Abstract
TMEM16A was found recently to be a calcium-activated Cl− channel (CaCC). CaCCs perform important functions in cell physiology, including regulation of epithelial secretion, cardiac and neuronal excitability, and smooth muscle contraction. CaCC modulators are of potential utility for treatment of hypertension, diarrhea, and cystic fibrosis. Screening of drug and natural product collections identified tannic acid as an inhibitor of TMEM16A, with IC50 ∼ 6 μM and ∼100% inhibition at higher concentrations. Tannic acid inhibited CaCCs in multiple cell types but did not affect CFTR Cl− channels. Structure-activity analysis indicated the requirement of gallic or digallic acid substituents on a macromolecular scaffold (gallotannins), as are present in green tea and red wine. Other polyphenolic components of teas and wines, including epicatechin, catechin, and malvidin-3-glucoside, poorly inhibited CaCCs. Remarkably, a 1000-fold dilution of red wine and 100-fold dilution of green tea inhibited CaCCs by >50%. Tannic acid, red wine, and green tea inhibited arterial smooth muscle contraction and intestinal Cl− secretion. Gallotannins are thus potent CaCC inhibitors whose biological activity provides a potential molecular basis for the cardioprotective and antisecretory benefits of red wine and green tea.—Namkung, W., Thiagarajah, J. R., Phuan, P.-W., Verkman, A. S. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea.
Keywords: CaCC, TMEM16A, tannic acid, CFTR, diarrhea, hypertension
Calcium-activated chloride channels (CaCCs) are involved in many physiological functions, including transepithelial fluid secretion, smooth muscle contraction, and sensory signal transduction (1, 2). A variety of stimuli elevate cytoplasmic calcium, resulting in CaCC activation by direct action or through calmodulin signaling (3). The consequences of CaCC activation are cell-type specific, including, for example, Cl− secretion in intestinal epithelia, arterial smooth muscle contraction, and action potential generation in olfactory receptor neurons. CaCCs are considered as potential targets for drug therapy of hypertension, secretory diarrheas, neuropathic pain, and certain tumors (2).
Until recently the molecular identity of CaCCs has been unclear, with candidate proteins including the bestrophins (Best1–Best4), CLCA family proteins, tweety family proteins (TTYH2 and TTYH3), and ClC-3 (1, 3). Recently, 3 laboratories reported compelling evidence that the TMEM16A (anoctamin-1) gene encodes a CaCC (4–6). Heterologous expression of TMEM16A produced calcium-activated Cl− currents with similar electrophysiological properties to those in native cells. TMEM16A is expressed broadly in tissues having strong CaCC activity. TMEM16A knockdown reduces CaCC currents, and TMEM16A knockout in mice causes developmental tracheomalacia resulting in neonatal death (6, 7). The role of TMEM16A in mature organs has not been determined, in part because of the lack of potent and selective inhibitors.
We report here a screen to identify TMEM16A Cl− channel modulators as pharmacological tools to study TMEM16A and as potential therapeutics. We identified tannic acid and related gallotannins as potent TMEM16A/CaCC inhibitors and provide evidence that Cl− channel inhibition by gallotannins in green tea and red wine may be responsible for their health benefits.
MATERIALS AND METHODS
Compounds, teas, and wines
Screening was done with ∼3200 approved/investigational drugs and related natural compounds (Microsource Spectrum Library, Gaylordsville, CT, USA; National Institutes of Health Clinical Collection, South San Francisco, CA, USA; Iconix Library, Foster City, CA, USA). Tannic acid (compound 1) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Tannic acid analogs 2 and 3 were purchased from CarboSynth Ltd. (Compton, UK), 4 from Sigma-Aldrich, and 5 and 6 from AnalytiCon Discovery (Potsdam, Germany). Grape tannin and wine tannin were purchased from E. C. Kraus (Independence, MO, USA). Gambogic acid (−)-epigallocatechin-3-gallate, (−)-epicatechin-3-gallate, (−)-epigallocatechin, (−)-epicatechin, catechin, gallocatechin, and malvidin-3-glucoside were purchased from Sigma-Aldrich. Candesartan was purchased from Tecoland Corp. (Edison, NJ, USA). Gossypol and dichlorophene were purchased from MP Biomedicals (Solon, OH, USA). Green teas were purchased from Dongwon F&B (Seoul, Korea), Amorepecific (Choongbuk, Korea), Yamama Masudaen (Shizuoka, Japan), Yamamotoyama (Pomona, CA, USA), and Triple Leaf Tea (South San Francisco, CA, USA). Black teas were purchased from Whittard of Chelsea (London, UK). Red wines were purchased from La Fleur Bibian (Bordeaux, France), Falesco (Montecchio, Italy), Los Vascos (Santa Cruz, Chile), Preludio (Mendoza, Argentina), and Robert Mondavi Winery (Oakville, CA, USA). White wines were purchased from Snoqualmie (Paterson, WA, USA) and Chateau haut Rian (Bordeaux, France). Green tea (2.5 g) was boiled in 30 ml water for 10 min, with 1.25 g green tea in 300 ml water considered as normal (consumption-strength) green tea. Ethanol was removed from red wine by boiling to reduce volume by one-third. Aqueous green tea and wine were filtered with 0.2-μm filters.
Cell lines and culture
FRT cells were stably transfected with human TMEM16A (abc isoform, cDNA provided by Dr. Luis Galietta, Gaslini Institute, Genoa, Italy) and the halide sensor YFP-F46L/H148Q/I152L. Cells were plated in 96-well black-walled microplates (Corning, Corning, NY, USA) at a density of 20,000 cells/well in Coon's modified F12 medium supplemented with 5% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Assays were done at 48 h after plating, when cells were just confluent. Some measurements were made on T84 human intestinal epithelial cells and primary cultures of normal- and ΔF508-CFTR-expressing human bronchial epithelial cells, obtained and grown as described previously (8).
Screening procedures
Assays were done using an automated screening platform (Beckman, Fullerton, CA, USA) equipped with FluoStar fluorescence plate readers (BMG Lab Technologies, Durham, NC, USA), as described previously (9). Each well of a 96-well plate was washed 3 times in PBS (200 μl/wash), leaving 50 μl PBS. Test compounds (0.5 μl) were added to each well at 25 μM final concentration. After 10 min, 96-well plates were transferred to a plate reader for fluorescence assay. Each well was assayed individually for TMEM16A-mediated I− influx by recording fluorescence continuously (400 ms/point) for 2 s (baseline), then 50 μl of 140 mM I− solution was added at 2 s, and then 50 μl of 70 mM I− solution containing 300 μM ATP was added at 6.4 s. The 70 mM I− solution consisted of a 1:1 mixture of PBS and the 140 mM I− solution. The initial rate of I− influx following each of the solution additions was computed from fluorescence data by nonlinear regression.
Short-circuit current
Snapwell inserts containing TMEM16A-expressing FRT cells, T84 cells, or human bronchial epithelial cells were mounted in Ussing chambers (Physiological Instruments, San Diego, CA, USA). For FRT cells, the hemichambers were filled with 5 ml of half-Cl− solution (apical), and HCO3−-buffered solution (basolateral). CFTRinh-172, UTP, ATP, tannic acid, and gallotannins were added to the apical solution, and equal volume of vehicle was added simultaneously to the basolateral solution. The HCO3−-buffered solution contained (in mM) 120 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 d-glucose, 5 HEPES, and 25 NaHCO3 (pH 7.4). In the half-Cl− solution, 65 mM NaCl in the HCO3−-buffered solution was replaced with Na gluconate. Symmetrical HCO3−-buffered solutions were used for T84 cells and human bronchial epithelial cells. Cells were bathed for a 10-min stabilization period and aerated with 95% O2/5% CO2 at 37°C or room temperature. ENaC activity was measured as amiloride-sensitive short-circuit current (Isc). Short-circuit current was measured using an EVC4000 Multi-Channel V/I Clamp (World Precision Instruments, Sarasota, FL) and recorded using PowerLab/8sp (AD Instruments, Castle Hill, Australia).
Patch clamp
Whole-cell recordings were made at room temperature on FRT cells stably expressing TMEM16A. The pipette solution contained (in mM) 130 CsCl, 0.5 EGTA, 1 MgCl2, 1 Tris-ATP, and 10 HEPES (pH 7.2). The bath solution contained (in mM) 140 NMDG-Cl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (pH 7.4). Pipettes were pulled from borosilicate glass and had resistances of 3–5 MΩ after fire polishing. Seal resistances were between 3 and 10 GΩ. After establishing the whole-cell configuration, TMEM16A was activated by ATP. Whole-cell currents were elicited by applying hyperpolarizing and depolarizing voltage pulses from a holding potential of 0 mV to potentials between −100 and +100 mV in steps of 20 mV. Recordings were performed at room temperature using an Axopatch-200B (Axon Instruments, Foster City, CA, USA). Currents were digitized with a Digidata 1440A AC/DC converter (Axon Instruments), filtered at 5 kHz, and sampled at 1 kHz.
Calcium signaling
FRT cells in 96-well black-walled microplates were loaded with Fluo-4 NW (Invitrogen, Carlsbad, CA, USA) at 48 h after plating, according to the manufacturer's recommendation. Fluo-4 fluorescence was measured with a FLUOstar Optima fluorescence plate reader (BMG Labtechnologies) equipped with syringe pumps and custom excitation/emission filters (485/538 nm).
Arterial smooth muscle contraction
Wild-type CD1 mice (age 8–10 wk) were killed by avertin overdose (200 mg/kg). Protocols were approved by the University of California–San Francisco Committee on Animal Research. Following thoracotomy, the aorta was gently removed, dissected from fat and connective tissues, and cut transversely into rings of length 4–5 mm. Care was taken not to damage the endothelium in the rings. The rings were mounted between 2 stainless steel wire hooks and suspended in a 20-ml organ bath (Biopac Systems, Goleta, CA, USA) containing Krebs-Henseleit buffer (in mM): 118 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose, 2.5 CaCl2, and 0.01 indomethacin (pH adjusted to 7.4 with 95% O2-5% CO2 at 37°C). Aortic rings were equilibrated for 60 min with a resting force of 5 mN, with changes of the bathing solution at 15-min intervals. At the end of equilibration period, 50 mM KCl was applied to confirm aortic ring contractility, which was washed out with Krebs-Henseleit buffer. Tension was monitored continuously with a fixed range precision force transducer (TSD, 125 C; Biopac Systems) connected to a differential amplifier (DA 100B; Biopac Systems). Data were recorded using a MP100 Biopac digital acquisition system and analyzed using Acknowledge 3.5.7 software (Biopac Systems). Prior to compound testing, the integrity of the vascular endothelium was assessed by acetylcholine (1 μM)-induced relaxation after contraction with phenylephrine (1 μM). Aortic rings were then washed several times with Krebs-Henseleit solution and allowed to equilibrate for 30 min before the experimental protocol, which involved addition of phenylephrine (1 μM) to induce maximum contraction followed by addition of test compounds.
Intestinal short-circuit current measurements
Wild-type CD1 mice (age 8–10 wk) were killed as above, and the colon was removed and washed with ice-cold Krebs buffer. The intestine was opened along the mesenteric border and mounted in Ussing chambers (Physiological Instruments). Chambers were filled with symmetric solutions of a modified Krebs-bicarbonate solution containing (in mM) 120 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 d-glucose, 5 HEPES, 25 NaHCO3, 0.01 indomethacin, and 0.0001 tetrodotoxin, and maintained at pH 7.4 with 95% O2-5% CO2 at 37°C. Short-circuit current Isc was measured in the presence of 50 μM amiloride as described above.
RESULTS
Identification of tannic acid as a TMEM16A inhibitor
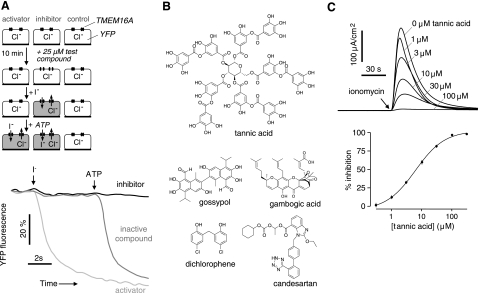
As shown in Fig. 1A (top panel), a screening assay was developed to identify TMEM16A modulators. TMEM16A activity was measured from the kinetics of I− influx in TMEM16A-expressing Fisher rat thyroid epithelial (FRT) cells, using as readout the fluorescence of the I−-sensing fluorescent protein YFP-F46L/H148Q/I152L. Following incubation of cells with test compounds in an I−-free buffer, fluorescence was measured in response to serial additions of I− and ATP. I− addition produced little YFP fluorescence quenching in the absence of TMEM16A agonists because of the low basal I− permeability of FRT cells. Examples of original screening data are shown in Fig. 1A (bottom panel). A putative TMEM16A activator was identified by YFP fluorescence quenching immediately following I− addition, while a TMEM16A inhibitor was identified by reduced YFP fluorescence quenching in response to ATP, which activates TMEM16A through purinergic receptor/Ca2+ signaling. The screening assay was robust, with a Z′ factor > 0.7 for identification of TMEM16A activators and inhibitors.
Figure 1.
Identification of inhibitors of TMEM16A Cl− conductance. A) Top panel: screening assay. FRT cells stably expressing TMEM16A and the I−-sensitive cytoplasmic fluorescent sensor YFP-F46L/H148Q/I152L were incubated for 10 min with test compound. Fluorescence was monitored in response to serial additions of I− (to identify activators) and then ATP (to identify inhibitors). Bottom panel: representative screening data (fluorescence from individual wells of 96-well plates), showing examples of a putative activator and inhibitor. B) Chemical structures of TMEM16A inhibitors. C) Short-circuit analysis of TMEM16A-expressing FRT cells. Top panel: representative current traces showing ionomycin-stimulated TMEM16A Cl− current in cells pretreated with indicated concentrations of tannic acid for 10 min. Bottom panel: dose-response summary (means ± se, n=3). IC50=6.1 μM.
Here 3200 compounds from collections of approved/investigational drugs and related natural compounds were screened at a concentration of 25 μM. Five compounds had apparent activator activity, as defined by >5-fold increased YFP fluorescence quenching following I− addition. However, all 5 compounds (tyrothricin, sapindoside A, digitonin, chrysanthellin A, and nordihydroguaiaretic acid) were of little interest because they increased cellular I− influx by trivial detergent or ionophoric actions or had poor potency. Ten compounds had apparent inhibitor activity, as defined by >70% reduction in the rate of YFP fluorescence quenching following ATP addition. Five compounds, including tannic acid, gossypol, dichlorophene, gambogic acid, and candesartan (Fig. 1B), were confirmed from electrophysiological studies as bona fide TMEM16A inhibitors, with IC50 values of 6.1, 6.1, 4.5, 3.9, and 3.7 μM, respectively. However, with the exception of tannic acid, these TMEM16A inhibitors showed partial or weak inhibition of native CaCC Cl− currents in T84 cells. Figure 1C shows short-circuit current data and the deduced dose-response relation for tannic acid inhibition of ionomycin-stimulated TMEM16A activity. Tannic acid was further studied because of its high inhibition potency and efficacy, and because tannic acid and related gallotannins are present in a number of widely consumed food products.
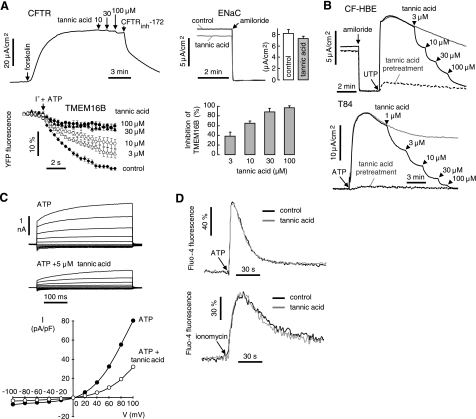
Tannic acid selectivity was studied (Fig. 2A). Tannic acid at a concentration that inhibited TMEM16A by >95% (100 μM) had little effect on CFTR Cl− conductance (8.8±0.8% inhibition) or on ENaC Na+ conductance (11±5% inhibition). Tannic acid strongly inhibited another TMEM16 isoform (TMEM16B) that has been associated with Cl− channel activity (5), with similar IC50 to that for inhibition of TMEM16A. Tannic acid was tested for inhibition of CaCC Cl− conductance in native tissue-derived cells, including primary cultures of human bronchial epithelial cells and human T84 colonic epithelial cells (Fig. 2B). Strong CaCC inhibition was found with IC50 of 11.2 and 3.1 μM, respectively, and near-complete inhibition at higher concentrations.
Figure 2.
Specificity and mechanism of tannic acid inhibition of TMEM16A. A) Effect of tannic acid on CFTR (T84 colonic cells), ENaC (HBE cells), and mouse TMEM16B (FRT cells). B) Tannic acid inhibition of native CaCC Cl− currents in human epithelial cell cultures, including bronchial cells (top panel) and T84 cells (bottom panel). ENaC and CFTR were inhibited by 10 μM amiloride and 20 μM CFTRinh-172, respectively. C) Top panel: whole-cell TMEM16A current recorded at a holding potential at 0 mV, and pulsing to voltages between ±100 mV (in steps of 20 mV) in the absence and presence of 5 μM tannic acid. TMEM16A was stimulated by 100 μM ATP. Bottom panel: current/voltage (I/V) plot of mean currents normalized as current densities (pA/pF). D) Cytoplasmic [Ca2+] measured by Fluo-4 fluorescence in TMEM16A-expressing FRT cells, showing no significant effects of 100 μM tannic acid on resting [Ca2+] or [Ca2+] elevation following 100 μM ATP (top panel) or 1 μM ionomycin (bottom panel).
Patch-clamp measurement of whole-cell currents in TMEM16A-expressing FRT cells showed a characteristic outwardly rectifying Cl− conductance (Fig. 2C), as reported for heterologously expressed TMEM16A (4). Tannic acid at 5 μM inhibited ATP-induced TMEM16A Cl− current by ∼50% at all voltages, indicating a voltage-independent block mechanism. Figure 2D shows that tannic acid at 100 μM did not alter basal cytoplasmic [Ca2+] or the increase in [Ca2+] following ATP or ionomycin, supporting its direct action on TMEM16A.
Structural determinants of TMEM16A inhibition
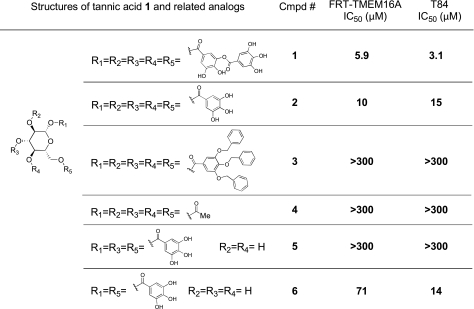
Several tannic acid analogs (Fig. 3) were tested to investigate the structural determinants of TMEM16A inhibition. Tannic acid 1 consists of α-d-glucose linked with 5 digallic acid moieties. To assess the importance of the digalloyl groups, the digalloyl substituents of tannic acid were substituted with galloyl, benzyl-protected galloyl, and acetate-protected groups in analogs 2, 3, and 4, respectively. Analogs 5 and 6, having 3 and 2 galloyl groups on the glucose core, respectively, were also studied.
Figure 3.
Structure-activity analysis of tannic acid analogs. IC50 was determined from concentration-inhibition data from short-circuit current measurements.
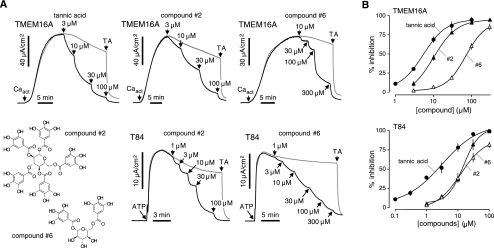
Compounds were tested for inhibition of Cl− current in TMEM16A-expressing FRT cells and in T84 cells. TMEM16A was activated by a small-molecule activator (Caact) that produces a sustained elevation of intracellular calcium (Supplemental Fig. 1). The IC50 for tannic acid in this assay was 5.9 μM, similar to that of 6.1 μM found with ionomycin as agonist in Fig. 1C. Figure 4A shows representative short-circuit current data in the TMEM16A-expressing FRT cells and T84 cells. Dose-response data are shown in Fig. 4B and summarized in Fig. 3. Penta-1,2,3,4,6-O-galloyl-β-d-glucose 2, which contains galloyl instead of digalloyl functions, strongly inhibited TMEM16A, although with slightly lower potency than tannic acid (IC50 10.0 and 15.0 μM). Glucopyranoses 3 and 4, which are identical to glucopyranose 2 except that the free phenol functions on the galloyl ring are protected with benzyl and acetate groups, respectively, were inactive (IC50>300 μM). Glucopyranoses 5 and 6, which contain only 3 and 2 galloyl substituents, respectively, were less active than glucopyranoses 1 and 2. The differential effects of some tannic acid analogs (and other compounds in Fig. 1B) on TMEM16A in FRT cells vs. CaCCs in T84 cells suggests that CaCC current in T84 cells may be carried by other, as yet unidentified, CaCCs.
Figure 4.
Structure-activity analysis of tannic acid analogs. A) Representative short-circuit current data in TMEM16A-expressing FRT cells showing inhibition following activation of TMEM16A Cl− current by a small-molecule calcium agonist (Caact, 5 μM). Tannic acid or indicated analogs added as shown. Tannic acid (TA; 100 μM) was added where indicated. B) Dose-response data for inhibition of Cl− current in TMEM16A-expressing FRT cells (top panel) and T84 cells (bottom panel) (means ± se, n=3–4). Some error bars are smaller than symbol size. IC50 values are summarized in Fig. 3.
TMEM16A/CaCC inhibition by red wine and green tea gallotannins
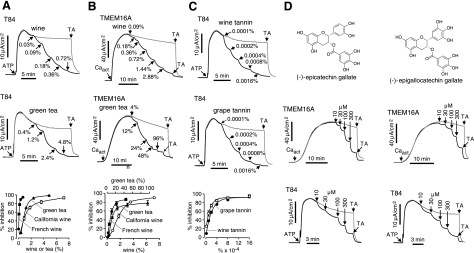
Because tannic acid-related gallotannins and polyphenols are present in relatively high concentrations in red wines and green teas and have been proposed, without a specific mechanism, to be involved in multiple beneficial health effects (10, 11), we tested the efficacy of red wines and green teas for TMEM16A/CaCC inhibition. We found that each of 5 red wines and 5 green teas tested strongly inhibited Ca2+-activated Cl− currents in T84 cells (Fig. 5A and Supplemental Fig. 2). White wines, however, were ineffective. A French red wine at 0.09% (1100-fold dilution) produced >50% inhibition (Fig. 5A). A green tea, prepared as for ingestion by boiling in water, produced >60% inhibition at 1.2% (83-fold dilution). Potent inhibition of Cl− current by red wines and green teas was also found in the TMEM16A-expressing FRT cells (Fig. 5B). Wine tannin and grape-derived tannins each strongly inhibited CaCC Cl− current in T84 cells (Fig. 5C), with 0.0001% (w/v) producing ∼50% inhibition. Red wines and green teas did not inhibit CFTR Cl− conductance (Supplemental Fig. 3).
Figure 5.
Potent inhibition of TMEM16A/CaCC Cl− conductance by red wine and green tea. A) Short-circuit current data (top and middle panels) and dose-response summary (bottom panel) for inhibition of ATP-induced CaCC Cl− current in T84 cells by a red wine and a green tea. CFTRinh-172 (20 μM) was present to inhibit CFTR. B) Inhibition of Cl− current in TMEM16A-expressing FRT cells. C) Inhibition of ATP-induced CaCC Cl− current in T84 cells by wine and grape tannins. D) Inhibition of Cl− current by 2 major polyphenols present in green tea, epigallocatechin-3-gallate (EGCG), and epicatechin-3-gallate (ECG) (top panels), in TMEM16A-expressing FRT cells (middle panels) and T84 cells (bottom panels).
Red wines and green teas strongly inhibited TMEM16A and CaCC Cl− currents in T84 cells. Tannic acid itself is found in red wine but not in green tea, although green tea contains other gallotannins and polyphenols. To investigate the tannic acid-related molecules that may be responsible for TMEM16A/CaCC inhibition, we tested major polyphenols of green tea and red wine, including epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), epigallocatechin, epicatechin, catechin, gallocatechin, and malvidin-3-glucoside. ECG and EGCG, the main constituents of green tea, more strongly inhibited Cl− currents than the other polyphenols (Fig. 5D and Supplemental Fig. 4).
Red wine and green tea inhibit arterial smooth muscle contraction and intestinal Cl− secretion
Prior studies have suggested the involvement of CaCCs in vascular smooth muscle contraction (12, 13). Motivated by the potent inhibition of CaCC-mediated currents in epithelia by gallotannin constituents of red wines and green teas, we measured inhibition of phenylephrine-induced aortic smooth muscle contraction. Figure 6A shows concentration-dependent relaxation of aortic smooth muscle contraction by a red wine and a green tea. A French red wine was ∼4-fold more potent than a California red wine, as found in the TMEM16A-expressing FRT cells. Figure 6A also shows relaxation of aortic smooth muscle contraction by tannic acid and EGCG.
Figure 6.
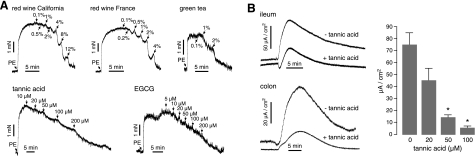
Gallotannins inhibit arterial smooth muscle contraction and intestinal Cl− secretion. A) Mouse aortic smooth muscle contraction. Contraction-relaxation data showing inhibition of phenylephrine (PE, 1 μM) induced contraction by a California and French red wine and a green tea (top panel), and tannic acid and EGCG (bottom panel). Control experiments with only PE added showed sustained contraction with <2% decrease in arterial tension over 45 min. Representative of 3 sets of experiments. B) Left panel: short-circuit current of mouse ileum and colon showing inhibition of carbachol (1 mM)-stimulated Cl− current by 10 min tannic acid pretreatment (100 μM, ileum; 50 μM, colon). Right panel: summary of peak carbachol-stimulated current by tannic acid in mouse colon (means ± se, n=5). *P < 0.001 vs. 0 tannic acid, ANOVA with Tukey-Kramer post hoc test.
Figure 6B shows inhibition of carbachol-stimulated short-circuit current in mouse ileum and colon. Carbachol produces a transient increase in short-circuit current in the intestine (14, 15). Tannic acid greatly reduced peak current in a dose-dependent manner.
DISCUSSION
The principal discovery here is that tannic acid and related gallotannins are strong CaCC inhibitors. CaCC Cl− currents were inhibited in TMEM16A-transfected cells and various native cell cultures and tissues. Gallotannins are prominent constituents of red wines and green teas, along with other polyphenolic compounds (10, 11). Their potent inhibition of CaCC Cl− currents, aortic smooth muscle contraction, and epithelial Cl− secretion may provide a molecular basis for some of their health benefits, including reduced risk of cardiovascular disease and antidiarrheal properties.
The recent discovery of TMEM16A as a CaCC has motivated investigation of the physiological role of CaCCs in different cell types and organ systems (16–19). However, these studies have been limited by the neonatal mortality of TMEM16A-null mice and the lack of potent and selective inhibitors (7, 20). The original aim of our study was to identify selective and potent inhibitors of TMEM16A, both as tools to investigate CaCC function and as potential therapeutics. We found that the natural substances tannic acid 1 and penta-1,2,3,4,6-O-galloyl-β-d-glucose 2 are potent CaCC inhibitors. CaCC Cl− currents were inhibited in TMEM16A-transfected cells and in various native cell cultures and tissues (Figs. 2B and 6 and Supplemental Fig. 5). Tannic acid did not block CFTR or ENaC sodium channels in epithelia, allowing its use as a pharmacological tool to isolate the role of CaCCs in fluid transport.
The CaCC targets of tannic acid include TMEM16 and probably other, as yet unidentified, CaCCs. We found by RT-PCR that T84 cells express transcripts encoding CLCA1 and Best1, and that HBE cells express CLCA2, CLCA4, and Best1 (data not shown). However, we were unable to demonstrate significant Cl− transport by human CLCA1, CLCA2, CLCA4, and Best1 following transfection into YFP-F46L/H148Q/I152L expressing FRT cells. Recent studies concluded that CLCA and Best1 proteins do not form independent CaCC channels, but may regulate CaCC channels as a part of protein complexes with CaCCs or by modulation of cytosolic calcium (21, 22).
Tannic acid belongs to a general class of related compounds, the gallotannins, which are relatively abundant in a wide range of natural products and extracts. Gallotannins and other plant polyphenols, such as procyandins, flavanoids, and catechins, have been reported to have a wide range of diverse biological activities, including reduced incidence of cardiovascular disease, diabetes, cancer, stroke, and cataracts, and inhibition of tumor growth, inflammation, and fat absorption (11, 23). We found that unlike tannic acid and gallic acid derivatives (such as EGCG), other major plant polyphenols (such as catechins and malvadin-3-glucoside) had little or activity against CaCCs, suggesting that the galloyl moiety on a macromolecular scaffold was important for CaCC inhibition. Structure-activity analysis of tannic acid analogs established that free phenols on the galloyl ring are needed for TMEM16A inhibition, as evidenced by the lack of activity of protected tannic acid analogs 3 and 4, and the reduced activity of analogs 5 and 6 containing fewer galloyl groups.
Anecdotal literature and controlled clinical studies suggest multiple health benefits of red wine and green tea, including reduced risk of cardiovascular disease, anticancer and antidiarrheal properties, body weight control, antibacterial and antiviral activity, increased bone mineral density, antifibrotic properties, and neuroprotection (24, 25). It is well established that moderate consumption of red wine reduces the risk of coronary artery disease (26–28), and it has been suggested that red wine consumption is responsible for the reduced cardiovascular risk in populations in which red wine consumption is relatively high. High green tea consumption in Asian populations has been correlated with reduced cardiovascular risk as well (24, 29). Tannic acid and other hydrolyzable tannins (gallotannins) are found abundantly in bark from wood such as oak and redwood, and their abundance in red wine is largely ascribed to the aging process in wood barrels. Our initial finding of strong inhibition of TMEM16A/CaCC by galloyl-containing analogs of tannic acid led to testing of green teas because of their abundance of chemically similar compounds. Green tea contains a number of different polyphenols with galloyl-containing catechins (EGCG, ECG, etc.) the most abundant class making up 15–30% of the total dry weight (30).
We found that all the red wines tested showed strong inhibition of CaCC Cl− current, but the white wines did not, which is consistent with the much higher concentrations of tannic acid and related gallotannins in red vs. white wines (10, 31) (Fig. 5A and Supplemental Fig. 2A). The variation in CaCC inhibition by different red wines is probably explained by differences in duration and conditions of aging, barrel type, grape variety, and other idiosyncrasies of the wine-making process (10, 32). Green and black teas were also tested (Supplemental Fig. 2B). Black teas were ∼4-fold less potent than green teas, which is likely explained by the lower concentration of gallotannins such as EGCG and ECG in black teas than in green teas (30, 31).
Previous studies have shown that red wine causes an endothelium-dependent vasorelaxation in blood vessels (32, 33). Tannic acid has been shown to produce a concentration-dependent inhibition of smooth muscle contraction, although no specific mechanisms have been determined (32). Interestingly, red wines produced by older, more traditional methods (en barrique) using oak barrels, such as those produced in southern France and Italy, have been shown to be more vasoactive and contain greater concentrations of tannic acid, consistent with our finding of more potent CaCC inhibition with wines from those regions. The role of CaCCs in vascular smooth muscle contraction has been suggested previously by the partial inhibition of noradrenaline-induced contraction in rat aorta by niflumic acid (34). Our results here show that red wine and green tea cause an endothelium-dependent vasorelaxation in aortic smooth muscle that replicates the pattern found in TMEM16A-transfected epithelia. As in epithelia we also found that tannic acid and the gallotannin EGCG produce dose-dependent vasorelaxation, providing further support for the involvement of smooth muscle CaCCs.
CaCC Cl− transport is thought to be involved in fluid secretion in airway and intestinal epithelia, although its relative contribution vs. CFTR Cl− channels has been uncertain. Excessive intestinal fluid secretion in rotaviral diarrhea is thought to involve CaCCs in enterocytes via elaboration of the viral enterotoxin NSP4 (35, 36). It has been reported that tannic acid and gallic acid derivatives have antidiarrheal activity in animals and humans (37). We found here that tannic acid reduced calcium-induced Cl− secretion in human intestinal cells and intact mouse intestine, providing a possible mechanism for the observed antidiarrheal properties of gallotannins (38). Tannic acid and related nonabsorbable gallotannins may thus have utility for therapy of certain secretory diarrheas.
The bioavailability of the polyphenolic constituents in tea and wine is an important determinant of the clinical relevance of gallotannin inhibition of CaCCs. Previous studies have shown that peak plasma and tissue levels of individual polyphenols reach sub- to low-micromolar concentrations (39, 40). We found here that 0.03% (a 3333-fold dilution) red wine, 0.0001% wine or grape tannin, and low-micromolar concentration of tannic acid and penta-1,2,3,4,6-O-galloyl-β-d-glucose are sufficient to inhibit CaCC Cl− current in cell cultures. The inhibitory concentrations were higher in intact mouse aorta and intestine, which is probably related to limited tissue access of these large polar molecules under the conditions of our experiments. Analysis of the tissue concentration of specific CaCC-inhibiting gallotannins is needed to further evaluate the role of CaCC inhibition in the various biological effects of red wine and green tea consumption.
In summary, we found that gallotannins are potent inhibitors of calcium-activated Cl− channels and suggest that inhibition of Cl− conductance by constituents in green tea and red wine may provide a molecular explanation for some of their reported health benefits. Our findings also provide a rational basis for the use of gallotannins or more potent synthetic derivatives as potential therapeutics in hypertension, diarrhea, cancer, and asthma.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grants HL73856, DK72517, DK86125, DK35124, EB00415, and EY13574, and Research Development Program and Drug Discovery grants from the Cystic Fibrosis Foundation.
REFERENCES
- 1.Hartzell H. C., Yu K., Xiao Q., Chien L. T., Qu Z. (2009) Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J. Physiol. 587, 2127– 2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkman A. S., Galietta L. J. (2009) Chloride channels as drug targets. Nat. Rev. Drug Discov. 8, 153– 171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggermont J. (2004) Calcium-activated chloride channels: (un) known, (un)loved? Proc. Am. Thorac. Soc. 1, 22– 27 [DOI] [PubMed] [Google Scholar]
- 4.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590– 594 [DOI] [PubMed] [Google Scholar]
- 5.Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y. (2008) Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019– 1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210– 1215 [DOI] [PubMed] [Google Scholar]
- 7.Rock J. R., Futtner C. R., Harfe B. D. (2008) The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev. Biol. 321, 141– 149 [DOI] [PubMed] [Google Scholar]
- 8.Namkung W., Song Y., Mills A. D., Padmawar P., Finkbeiner W. E., Verkman A. S. (2009) In situ measurement of airway surface liquid [K+] using a ratioable K+-sensitive fluorescent dye. J. Biol. Chem. 284, 15916– 15926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 110, 1651– 1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterhouse A. L. (2002) Wine phenolics. Ann. N. Y. Acad. Sci. 957, 21– 36 [DOI] [PubMed] [Google Scholar]
- 11.Crozier A., Jaganath I. B., Clifford M. N. (2009) Dietary phenolics: chemistry, bioavailability and effects on health. Nat. Prod. Rep. 26, 1001– 1043 [DOI] [PubMed] [Google Scholar]
- 12.Droogmans G., Callewaert G., Declerck I., Casteels R. (1991) ATP-induced Ca2+ release and Cl− current in cultured smooth muscle cells from pig aorta. J. Physiol. 440, 623– 634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson M. T., Conway M. A., Knot H. J., Brayden J. E. (1997) Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. J. Physiol. 502, 259– 264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayer B., Lu J., Green C., Soderholm J. D., Akhtar M., McKay D. M. (2002) Dextran sodium sulphate-induced colitis perturbs muscarinic cholinergic control of colonic epithelial ion transport. Br. J. Pharmacol. 135, 1794– 1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolfe V. E., Brand M. P., Heales S. J., Lindley K. J., Milla P. J. (1997) Tetrahydrobiopterin regulates cyclic GMP-dependent electrogenic Cl− secretion in mouse ileum in vitro. J. Physiol. 503, 347– 352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang F., Rock J. R., Harfe B. D., Cheng T., Huang X., Jan Y. N., Jan L. Y. (2009) Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl. Acad. Sci. U. S. A. 106, 21413– 21418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rock J. R., O'Neal W. K., Gabriel S. E., Randell S. H., Harfe B. D., Boucher R. C., Grubb B. R. (2009) Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J. Biol. Chem. 284, 14875– 14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ousingsawat J., Martins J. R., Schreiber R., Rock J. R., Harfe B. D., Kunzelmann K. (2009) Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J. Biol. Chem. 284, 28698– 28703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Pinilla P. J., Gibbons S. J., Bardsley M. R., Lorincz A., Pozo M. J., Pasricha P. J., Van de Rijn M., West R. B., Sarr M. G., Kendrick M. L., Cima R. R., Dozois E. J., Larson D. W., Ordog T., Farrugia G. (2009) Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1370– G1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galietta L. J. (2009) The TMEM16 protein family: a new class of chloride channels? Biophys. J. 97, 3047– 3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loewen M. E., Forsyth G. W. (2005) Structure and function of CLCA proteins. Physiol. Rev. 85, 1061– 1092 [DOI] [PubMed] [Google Scholar]
- 22.Kunzelmann K., Kongsuphol P., Aldehni F., Tian Y., Ousingsawat J., Warth R., Schreiber R. (2009) Bestrophin and TMEM16-Ca2+ activated Cl− channels with different functions. Cell Calcium 46, 233– 241 [DOI] [PubMed] [Google Scholar]
- 23.Scalbert A., Manach C., Morand C., Remesy C., Jimenez L. (2005) Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 45, 287– 306 [DOI] [PubMed] [Google Scholar]
- 24.Sumpio B. E., Cordova A. C., Berke-Schlessel D. W., Qin F., Chen Q. H. (2006) Green tea, the “Asian paradox,” and cardiovascular disease. J. Am. Coll. Surg. 202, 813– 825 [DOI] [PubMed] [Google Scholar]
- 25.Cordova A. C., Jackson L. S., Berke-Schlessel D. W., Sumpio B. E. (2005) The cardiovascular protective effect of red wine. J. Am. Coll. Surg. 200, 428– 439 [DOI] [PubMed] [Google Scholar]
- 26.Aviram M., Fuhrman B. (2002) Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann. N. Y. Acad. Sci. 957, 146– 161 [DOI] [PubMed] [Google Scholar]
- 27.Frankel E. N., Kanner J., German J. B., Parks E., Kinsella J. E. (1993) Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 341, 454– 457 [DOI] [PubMed] [Google Scholar]
- 28.Maxwell S., Cruickshank A., Thorpe G. (1994) Red wine and antioxidant activity in serum. Lancet 344, 193– 194 [DOI] [PubMed] [Google Scholar]
- 29.Moore R. J., Jackson K. G., Minihane A. M. (2009) Green tea (Camellia sinensis) catechins and vascular function. Br. J. Nutr. 102, 1790– 1802 [DOI] [PubMed] [Google Scholar]
- 30.Dufresne C. J., Farnworth E. R. (2001) A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 12, 404– 421 [DOI] [PubMed] [Google Scholar]
- 31.Actis-Goretta L., Ottaviani J. I., Fraga C. G. (2006) Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J. Agric. Food Chem. 54, 229– 234 [DOI] [PubMed] [Google Scholar]
- 32.Flesch M., Schwarz A., Bohm M. (1998) Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am. J. Physiol. 275, H1183– H1190 [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick D. F., Hirschfield S. L., Coffey R. G. (1993) Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 265, H774– H778 [DOI] [PubMed] [Google Scholar]
- 34.Criddle D. N., de Moura R. S., Greenwood I. A., Large W. A. (1996) Effect of niflumic acid on noradrenaline-induced contractions of the rat aorta. Br. J. Pharmacol. 118, 1065– 1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball J. M., Tian P., Zeng C. Q., Morris A. P., Estes M. K. (1996) Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272, 101– 104 [DOI] [PubMed] [Google Scholar]
- 36.Morris A. P., Scott J. K., Ball J. M., Zeng C. Q., O'Neal W. K., Estes M. K. (1999) NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am. J. Physiol. 277, G431– G444 [DOI] [PubMed] [Google Scholar]
- 37.Powell D. W., Field M. (1980) Pharmacological approaches to treatment of secretory diarrhea. In Secretory Diarrhea ( Field M., Fordtran J.S., Schultz S.G., eds) pp. 187– 209, American Physiological Society, Bethesda, MD, USA [Google Scholar]
- 38.Palombo E. A. (2006) Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother. Res. 20, 717– 724 [DOI] [PubMed] [Google Scholar]
- 39.Yang C. S., Sang S., Lambert J. D., Lee M. J. (2008) Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 52, S139– S151 [DOI] [PubMed] [Google Scholar]
- 40.Scalbert A., Williamson G. (2000) Dietary intake and bioavailability of polyphenols. J. Nutr. 130, 2073S– 2085S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.