrillary pathology with antibodies to NFs may be due to the crossreactivity with phosphorylated epitopes in phospho-Tau. However, numerous studies indicate that in addition to Tau, NFs are involved in neurofibrillary pathology, which can be exemplified by the cellular basis for selective neuronal vulnerability in AD. With respect to AD, there is strong evidence that NF-triplet-containing neurons in association with neocortices as well as middle temporal lobe structures show a high degree of vulnerability to develop NFTs (44) and degenerate (45). The cortical neurons that contain this cytoskeletal protein are principally a subset of pyramidal neurons (45). Recently, loss of non-phosphorylated neurofilament immunoreactivity (SMI32) in temporal cortical areas of AD was reported, suggesting that NFs are hyperphosphorylated in AD (46).
The iTRAQ analysis reported here has resulted in a remarkably high degree of characterization of the phosphorylation sites of human NF-M and NF-H from control and AD brain. This forms the first report of quantitative characterization of highly phosphorylated protein at multiple sites. The identification of the individual phosphorylation sites of NF-M and NF-H helps to raise specific antibodies for these peptides that could have diagnostic value and also may lead to the development of therapeutic approaches to a wide range of neurodegenerative diseases associated with aberrant neurofilament phosphorylation.
Acknowledgments
This work was supported by the National Institutes of Health Intramural research programs of the National Institute of Neurological Diseases and Stroke.
The authors thank the Harvard Brain Resource Center (Boston, MA, USA) and the National Institute of Child Health and Human Development Brain and Tissue Bank (Bethesda, MD, USA) for providing human brain tissue.
REFERENCES
- 1. Sternberger N. H., Sternberger L. A., Ulrich J. (1985) Aberrant neurofilament phosphorylation in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 82, 4274– 4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cook L. C., Steinberger N. H., Steinberger L. A., Casonva M. F., Struble R. G. (1986) Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 45, 56– 64 [DOI] [PubMed] [Google Scholar]
- 3. Zhang H., Sternberger N. H., Rubinstein L. J., Herman M. H., Binder L. I., Sternberger L. A. (1989) Abnormal processing of multiple proteins in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 86, 8045– 8049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forno L. S., Steinberger L. A., Steinberger N. H., Strefling A. M., Swanson K., Eng L. F. (1986) Reaction of Lewy bodies with antibodies to phosphorylated and non-phosphorylated neurofilaments. Neurosci. Lett. 64, 253– 258 [DOI] [PubMed] [Google Scholar]
- 5. Pollanen M. S., Bergeron C., Weyer L. (1994) Alzheimer paired helical filaments: a comparison with the twisted ribbon model. Acta Neuropathol. 88, 1– 6 [DOI] [PubMed] [Google Scholar]
- 6. Manetto N. H., Steinberger N. H., Perry G., Steinberger L. A., Gambetti P. (1988) Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 47, 642– 653 [DOI] [PubMed] [Google Scholar]
- 7. Munoz D. G., Grein C., Perl D. P., Selkoe D. J. (1988) Accumulation of phosphorylated neurofilaments in anterior horn motor neurons of amyotrophic lateral sclerosis patients. J. Neuropathol. Exp. Neuropathol. 47, 9– 18 [DOI] [PubMed] [Google Scholar]
- 8. Sobue G., Hashizumi Y., Yasude T., Mukai E., Kumagai T., Trojanowski J.Q. (1990) Phosphorylated high molecular weight neurofilament protein in lower motor neurons in amyotrophic lateral sclerosis and other neurodegenerative diseases involving ventral horn cells. Acta Neuropathol. 79, 402– 408 [DOI] [PubMed] [Google Scholar]
- 9. Pant H.C., Veeranna (1995) Neurofilament phosphorylation. Biochem. Cell Biol. 73, 575– 592 [DOI] [PubMed] [Google Scholar]
- 10. Julien J. P., Mushinski W. E. (1983) Multiple phosphorylation sites in mammalian neurofilament polypeptides. J. Biol. Chem. 258, 4019– 4025 [PubMed] [Google Scholar]
- 11. Lees J. F., Shneidman P. S., Skuntz S. F., Carden M. J., Lazzarini R. (1988) The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 7, 1947– 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elhanany E., Jaffe H., Link W. T., Sheeley D. M., Gainer H., Pant H. C. (1994) Identification of endogenously phosphorylated KSP sites in the high-molecular-weight rat neurofilament protein. J. Neurochem. 63, 2324– 2335 [DOI] [PubMed] [Google Scholar]
- 13. Yamasaki H., Itakura C., Mizutani M. (1991) Hereditary hypotrophic axonopathy with neurofilament deficiency in a mutant strain of the Japanese quail. Acta Neuropathol. 82, 427– 434 [DOI] [PubMed] [Google Scholar]
- 14. Mata M., Kupina M., Fink D. J. (1992) Phosphorylation-dependent neurofilament epitopes are reduced in nodes of Ranvier. Neurocytology 21, 199– 210 [DOI] [PubMed] [Google Scholar]
- 15. O'Hara O., Gahara Y., Miyake T., Teraoka H., Kitamura T. (1993) Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J. Cell Biol. 121, 387– 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeWaegh S. M., Lee V. M. Y., Brady S. T. (1992) Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 68, 451– 463 [DOI] [PubMed] [Google Scholar]
- 17. Eyer J., Peterson A. (1994) Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-beta-galactosidase fusion protein. Neuron 12, 389– 405 [DOI] [PubMed] [Google Scholar]
- 18. Goldstein M. E., Sternberger N. H., Sternberger L. A. (1987) Phosphorylation protects neurofilaments againsts proteolysis. J. Neuroimmunol. 14, 149– 160 [DOI] [PubMed] [Google Scholar]
- 19. Pant H. C. (1988) Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 256, 665– 668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krinks M. H., Klee C. B., Pant H. C., Gainer H. (1988) Identification and quantification of calcium binding proteins in squid axoplasm. J. Neurosci. 8, 2172– 2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis S. E., Nixon R. A. (1988) Multiple phosphorylation variants of the high molecular mass subunit of neurofilaments in axons of retinal cell neurons: characterization and evidence for their differential association with stationary and moving neurofilaments. J. Cell Biol. 107, 2689– 2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong P. C., Marszalek J., Crawford T. O., Xu Z., Hsieh S. T., Griffin J. W., Cleveland D. W. (1995) Increasing neurofilament subunit NF-M expression reduces axonal NF-H, inhibits radial growth, and results in neurofilamentous accumulation in motor neurons. J. Cell Biol. 130, 1413– 1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shea T. B., Zheng Y. L., Ortiz D., Pant H. C. (2004) Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport. J. Neurosci. Res. 76, 795– 800 [DOI] [PubMed] [Google Scholar]
- 24. Lee V. M. Y., Carden M., Schlaepfer W., Trojanski J. (1987) Identification of the major multiphosphorylation site in mammalian neurofilaments. J. Neurosci. 7, 3474– 3488 3119789 [Google Scholar]
- 25. Munton R. P., Tweedie-Cullen R., Livingstone-Zatchej M., Weinandy F., Waidelich M., Longo D., Gehrig P., Potthast F., Rutishauser D., Gerrits B., Panse C., Schlapbach R., Mansuy I. M. (2007) Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol. Cell. Proteomics 6, 283– 293 [DOI] [PubMed] [Google Scholar]
- 26. Tannu N. S., Hemby S. E. (2006) Methods for proteomics in neuroscience. Prog. Brain Res. 158, 41– 82 [DOI] [PubMed] [Google Scholar]
- 27. Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76– 85 [DOI] [PubMed] [Google Scholar]
- 28. Rudrabhatla P., Albers W., Pant H. C. (2009) Peptidyl-prolyl isomerase 1 regulates protein phosphatase 2A-mediated topographic phosphorylation of neurofilament proteins. J. Neurosci. 29, 14869– 14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone K.L., Williams K.R. (1993) Enzymatic digestion of proteins and HPLC peptide isolation. In A Practical Guide to Protein and Peptide Purification for Microsequencing (Matsudaira P. ed) pp. 43– 69, Academic Press, San Diego, CA, USA: [Google Scholar]
- 30. Dosemeci A., Jaffe H. (2010) Regulation of phosphorylation at the postsynaptic density during different activity states of Ca2+/calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Com. 391, 78– 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Q., Shakey W., Liu A., Schuller, Follettie M.T. (2007) Global profiling of phosphopeptides by titania affinity enrichment. J. Proteome Res. 6, 4684– 4689 [DOI] [PubMed] [Google Scholar]
- 32. Griffin T. J., Xie H., Bandhakavi S., Popko J., Mohan A., Carlis J. V., Higgins L. (2007) iTRAQ reagent-based quantitative proteomic analysis on a linear ion trap mass spectrometer. J. Proteome Res. 6, 4200– 4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghoshal N., Smiley J.F., DeMaggio A.J., Hoekstra M.F., Cochran E.J., Binder L. I., Kuret J. (1999) New molecular link between the fibrillar and granulovacuolar lesions of Alzheimer's disease. Am. J. Pathol. 155, 1163– 1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaffe H., Veeranna, Shetty K. T., Pant H. C. (1998) Characterization of the phosphorylation sites of human high molecular weight neurofilament protein by electrospray ionization tandem mass spectrometry and database searching. Biochemistry 17, 3931– 3940 [DOI] [PubMed] [Google Scholar]
- 35. Pant A. C., Veeranna, Pant H. C., Amin N. (1997) Phosphorylation of human high molecular weight neurofilament protein (hNF-H) by neuronal cyclin-dependent kinase 5 (cdk5). Brain Res. 765, 259– 266 [DOI] [PubMed] [Google Scholar]
- 36. Veeranna, Lee J. H., Pareek T. K., Jaffee H., Boland B., Vinod K. Y., Amin N., Kulkarni A. B., Pant H. C., Nixon R. A. (2008) Neurofilament tail phosphorylation: identity of the RT-97 phosphoepitope and regulation in neurons by cross-talk among proline-directed kinases. J. Neurochem. 107, 35– 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brettschneider J., Petzold A., Schöttle D., Claus A., Riepe M., Tumani H. (2006) The neurofilament heavy chain (Nf-H SMI35) in the cerebrospinal fluid diagnosis of Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 21, 291– 295 [DOI] [PubMed] [Google Scholar]
- 38. Lewis S. B., Wolper R. A., Miralia L., Yang C., Shaw G. (2008) Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J. Cereb. Blood Flow Metab. 28, 1261– 1271 [DOI] [PubMed] [Google Scholar]
- 39. Dahl D., Selkoe D. J., Pero R. T., Bignami A. (1982) Immunostaining of neurofibrillary tangles in Alzheimer's senile dementia with a neurofilament antiserum. J. Neurosci. 2, 113– 119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perry G., Lipphardt S., Mulvihill P., Kancherla M., Mijares M., Gambetti P., Sharma S., Maggiora L., Cornette J., Lobl T. (1988) Amyloid precursor protein in senile plaques of Alzheimer disease. Lancet 2 746 [DOI] [PubMed] [Google Scholar]
- 41. Miller C. C., Brion J. P., Calvert R., Chin T. K., Eagles P. A., Downes M. J., Flament-Durand J., Haugh M., Kahn J., Probst A., Ulrich J., Anderton B. H. (1986) Alzheimer's paired helical filaments share epitopes with neurofilament side arms. EMBO J. 5, 269– 276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nukina N., Kosik K. S., Selkoe D. J. (1987) Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to cross-reaction with tau protein. Proc. Natl. Acad. Sci. U. S. A. 84, 3415– 3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kosik K. S., Joachim C. L., Selkoe D. J. (1986) Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 83, 4044– 4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vickers J. C., Chin D., Edwards A.-M., Sampson V., Harper C., Morrison J. (1996) Dystrophic neurite formation associated with age-related β amyloid deposition in the neocortex: clues to the genesis of neurofibrillary pathology. Exp. Neurol. 141, 1– 11 [DOI] [PubMed] [Google Scholar]
- 45. Morrison J. H., Lewis D. A., Campbell M. J., Huntley G. W., Benson D. L., Bouras C. (1987) A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer's disease. Brain Res. 416, 331– 336 [DOI] [PubMed] [Google Scholar]
- 46. Thangavel S. K., Sahu G. W., Hoesen V., Zaheer A. (2009) Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in Alzheimer's disease. Neuroscience 160, 427– 433 [DOI] [PMC free article] [PubMed] [Google Scholar]