Abstract
The transcription factor TonEBP/OREBP promotes cell survival during osmotic stress. High NaCl-induced phosphorylation of TonEBP/OREBP at tyrosine-143 was known to be an important factor in increasing its activity in cell culture. We now find that TonEBP/OREBP also is phosphorylated at tyrosine-143 in rat renal inner medulla, dependent on the interstitial osmolality. c-Abl seemed likely to be the kinase that phosphorylates TonEBP/OREBP because Y143 is in a consensus c-Abl phosphorylation site. We now confirm that, as follows. High NaCl increases c-Abl activity. Specific inhibition of c-Abl by imatinib, siRNA, or c-Abl kinase dead drastically reduces high NaCl-induced TonEBP/OREBP activity by reducing its nuclear location and transactivating activity. c-Abl associates with TonEBP/OREBP (coimmunoprecipitation) and phosphorylates TonEBP/OREBP-Y143 both in cell and in vitro. High NaCl-induced activation of ataxia telangiectasia mutated, previously known to contribute to activation of TonEBP/OREBP, depends on c-Abl activity. Thus, c-Abl is the kinase responsible for high NaCl-induced phosphorylation of TonEBP/OREBP-Y143, which contributes to its increased activity.—Gallazzini, M., Yu, M.-J., Gunaratne, R., Burg, M. B., Ferraris, J. D. c-Abl mediates high NaCl-induced phosphorylation and activation of the transcription factor TonEBP/OREBP.
Keywords: hypertonicity, kinase, NFAT5, signaling
High NaCl activates TonEBP/OREBP (tonicity enhancer binding protein/osmotic response element binding protein; also called NFAT5), which is a Rel-family transcription factor that is central to osmoprotection (1, 2). The increase in transcription of its target genes causes accumulation of osmoprotective organic osmolytes and heat shock proteins (reviewed in ref. 3). NaCl concentration is high in renal medullary interstitial fluid, which energizes urinary concentration. TonEBP/OREBP protects renal medullary cells from the high NaCl.
High NaCl concentration rapidly increases phosphorylation of TonEBP/OREBP (4), which contributes to its high NaCl-induced activation (5). Numerous kinases were previously discovered to be involved in high NaCl-induced activation of TonEBP/OREBP, including protein kinase A (PKA), ataxia telangiectasia mutated (ATM), phosphatidylinositol 3- kinase class IA (PI3K-IA), Fyn, and p38 (reviewed in ref. 3). However, direct evidence had been lacking as to which particular amino acids in TonEBP/OREBP are phosphorylated, until we recently discovered that high NaCl induces phosphorylation of TonEBP/OREBP at tyrosine-143, making it a binding site for phospholipase C-γ1 (PLC-γ1) (6). PLC-γ1 and phosphorylation at Y143 are both necessary for high NaCl-induced increase of TonEBP/OREBP nuclear localization, transcriptional activity, and transactivating activity (6). The present studies were aimed at identifying the high NaCl-activated kinase responsible for phosphorylating TonEBP/OREBP-Y143.
TonEBP/OREBP-Y143 is a predicted c-Abl phosphorylation site (Minimotif Miner; http://mnm.engr.uconn.edu/MNM/SMSSearchServlet). c-Abl kinase preferentially phosphorylates peptides with the consensus YXXP, where Y is tyrosine, X is any amino acid, and P is proline (7). The corresponding sequence in TonEBP/OREBP is HTVLY(143)ISPPPEDLLDNSR. With this in mind, we conducted the following studies, which show that high NaCl concentration activates c-Abl, leading to phosphorylation of TonEBP/OREBP-Y143, and thus contributing to increased TonEBP/OREBP nuclear localization, transcriptional activity, and transactivating activity.
MATERIALS AND METHODS
Animal studies
Pathogen-free male Sprague-Dawley rats (Taconic Farms, Germantown, NY, USA) or homozygous Brattleboro rats (Harlan Sprague-Dawley, Indianapolis, IN, USA) weighing 150–200 g were used in these studies. All experiments were conducted in accord with animal protocol H-0110R1 approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. The urine osmolality was measured before and after dDAVP/vehicle treatment using a vapor pressure osmometer (Vapro 5520; Wescor, Logan, UT, USA).
Immunofluorescence microscopy of kidney sections
Brattleboro rats were injected intramuscularly with 2 nmol of dDAVP (Bachem, Ludger, Germany) or vehicle (as control), and their kidneys were harvested 1 h later. Tissue sections from kidneys were prepared and immunostained, as described previously (8). In brief, rats were anesthetized and surgically prepared for retrograde perfusion of the kidneys via the abdominal aorta. The kidneys were perfused first with PBS for 10 s to wash out the blood, followed by ice-cold 4% paraformaldehyde for 5 min. The fixed kidneys were trimmed to expose cortex and inner medulla, embedded in paraffin, and sectioned (4 μm) for immunofluorescence study. Primary antibodies were anti-TonEBP/OREBP and anti-TonEBP/OREBP-phospho-Y143 (6). The secondary antibodies were conjugated with Alexa 488 fluorophore (Invitrogen, Carlsbad, CA, USA). For negative controls, some tissue slices were processed as described above except that omission of the primary antibody yielded no detectable staining. For visualization purposes the green signal from Alexa 488 fluorophore was transformed in red for images with anti-TonEBP/OREBP-phospho-Y143 antibody.
Western blot analysis of renal inner medulla
Homogenates were prepared from the inner medullas, in Laemmli buffer (1.5% SDS, 10 mM Tris, pH 6.8), containing a protease/phosphatase inhibitor cocktail (Pierce, Rockland, IL, USA). Following determination of total protein concentration (BCA; Pierce), samples were heated to 60°C for 15 min, then stored in 6% glycerol plus 40 mM DTT. Primary antibodies were anti-TonEBP/OREBP and anti-TonEBP/OREBP-phospho-Y143 (6). The secondary antibodies were conjugated with Alexa 488 fluorophore (Invitrogen). Nuclei were counterstained with DAPI (10 μl of 1 mg/ml DAPI stock solution diluted in 10 ml PBS).
Immunofluorescence studies of HeLa cells
The cells were fixed for immunofluorescence with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 PBS, then blocked with 1% BSA. Primary antibody was anti-TonEBP/ORERBP. The secondary was conjugated to Alexa 488 fluorophore (Invitrogen).
Confocal microscopy
Images were acquired using a Zeiss LSM 510 META microscope with a ×63, NA = 1.4, oil-immersion objective for kidney sections, and ×40, NA = 1.3, oil-immersion objective for HeLa cells (Carl Zeiss MicroImaging, Thornwood, NY, USA).
Cell culture and treatment
HEK293 cells were grown in Eagle’s modified essential medium (EMEM; American Type Culture Collection, Manassas, VA, USA) supplemented with 10% FBS (HyClone Laboratories, Logan, UT, USA) as described previously (9). AT cells (GM09607 B), in which ATM protein is inactive (Coriell Cell Repositories, Camden, NJ, USA), were maintained according to the supplier’s instructions. HEK293 Tet on cells stably transfected with c-Abl wild type (WT) or kinase dead were kindly provided by Dr. Zhi-Min Yuan (Harvard School of Public Health, Boston, MA, USA). We grew the Tet-on HEK293 cells in Dulbecco’s modified Eagle medium (DMEM; American Type Culture Collection), supplemented with 10% Tet System Approved FBS (BD Biosciences, San Jose, CA), adding 2.0 μg/ml doxycycline for induction (Sigma-Aldrich, St. Louis, MO, USA). Osmolality of the control medium was 300 mosmol/kg, and at experiment-specific time points, medium was replaced with one at 300 mosmol/kg, 200 mosmol/kg (NaCl added to NaCl-free medium; Biofluids, Rockville, MD, USA), or 500 mosmol/kg (NaCl added). The c-Abl inhibitor imatinib mesylate was from LC Laboratories (Woburn, MA, USA), the ATM/ATR inhibitor (2,2-diphenyl-N-{2,2,2-trichloro-1-[3-(4-fluoro-3-nitro-phenyl)-thioureido]- ethyl}-acetamide) was from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). They were added to medium bathing HEK293 cells 60 min before changing the osmolality. All cells were cultured at 37°C in 5% CO2 and were studied while subconfluent. Anti-TonEBP/OREBP, anti-BRG1, anti-aldose reductase, anti-p-Tyr, and anti-ATM antibodies were purchased from Santa Cruz Biotechnologies and anti-p-S1981-ATM from Rockland (Gilbertsville, PA, USA). Anti-V5 was from AbD Serotec (Raleigh, NC, USA). Anti-phospho-Y245-c-Abl and anti-phospho-Y412-c-Abl antibodies were from Sigma-Aldrich. Anti-c-Abl was from Calbiochem (San Diego, CA, USA). The rabbit anti-TonEBP/OREBP-Phospho-Y143 was described previously (6).
Plasmids and siRNA
c-Abl-FLAG WT or kinase dead (KD) dominant negative were kindly provided by Dr. Zhi-Min Yuan (Harvard School of Public Health, Boston, MA, USA). The pcDNAcAbl-1q2q3q (c-Ablqqq-WT), pcDNA-cAbl-1q2q3q-K290M (c-Ablqqq-KD) (10) constructs were a generous gift from Dr. Martin Playford (U.S. National Institutes of Health, Bethesda, MD, USA). The ORE-X luciferase reporter construct contains 2 copies of human ORE-X (11) within a minimal IL-2 promoter (12) (hTonE-GL3, a gift from Dr. S. N. Ho, University of California at San Diego, La Jolla, CA, USA). WT and S1981A mutant Flag-ATM (13) were gifts of Dr. M. B. Kastan (St. Jude Children’s Research Hospital, Memphis, TN, USA). Constructs containing TonEBP/OREBP aa 1-1531 were described previously (9). Mutants Y143A and Y143D of TonEBP-1-1531-V5 were prepared by site-directed mutagenesis (Quik Change; Stratagene, La Jolla, CA, USA). The binary GAL4/TAD reporter system was previously described (5). The control and c-Abl siRNA were from Applied Biosystems/Ambion (Austin, TX, USA; Silencer Select Negative Control #1 siRNA AM4620 and c-Abl Silencer siRNA S864).
Transfection and luciferase assays
Cells were transfected with Lipofectamine 2000 (Invitrogen), according to the supplier’s instructions. For assay of the transcriptional activity of native TonEBP/OREBP, cells were transfected with ORE-X or promoter only (control) reporters (9). For assay of TonEBP/OREBP transactivating activity, cells were cotransfected with the GAL4 reporter (pFR-Luc) and GAL4dbd-548–1531, which contains the recombinant TonEBP/OREBP transactivation domain (5). For knockdown by siRNA, cells were transfected with 20 nM siRNA.
Immunoprecipitation
After washing, cells were collected in buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, protease inhibitor cocktail tablet (Roche, Indianapolis, IN, USA), and phosphatase cocktail inhibitor I and II (Sigma-Aldrich). Cell extracts were precleared with mouse IgG plus Protein A/G-PLUS Agarose (Santa Cruz Biotechnologies) at 4°C for 1 h and then incubated with mouse anti-V5 antibody or anti-c-Abl antibody plus Protein A/G-PLUS Agarose at 4°C overnight. The agarose beads were washed gently once with the lysis buffer. Proteins were eluted with the SDS loading buffer, boiled for 5 min, then subjected to Western blot analysis, or kept at 4°C for kinase assay.
c-Abl kinase assay
Beads containing c-Abl immunoprecipitated from cell extracts were suspended in kinase buffer (Cell Signaling Technology, Danvers, MA, USA), then incubated with TonEBP-Y143 peptide (138-RHTVLYISPPPED-150) (1 μg) or GST-CRKII (1 μg) in assay buffer supplemented with 200μM ATP (Cell Signaling Technology) at 30°C for 30 min. Supernatants were analyzed by dot-blot for phosphorylated TonEBP-Y143 peptide and both dot-blot and Western blot for phosphorylated-CRKII-GST. The relative amounts of c-Abl present on the beads were measured by Western blot analysis after decanting the supernatant.
Nuclear to cytoplasmic ratio
Nuclear to cytoplasmic ratio was determined as described previously (14). Cytoplasmic and nuclear proteins were extracted separately by using NE-PER (Pierce). Specific proteins in each fraction were measured by Western blot analysis. The relative amounts of TonEBP/OREBP or c-Abl in the cytoplasmic and nuclear fractions and the nuclear/cytoplasmic ratio were calculated from the relative concentrations of the respective protein in each cytoplasmic or nuclear extract and the relative volumes of the extracts. BRG1 and aldose reductase were used as nuclear and cytoplasmic markers, respectively.
Western blot analysis
Western blot analysis was performed as described previously (6). Briefly, total protein concentration was measured in cell extracts by BCA assay (Pierce), and proteins were separated on 4–12% gradient Novex Bis-Tris or Tris-Acetate gels and transferred electrophoretically to nitrocellulose membranes (Invitrogen). Nonspecific binding was blocked by incubating membranes for 1 h at 4°C with Odyssey Blocking Buffer (Li-COR Biosciences, Lincoln, NE, USA). Membranes then were incubated with various primary antibodies overnight at 4°C. After washing with 0.1% Tween-20 in PBS, blots were incubated with Alexa Fluor 680 goat anti-rabbit IgG or Alexa Fluor 780 goat anti-mouse IgG (Molecular Probes, Carlsbad, CA, USA). Blots were visualized and quantitated using an Odyssey Infrared Imager (Li-COR Biosciences).
Statistical analysis
Data were compared by ANOVA, followed by the Student-Newman-Keuls posttest. Normalized data were transformed prior to ANOVA. Results are expressed as means ± se (n=number of independent experiments). Differences were considered significant for P < 0.05.
RESULTS
High osmolality increases phosphorylation of TonEBP/OREBP on Y143 in the renal inner medulla in vivo
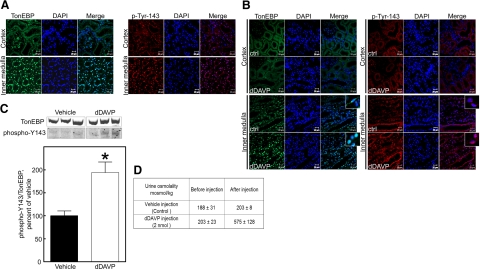
We previously observed that high NaCl concentration increases TonEBP/OREBP phosphorylation on Y143 in cell cultures (6), implying that the normally high NaCl concentration in the renal inner medulla might do so also. To test that directly in the present studies, we measured phosphorylation of TonEBP/OREBP on Y143 by immunocytochemistry in rat renal inner medullas and cortex, using a specific anti-TonEBP/OREBP-phospho-Y143 antibody (6). TonEBP/OREBP is normally localized in nuclei of cells in the renal inner medulla because of the high interstitial NaCl concentration (15). In agreement, we find it mainly in the nuclei of cells in rat renal inner medullas, in contrast to a diffuse localization in the renal cortex cells (Fig. 1A), where NaCl is not high. Further, in the nuclei, TonEBP/OREBP is phosphorylated on Y143.
Figure 1.
TonEBP/OREBP is phosphorylated on Y143 in the nuclei of rat renal inner medullary cells in vivo. A) Immunocytochemistry of normal rat kidney, using antibodies against TonEBP/OREBP and TonEBP/OREBP-phospho-Y143. Nuclei are marked by DAPI staining. Expression of TonEBP/OREBP in the inner medulla is mainly in nuclei, whereas it is more diffuse in the cortex, and TonEBP/OREBP in inner medullary nuclei is phosphorylated on Y143 (representative of 3 experiments). B) Administration of 2 nmol of dDAVP to Brattleboro rats for 1 h increases nuclear localization of TonEBP/OREBP and its phosphorylation on Y143 in nuclei of cells in the renal inner medulla, but not in the cortex (representative of 3 experiments). C) Administration of dDAVP to the Brattleboro rats increases phosphorylation of TonEBP/OREBP at Y143 in the renal inner medulla (Western blot analysis, using anti-TonEBP/OREBP and anti-TonEBP/OREBP-phospho-Y143 antibodies, n=3) *P < 0.05. D) Increase of urine osmolality after injection of the dDAVP in Brattleboro rats. Values are means ± se, n = 3.
Brattleboro rats, being deficient in vasopressin, do not concentrate their urine and do not maintain a high renal medullary interstitial NaCl concentration (16). However, osmolality of their urine and inner medullary interstitial fluid increases rapidly when vasopressin is injected. Accordingly, we find that in renal inner medullary cells of untreated Brattleboro rats TonEBP/OREBP is distributed between cytoplasm and nucleus and is not heavily phosphorylated on Y143 (Fig. 1B, Ctrl). In contrast, within 1 h after vasopressin (dDAVP) is injected, TonEBP/OREBP moves into the nuclei, where it is phosphorylated on Y143 (Fig. 1B). Phosphorylation of TonEBP/OREBP on Y143 increases significantly after injection of dDAVP (Fig. 1C), accompanying the increase of urine osmolality (Fig. 1D). dDAVP does not increase nuclear localization or phosphorylation of TonEBP/OREBP in the renal cortex of the Brattleboro rats (Fig. 1B). Thus, high NaCl increases phosphorylation of TonEBP/OREBP on Y143 in the renal inner medulla in vivo.
High NaCl increases c-Abl tyrosine kinase activity
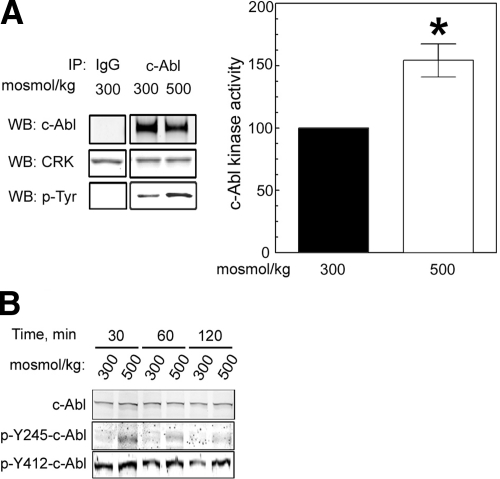
Given that TonEBP/OREBP-Y143 is located within a consensus c-Abl phosphorylation site, we used cell cultures to investigate the possible role of c-Abl in high NaCl-induced phosphorylation at that site and activation of TonEBP/OREBP. HEK293 cells were exposed to medium kept at 300 mosmol/kg or increased to 500 mosmol/kg by adding NaCl. Protein extracts were immunoprecipitated either with IgG (control) or with anti-c-Abl antibody. The immunoprecipitates were then incubated with a c-Abl-specific substrate, GST-CRKII, and phosphorylation of GST-CRKII was measured by Western blot analysis (using a phosphospecific antibody) to determine c-Abl kinase activity. High NaCl increases c-Abl activity within 1 h (Fig. 2A). c-Abl is stimulated to full catalytic activity by sequential phosphorylation at Tyr-412 and Tyr-245 (17). High NaCl increases phosphorylation at both sites within 30 min (Fig. 2B and Supplemental Fig. 1). The phosphorylation decreases with time and is already much reduced within 1 h after NaCl is increased (Supplemental Fig. 1). We conclude that high NaCl increases c-Abl kinase activity.
Figure 2.
High NaCl increases c-Abl kinase activity. A) Osmolality bathing HEK293 cells was increased to 500 mosmol/kg (NaCl added) for 1 h or kept at 300 mosmol/kg. Proteins were immunoprecipitated using anti-c-Abl antibody (IP c-Abl) or IgG (IP IgG, as control). Kinase activity of the immunoprecipitates was determined from phosphorylation of the GST-CRKII (CRK) substrate, measured with anti-phospho-tyrosine antibody (p-Tyr). Left panel: representative Western blot of immunoprecipitated c-Abl, of GST-CRKII, and of tyrosine phosphorylation of CRKII (p-Tyr). Right panel: relative kinase activity (mean±se, n=3). *P < 0.05. B) Osmolality bathing HEK293 cells was increased to 500 mosmol/kg (NaCl added) or kept at 300 mosmol/kg for the times shown, and protein extracts were assayed by Western blot analysis for c-Abl-phospho-Tyr-245 (p-Y245, required for c-Abl kinase activity) and phospho-Tyr-412 c-Abl (p-Y412, required for c-Abl kinase activation). Western blot is representative of 3 experiments (see Supplemental Fig. 1).
c-Abl contributes to high NaCl-induced phosphorylation of TonEBP/OREBP at tyrosine-143
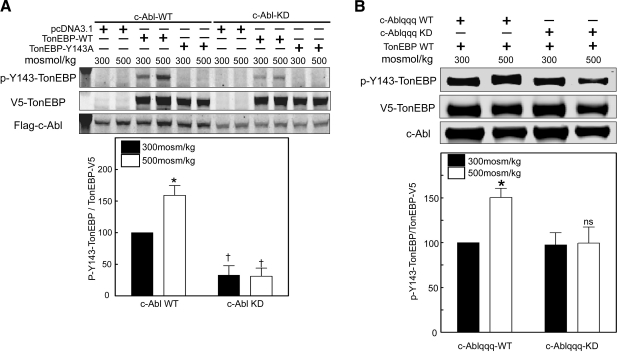
In the context of its surrounding amino acids, Y143 is a likely site for phosphorylation catalyzed by c-Abl. To test directly whether c-Abl contributes to TonEBP/OREBP-Y143 phosphorylation, we transfected HEK293 cells with either c-Abl WT or c-Abl KD. When c-Abl WT is overexpressed, TonEBP/OREBP-Y143 is phosphorylated, and high NaCl increases the phosphorylation (Fig. 3A). In contrast, c-Abl KD overexpression greatly reduces phosphorylation of Y143 at 300 and 500 mosmol/kg and eliminates the high NaCl-induced increase in phosphorylation. TonEBP/OREBP-Y143A is not phosphorylated.
Figure 3.
Role of c-Abl kinase in high NaCl-induced phosphorylation of TonEBP/OREBP on Y143. A) c-Abl kinase activity is necessary for high NaCl-induced phosphorylation of TonEBP/OREBP-Y143. HEK293 cells were transiently transfected with empty vector (pcDNA3.1), WT TonEBP/OREBP-V5 (TonEBP-WT), or Y143A-TonEBP mutant (TonEBP-Y143A) and either c-Abl-WT or c-Abl-KD for 48 h, then osmolality was increased to 500 mosmol/kg (NaCl added) for 1 h or kept at 300 mosmol/kg. Top panel: representative Western blot analysis of phosphorylation of TonEBP/OREBP-Y143. Bottom panel: effect of c-Abl-KD on phosphorylation of TonEBP/OREBP at Y143. B) HEK293 cells were transiently transfected with TonEBP-WT and either WT c-Abl with a mutated NLS (c-Ablqqq-WT) or c-Abl with a mutated NLS KD dominant negative (c-Ablqqq-KD) for 48 h, then osmolality was increased to 500 mosmol/kg (NaCl added) for 1 h or kept at 300 mosmol/kg. Top panel: representative Western blot analysis of phosphorylation of TonEBP/OREBP-Y143. Bottom panel: effect of c-Ablqqq-KD on phosphorylation of TonEBP/OREBP at Y143. Values are means± se, n = 3.*P < 0.05 vs. 300 mosmol/kg; †P < 0.05 vs. WT.
To determine where in the cell c-Abl phosphorylates Y143, we transfected HEK293 cells with c-Abl constructs mutated in their nuclear localization signals (c-Abl-qqq), so they remain cytoplasmic. Overexpression of c-Ablqqq-KD abolishes the high NaCl-induced phosphorylation of Y143 in contrast to c-Ablqqq-WT, which does not (Fig. 3B). We conclude that c-Abl phosphorylates TonEBP/OREBP on Y143 mainly in the cytoplasm.
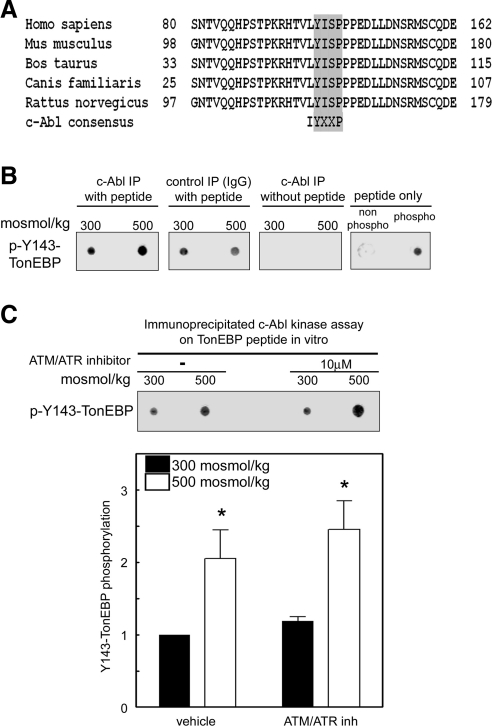
Furthermore, immunoprecipitated c-Abl increases phosphorylation of the TonEBP/OREBP-Y143 peptide in vitro, and this c-Abl kinase activity is greater in immunoprecipitates from cells exposed to high NaCl (Fig. 4B). Because ATM is reported to activate c-Abl kinase (18), and high NaCl activates ATM (9), we tested whether ATM is responsible for the high NaCl-induced activation of c-Abl and subsequent phosphorylation of Y143 (Fig. 4C) by adding ATM/ATR inhibitor (IC50 0.2 μM). Inhibition of ATM and ATR does not reduce the high NaCl-induced increase of phosphorylation of TonEBP/OREBP at Y143, showing that the phosphorylation does not depend on ATM, as might also be surmised from the fact that it occurs in the cytoplasm, whereas ATM is mainly nuclear.
Figure 4.
c-Abl directly phosphorylates TonEBP-Y143. A) The c-Abl consensus phosphorylation site in TonEBP/OREBP is conserved in various mammalian species. B) High NaCl increases c-Abl kinase activity. Medium bathing HEK293 cells was increased to 500 mosmol/kg (NaCl added) or kept at 300 mosmol/kg for 1 h, then cell extracts were immunoprecipitated with IgG (control) or anti-c-Abl antibody. Immunoprecipitated proteins were incubated with a TonEBP/OREBP peptide containing the Y143 c-Abl consensus. Dot blots of the reaction mixture were probed with anti-phospho-Y143-TonEBP antibody to measure the c-Abl kinase activity. Nonphospho-TonEBP and phospho-Y143-TonEBP peptide are controls for the specificity of the antibodies. C) c-Abl activation by high NaCl is ATM and ATR independent. Medium bathing HEK293 cells was increased to 500 mosmol/kg or kept at 300 mosmol/kg for 1 h, then cell extracts were immunoprecipitated with anti-c-Abl antibody. Immunoprecipitated proteins were incubated with a TonEBP/OREBP peptide containing the Y143 c-Abl consensus. Top panel: representative dot-blot from one experiment. Bottom panel: summary of 3 experiments. Values are means ± se, n = 3. *P < 0.05 vs. 300 mosmol/kg.
Contribution of c-Abl to high NaCl-induced nuclear localization of TonEBP/OREBP
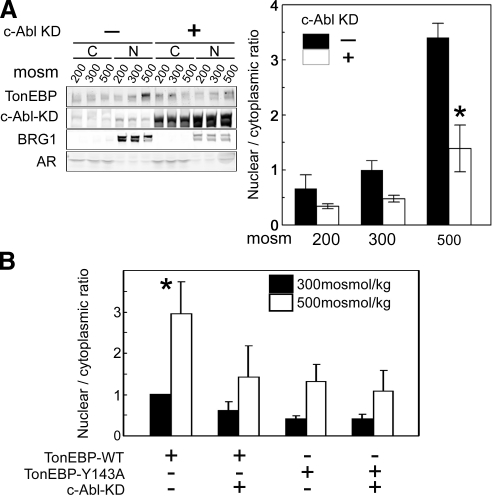
High NaCl increases the nuclear localization of native TonEBP/OREBP (Fig. 5A). We previously observed that mutation of Y143 to alanine, which prevents its phosphorylation, reduces high NaCl-induced nuclear localization of TonEBP/OREBP (6). We now find that inhibition of c-Abl kinase activity by conditional expression of c-Abl KD greatly reduces the high NaCl-induced nuclear localization of TonEBP/OREBP (Fig. 5A). High NaCl increases the nuclear to cytoplasmic ratio of recombinant mutant TonEBP/OREBP-Y143A-V5 less than that of WT TonEBP/OREBP-V5 (6; Fig. 5B), and expression of c-Abl KD reduces it to a similar extent, but the two effects are not additive. Inhibition of c-Abl kinase activity with 10 μM imatinib (IC50 0.5 μM) also reduces high NaCl-induced nuclear localization of TonEBP/OREBP (Fig. 6). In contrast, phospho-mimetic mutation to aspartate (Y143D) of TonEBP/OREBP does not inhibit it (Supplemental Fig. 2). We conclude that c-Abl contributes to high NaCl-induced nuclear localization of TonEBP/OREBP by phosphorylating TonEBP/OREBP on Y143.
Figure 5.
%c-Abl contributes to the high NaCl-induced nuclear localization of TonEBP/OREBP. A) Dominant negative inhibition of c-Abl kinase activity reduces high NaCl-induced nuclear localization of TonEBP/OREBP. HEK293 cells stably transfected with doxycycline-inducible c-Abl-KD dominant negative construct were incubated for 24 h with either 2 |gmg/ml of doxycycline or none. Then, osmolality was either maintained at 300 mosmol/kg or changed for 2 h to 200 or 500 mosmol/kg (NaCl varied). Western blot analysis was performed on proteins extracted from cytoplasm and nucleus, using antibodies against TonEBP/OREBP (TonEBP), c-Abl-KD, BRG1 (nuclear marker), or aldose reductase (AR, cytoplasmic marker). Left panel: representative Western blot. Right panel: summary data. B) Dominant negative inhibition of c-Abl kinase activity does not further decrease the already reduced nuclear localization of TonEBP/OREBP with Y143 mutated to alanine (TonEBP-Y143A). HEK293 cells stably transfected with doxycycline inducible c-Abl kinase dead (KD) dominant negative were transiently transfected with either TonEBP-WT or TonEBP-Y143A. One day later, the cells were exposed for 24 h with medium supplemented with 2 |gmg/ml of doxycycline or none. Then osmolality was either maintained at 300 mosmol/kg or increased to 500 mosmol/kg (NaCl added) for 2 h. Values are means ± se, n = 3. *P < 0.05.
Figure 6.
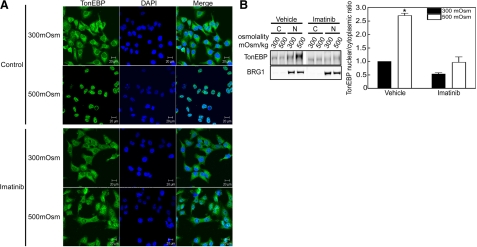
The c-Abl kinase inhibitor imatinib prevents high NaCl-induced nuclear localization of TonEBP/OREBP. A) HeLa cells were preincubated for 1 h at 300 mosmol/kg medium with vehicle or with 20 |gmM imatinib, then osmolality was increased to 500 mosmol/kg (NaCl added) for 1 h or kept at 300 mosmol/kg in the continued presence of imatinib or vehicle. Cells were fixed and stained with anti-TonEBP/OREBP antibody. At 500 mosmol/kg TonEBP/OREBP moves into the nuclei under control (vehicle) conditions, but not in the presence of imatinib. B) As in panel A, except that TonEBP/OREBP was measured by Western blot analysis in nuclear and cytoplasmic extracts from HEK293 cells for calculation of the nuclear to cytoplasmic ratio. Left panel: representative Western blot with antibodies against TonEBP/OREBP or BRG1 (nuclear marker). Right panel: summary data. Values are means ± se, n = 3. *P < 0.05 vs. 300 mosmol/kg; †P < 0.05 vs. vehicle control.
Association of c-Abl with TonEBP/OREBP
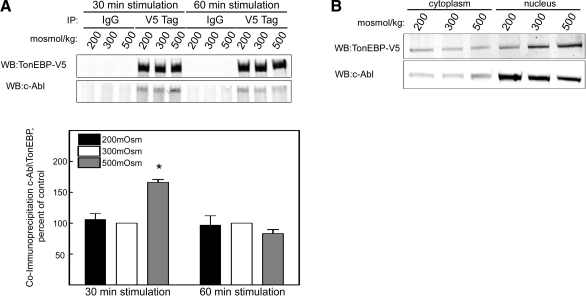
To test for direct interaction between c-Abl and TonEBP/OREBP, we immunoprecipitated recombinant TonEBP/OREBP-V5 from protein extracts of stably transfected HEK293 cells exposed for various times to different concentrations of NaCl. c-Abl coimmunoprecipitates with TonEBP-V5 (Fig. 7A). At 30 min after the level of NaCl is changed, the coimmunoprecipitation is greater at 500 mosmol/kg. However, the difference is gone by 60 min (Fig. 7A). Consistent with this time course, by 60 min TonEBP/OREBP has translocated to the nucleus (Fig. 5A), but the subcellular distribution of c-Abl is unchanged (Supplemental Fig. 3). Note that within 2 h after NaCl is elevated, the relative amount of c-Abl in the nucleus decreases (Fig. 7B and Supplemental Fig. 3).
Figure 7.
c-Abl coimmunoprecipitates with TonEBP/ OREBP, and high NaCl transiently increases the coimmunoprecipitation. A) HEK293 cells were transiently transfected with TonEBP/OREBP-V5 for 48 h, then osmolality was either maintained at 300 mosmol/kg or changed for 30 or 60 min to 200 or 500 mosmol/kg (NaCl varied). Immunoprecipitation was performed on whole-cell extracts, using IgG (control) or anti-V5 antibody, and TonEBP/OREBP-V5 and c-Abl were measured in the immunoprecipitates by Western blot analysis. Top panel: representative Western blot. Bottom panel: quantification of the coimmunoprecipitation. Values are means ± se, n = 3. *P < 0.05 vs. 300 mosmol/kg. B) Nuclear localization of c-Abl varies inversely with NaCl concentration. Osmolality bathing HEK293 cells was either maintained at 300 mosmol/kg or changed for 2 h to 200 or 500 mosmol/kg (NaCl varied). Representative Western blot of proteins extracted from cytoplasm and nucleus.
c-Abl contributes to high NaCl-induced increase of the transactivating activity of TonEBP/OREBP
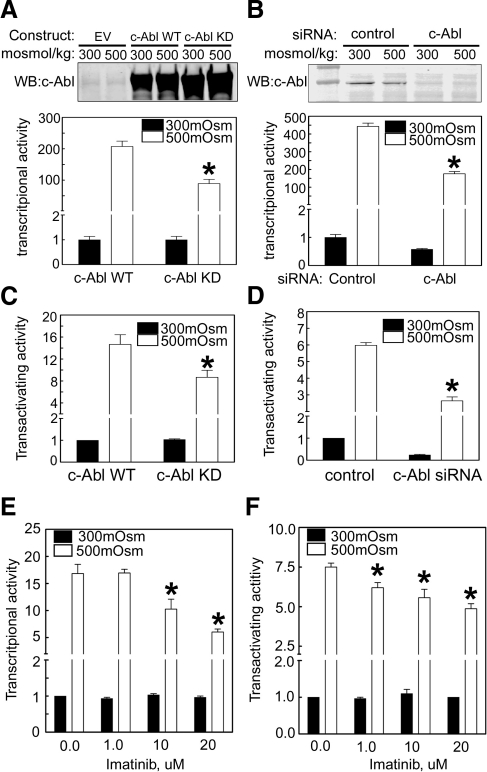
High NaCl can increase TonEBP/OREBP transcriptional activity bimodally. It increases TonEBP/OREBP nuclear accumulation (2), which should increase its transcriptional activity, and it increases its transactivating activity (5). Having established that c-Abl is necessary for high NaCl-induced TonEBP/OREBP nuclear accumulation, we first measured TonEBP/OREBP transcriptional activity in HEK293 cells stably transfected with a luciferase reporter whose transcription is driven by a TonEBP/OREBP-binding DNA element (ORE/TonE). Elevating osmolality from 300 to 500 mosmol/kg greatly increases TonEBP/OREBP transcriptional activity (Fig. 8A, B, E). The increase is reduced if c-Abl kinase activity is inhibited by either overexpression of c-Abl kinase dead (Fig. 8A), by c-Abl specific siRNA (Fig. 8B), or by adding imatinib to the medium (dose-dependent effect from 1 to 20 μM) (Fig. 8E). We conclude that c-Abl contributes to the high NaCl-induced increase of TonEBP/OREBP transcriptional activity. However, the c-Abl contribution to TonEBP/OREBP transcription might not be due exclusively to an increase in TonEBP/OREBP nuclear localization. Therefore, we also measured TonEBP/OREBP transactivating activity. High NaCl concentration greatly increases TonEBP/OREBP transactivating activity (Fig. 8C, D). The increase is reduced if c-Abl kinase activity is inhibited by overexpression of c-Abl KD (Fig. 8C) or c-Abl siRNA (Fig. 8D), or by adding imatinib to the medium (dose-dependent effect from 1 to 20 μM) (Fig. 8F). We conclude that c-Abl contributes to the high NaCl-induced increase of TonEBP/OREBP transactivating activity.
Figure 8.
%c-Abl contributes to the high NaCl-induced increases of TonEBP/OREBP transcriptional and transactivating activities. A, B) Inhibiting c-Abl kinase activity or knocking it down with a specific siRNA reduces the high NaCl-induced increase of TonEBP/OREBP transcriptional activity. HEK293 cells stably transfected with the ORE-X luciferase reporter of TonEBP/OREBP transcriptional activity were transiently transfected with c-Abl WT or c-Abl KD constructs (A) or with control or c-Abl specific siRNA (B). Top panels: representative Western blot analysis of c-Abl expression. Bottom panels: summary data. C, D) Inhibiting c-Abl kinase activity or knocking it down with a specific siRNA reduces the high NaCl-induced increase of TonEBP/OREBP transactivating activity. As in panels A, B, except that HEK293 cells stably transfected with a dual luciferase reporter of TonEBP/OREBP transactivating activity were used. E, F) Imatinib, a specific inhibitor of c-Abl kinase activity, reduces high NaCl-induced increase of TonEBP/OREBP transcriptional and transactivating activities. As in panels A–D, except that cells were treated with imatinib or vehicle control. Values are means ± se, n = 3. *P < 0.05 vs. control.
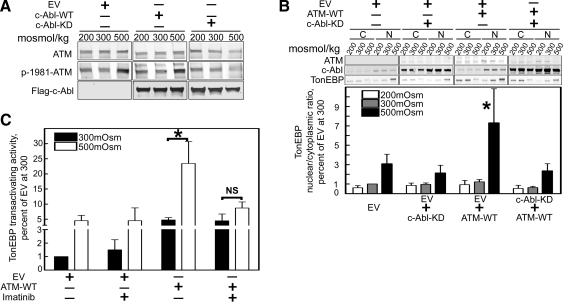
High NaCl-induced activation of ATM depends on c-Abl activity
That c-Abl contributes to both TonEBP/OREBP nuclear accumulation and transactivating activity raises a question. Indeed, c-Abl phosphorylates TonEBP/OREBP at amino acid Y143, but the transactivation reporter construct contains only TonEBP/OREBP amino acids 548-1531, where the high NaCl-dependent transactivation domain is located (5). We are unaware of a putative c-Abl consensus phosphorylation site among these amino acids. So it is unlikely that the c-Abl contribution to transactivating activity is due to a direct phosphorylation by c-Abl. We previously found that high NaCl activates ATM kinase, which contributes to the high NaCl-induced increases of the transactivating activity (9) and nuclear localization (19) of TonEBP/OREBP. Ionizing radiation activates both ATM and c-Abl, and the activation of c-Abl depends on ATM (20). With this in mind, we tested whether high NaCl-induced activation of c-Abl requires ATM. Surprisingly, we found that high NaCl-induced activation of c-Abl does not require ATM activity, but the activation of ATM requires c-Abl activity. Using phosphorylation of c-Abl on Y412 as an index of its activity (17), we find that high NaCl increases c-Abl activity in AT cells, which lack ATM activity (Supplemental Fig. 4). On the other hand we find, using phosphorylation of ATM-S1981 as an index of ATM activity (13), that inhibition of c-Abl kinase activity reduces ATM activation by high NaCl (Fig. 9A). ATM and c-Abl apparently act in the same pathway since high NaCl-induced nuclear localization of TonEBP/OREBP is greatly enhanced in AT cells when WT ATM (ATM-WT) is transfected, but this increase is eliminated by c-Abl dominant negative (Fig. 9B). Finally, the ATM-dependent high NaCl-induced increase in TonEBP/OREBP transactivating activity is prevented by imatinib (Fig. 9C). We conclude that high NaCl activates c-Abl, which in turn activates ATM, leading to activation of TonEBP/OREBP.
Figure 9.
High NaCl-induced increase of ATM activity and its effects on TonEBP/OREBP depend on c-Abl kinase. A) High NaCl-induced increase of ATM activity depends on c-Abl kinase. HEK293 cells were transiently transfected with empty vector (EV), c-Abl-WT, or c-Abl-KD for 48 h, then osmolality was either maintained at 300 mosmol/kg or changed for 60 min to 200 or 500 mosmol/kg (NaCl varied). Extracted proteins were assayed by Western blot for ATM activity by phosphorylation of serine 1981 (p-1981-ATM). Representative Western blot. B) Enhancement of high NaCl-induced nuclear localization of TonEBP/OREBP by ATM depends on c-Abl kinase. AT cells (deficient in ATM) were transiently transfected with empty vector (EV), WT ATM (ATM-WT), or c-Abl-KD for 48 h. Then TonEBP/OREBP protein was measured by Western blot analysis in extracts from cytoplasm and nucleus, and TonEBP/OREBP nuclear to cytoplasmic ratio was calculated. Top panel: representative Western blot. Bottom panel: quantification of the nuclear to cytoplasmic ratio. C) Inhibition of c-Abl kinase activity prevents the enhancement of high NaCl-induced transactivating activity of TonEBP/OREBP by ATM. AT cells (deficient in ATM) were transiently transfected with EV or ATM-WT, and with the dual luciferase reporter system that measures TonEBP/OREBP transactivating activity. After 48 h, the cells were preincubated for 30 min at 300 mosmol/kg medium with vehicle or with 20 |gmM imatinib, then osmolality was increased to 500 mosmol/kg (NaCl added) or kept at 300 mosmol/kg in the continued presence of imatinib or vehicle. Values are means ± se, n = 3. *P < 0.05 vs. vehicle.
DISCUSSION
Abl protein tyrosine kinases have been extensively studied and reviewed (7, 21, 22) because of their many important roles, including those in cancer and stress-induced apoptosis. c-Abl (ABL1) tyrosine kinase was originally identified as the normal cellular homologue of the constitutively active v-Abl oncogene product of Abelson murine leukemia virus. Another constitutively active form, BRC-ABL, causes human leukemia. In contrast to the constitutive activity of the oncogenic forms of Abl, the activity of normal c-Abl is tightly restricted in unstimulated cells. The differences between the tight restriction of normal c-Abl kinase activity and the elevated constitutive activity of the oncogenic forms are complex and not completely understood. The stress of ionizing radiation and other DNA damaging agents activates normal c-Abl (23). Our present study adds to understanding of the role of c-Abl in response to stress by revealing that high NaCl also activates c-Abl, which in turn phosphorylates and contributes to the activation of the osmoprotective transcription factor, TonEBP/OREBP.
c-Abl is activated by ionizing radiation (IR) and other genotoxic stresses (22), as well as by high NaCl (Fig. 2). IR increases phosphorylation of c-Abl at Ser-465, which increases its activity (20). The phosphorylation of c-Abl at Ser-465 is catalyzed by ATM kinase. IR does not activate c-Abl in AT cells (derived from patients who lack functional ATM) or in thymocytes or fibroblasts from ATM-deficient mice (18, 20). Ectopic expression of ATM corrects this defect. Further, IR and ATM kinase do not activate c-Abl in which Ser-465 is mutated to alanine. ATM binds c-Abl constitutively by interaction of the SH3 domain of c-Abl with a DPAPNPPHFP motif (residues 1373–1382) of ATM (18). These findings identified ATM as an upstream activator of the c-Abl tyrosine kinase in the cellular response to IR. Similar to IR, high NaCl also activates ATM (9). However, unlike IR, the high NaCl-induced increase of c-Abl activity does not require ATM because it occurs in AT cells (Supplemental Fig. 4) and is not prevented by inhibition of ATM (Fig. 4C). Rather, the opposite obtains because c-Abl is necessary for high NaCl-induced activation of ATM (Fig. 9A), not vice versa. We previously found that high NaCl-induced increase of ATM activity contributes to the activation of TonEBP/OREBP (9, 19), by increasing its nuclear localization and transactivating activity, as does c-Abl (Figs. 5, 6, 8). Thus, the contribution of c-Abl to high NaCl-induced activation of TonEBP/OREBP apparently is mediated by ATM.
Mediation by ATM helps explain an otherwise puzzling aspect of the role of c-Abl in high NaCl-induced increase of transactivating activity of TonEBP/OREBP. The dual luciferase reporter used to measure the transactivating activity contains the transactivation domain (TAD) within aa 548–1531 of TonEBP/OREBP (5), but does not contain the c-Abl phosphorylation site at Y143. Thus, the c-Abl catalyzed phosphorylation at Y143 should not directly affect the transactivating activity as measured with this reporter. ATM, on the other hand, contributes to the high NaCl-induced increase of TonEBP/OREBP transactivating activity (9). TonEBP/OREBP contains ATM consensus sites within its TAD, and mutation of TonEBP/OREBP-S1247 or -S1367 to alanine reduces its transcriptional activity, but only when ATM is active (9). We propose that the contribution of c-Abl to the high NaCl-induced increase of transactivating activity of TonEBP/OREBP is mediated through a direct effect of ATM on TonEBP/OREBP, but we do not yet know how c-Abl activates ATM.
High NaCl-induced activation of ATM requires at least one signal in addition to that from c-Abl, namely, from PI3K-IA. High NaCl activates PI3K-IA, and, if PI3K-IA is inhibited, the activation of ATM by either IR or high NaCl is reduced (24). High NaCl-induced increase in TonEBP/OREBP activity is reduced equally by inhibition of ATM or PI3K-IA, and the effects are not additive (24). Thus, PI3K-IA activity is necessary for both high NaCl- and IR-induced activation of ATM, and high NaCl activates PI3K-IA, which, in turn, contributes to the activation of TonEBP/OREBP via ATM.
It is important to note that, although IR and high NaCl both activate ATM, PI3K-IA, and c-Abl, IR does not activate TonEBP/OREBP (9). Evidently, additional effects of high NaCl besides activation of ATM, PI3K-IA, and c-Abl are necessary to activate TonEBP/OREBP. Numerous other proteins are known to contribute to high NaCl-induced activation of TonEBP/OREBP, including p38 (1, 25), Fyn (1), PARP (26), AP1 (27), and PKA (28).
Activity of c-Abl’s kinase domain is normally autoinhibited through intramolecular and intermolecular interactions. Activation is associated with phosphorylation at several tyrosines in c-Abl, including Y245 and Y412 (21). Phosphorylation of these and other tyrosines occurs either through autophosphorylation by the c-Abl kinase in trans- or phosphorylation by Src family kinases. In agreement, high NaCl-induced activation of c-Abl is accompanied by increased phosphorylation of c-Abl-Y245 and -Y412 (Fig. 2B and Supplemental Fig. 4).
c-Abl coimmunoprecipitates with TonEBP/OREBP (Fig. 6A), suggesting that they are physically associated in the cells, but we do not yet know what sites in the proteins are involved. c-Abl can bind to its substrates via its SH3 or SH2 domains (22). TonEBP/OREBP contains proline-rich regions that could act as binding sites for c-Abl SH3 domains. Alternatively (or in addition), SH2 domains in tyrosine protein kinases bind to tyrosine-phosphorylated residues.
When TonEBP/OREBP-Y143 becomes phosphorylated following an increase in NaCl, it becomes a binding site for PLC-γ1 (6). PLC-γ1 contributes to the high NaCl-induced increase in TonEBP/OREBP transcriptional activity by two mechanisms. High NaCl increases lipase activity of PLC-γ1, which contributes to the nuclear localization of TonEBP/OREBP. PLC-γ1 also contributes to the high NaCl-induced increase of TonEBP/OREBP transactivating activity, but this effect is independent of the lipase activity of PLC-γ1. A possible lipase-independent pathway is through increased PI3K-IA activity (24). PLC-γ1 acts as a GEF for the GTPase PIKE, which activates PI3K-IA (29, 30). A possible additional interaction between PLC-γ1 and c-Abl is suggested by the report that PLC-γ1 is required for c-Abl activation by platelet-derived growth factor. In that system, PLC-γ1 decreases the cellular level of phosphatidylinositol-4,5-bisphosphate by hydrolyzing it. Since phosphatidylinositol-4,5-bisphosphate inhibits c-Abl, lowering its abundance increases c-Abl kinase activity (31).
For a potential model, we suggest that high NaCl coordinately activates c-Abl, PLC-γ1, PI3K, and ATM, which in combination contribute to activation of TonEBP/OREBP; that c-Abl, while in the cytoplasm, phosphorylates TonEBP/OREBP on tyrosine 143; that PLC-γ1 binds via its C-SH2 domain to the TonEBP/OREBP at phospho-Y143; that the 2 proteins move into the nucleus, in a PLC-γ1 lipase-dependent manner, where they remain associated at the OREs/TonEs of TonEBP/OREBP target genes; that, while in the nucleus, PLC-γ1, through its SH3 domain and in a lipase-independent manner, acts as a GEF for the GTPase PIKE, which, in turn, activates PI3K; that PLC-γ1 and PI3K enhance the activation of c-Abl by decreasing the abundance of PIP2, an inhibitor of c-Abl; that c-Abl and PI3K together stimulate the activation of ATM, which contributes to nuclear localization of TonEBP/OREBP; and that activated ATM increases the transactivating activity of TonEBP/OREBP, likely through phosphorylation of TonEBP/OREBP S1247 and S1367.
Supplementary Material
Acknowledgments
The authors thank Dr. Martin Playford for suggestions on experimental design and Dr. Chris Combs and Dr. Daniela Malide of the National Heart, Lung, and Blood Institute (NHLBI) Light Microscopy Core Facility for help with microscopy.
This research was supported by the Intramural Research Program of NHLBI.
REFERENCES
- 1.Ko B. C., Lam A. K., Kapus A., Fan L., Chung S. K., Chung S. S. (2002) Fyn and p38 signaling are both required for maximal hypertonic activation of the OREBP/TonEBP. J. Biol. Chem. 277, 46085– 46092 [DOI] [PubMed] [Google Scholar]
- 2.Miyakawa H., Woo S. K., Dahl S. C., Handler J. S., Kwon H. M. (1999) Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. U. S. A. 96, 2538– 2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007) Cellular response to hyperosmotic stresses. Physiol. Rev. 87, 1441– 1474 [DOI] [PubMed] [Google Scholar]
- 4.Dahl S. C., Handler J. S., Kwon H. M. (2001) Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am. J. Physiol. Cell. Physiol. 280, C248– C253 [DOI] [PubMed] [Google Scholar]
- 5.Ferraris J. D., Williams C. K., Persaud P., Zhang Z., Chen Y., Burg M. B. (2002) Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc. Natl. Acad. Sci. U. S. A. 99, 739– 744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irarrazabal C. E., Gallazzini M., Schnetz M. P., Kunin M., Simons B. L., Williams C. K., Burg M. B., Ferraris J. D. (2010) PLC-γ1 contributes to signaling high NaCl-dependent activation of the osmoprotective transcription factor TonEBP/OREBP. Proc. Natl. Acad. Sci. U. S. A. 107, 906– 911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pendergast A. M. (2002) The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res. 85, 51– 100 [DOI] [PubMed] [Google Scholar]
- 8.Yu M. J., Pisitkun T., Wang G., Shen R. F., Knepper M. A. (2006) LC-MS/MS analysis of apical and basolateral plasma membranes of rat renal collecting duct cells. Mol. Cell. Proteomics 5, 2131– 2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irarrazabal C. E., Liu J. C., Burg M. B., Ferraris J. D. (2004) ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc. Natl. Acad. Sci. U. S. A. 101, 8809– 8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen S. T., Jackson P. K., Van Etten R. A. (1996) The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 15, 1583– 1595 [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraris J. D., Williams C. K., Jung K. Y., Bedford J. J., Burg M. B., Garcia-Perez A. (1996) ORE, a eukaryotic minimal essential osmotic response element: the aldose reductase gene in hyperosmotic stress. J. Biol. Chem. 271, 18318– 18321 [DOI] [PubMed] [Google Scholar]
- 12.Trama J., Lu Q., Hawley R. G., Ho S. N. (2000) The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J. Immunol. 165, 4884– 4894 [DOI] [PubMed] [Google Scholar]
- 13.Bakkenist C. J., Kastan M. B. (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499– 506 [DOI] [PubMed] [Google Scholar]
- 14.Ferraris J. D., Burg M. B. (2007) Tonicity-regulated gene expression. Methods Enzymol. 428, 279– 296 [DOI] [PubMed] [Google Scholar]
- 15.Cha J. H., Woo S. K., Han K. H., Kim Y. H., Handler J. S., Kim J., Kwon H. M. (2001) Hydration status affects nuclear distribution of transcription factor tonicity responsive enhancer binding protein in rat kidney. J. Am. Soc. Nephrol. 12, 2221– 2230 [DOI] [PubMed] [Google Scholar]
- 16.Valtin H., Schroeder H. A., Benirschke K., Sokol H. W. (1962) Familial hypothalamic diabetes insipidus in rats. Nature 196, 1109– 1110 [DOI] [PubMed] [Google Scholar]
- 17.Brasher B. B., Van Etten R. A. (2000) c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J. Biol. Chem. 275, 35631– 35637 [DOI] [PubMed] [Google Scholar]
- 18.Shafman T., Khanna K. K., Kedar P., Spring K., Kozlov S., Yen T., Hobson K., Gatei M., Zhang N., Watters D., Egerton M., Shiloh Y., Kharbanda S., Kufe D., Lavin M. F. (1997) Interaction between ATM protein and c-Abl in response to DNA damage. Nature 387, 520– 523 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z., Ferraris J., Irarrazabal C. E., Dmitireva N. I., Park J. H., Burg M. B. (2005) Ataxia-telangiectasia mutated (ATM), a DNA damage-inducible kinase, contributes to high NaCl-induced nuclear localization of the transcription factor TonEBP/OREBP. Am. J. Physiol. Renal Physiol. 289, F506–F511 [DOI] [PubMed] [Google Scholar]
- 20.Baskaran R., Wood L. D., Whitaker L. L., Canman C. E., Morgan S. E., Xu Y., Barlow C., Baltimore D., Wynshaw-Boris A., Kastan M. B., Wang J. Y. (1997) Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387, 516– 519 [DOI] [PubMed] [Google Scholar]
- 21.Gu J. J., Ryu J. R., Pendergast A. M. (2009) Abl tyrosine kinases in T-cell signaling. Immunol. Rev. 228, 170– 183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith J. M., Mayer B. J. (2002) Abl: mechanisms of regulation and activation. Front. Biosci. 7, d31–d42 [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda S., Ren R., Pandey P., Shafman T. D., Feller S. M., Weichselbaum R. R., Kufe D. W. (1995) Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature 376, 785– 788 [DOI] [PubMed] [Google Scholar]
- 24.Irarrazabal C. E., Burg M. B., Ward S. G., Ferraris J. D. (2006) Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: role in osmoprotective transcriptional regulation. Proc. Natl. Acad. Sci. U. S. A. 103, 8882– 8887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikh-Hamad D., Di Mari J., Suki W. N., Safirstein R., Watts B. A., III, Rouse D. (1998) p38 kinase activity is essential for osmotic induction of mRNAs for HSP70 and transporter for organic solute betaine in Madin-Darby canine kidney cells. J. Biol. Chem. 273, 1832– 1837 [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Schnetz M. P., Irarrazabal C. E., Shen R. F., Williams C. K., Burg M. B., Ferraris J. D. (2007) Proteomic identification of proteins associated with the osmoregulatory transcription factor TonEBP/OREBP: functional effects of Hsp90 and PARP-1. Am. J. Physiol. Renal Physiol. 292, F981–F992 [DOI] [PubMed] [Google Scholar]
- 27.Irarrazabal C. E., Williams C. K., Ely M. A., Birrer M. J., Garcia-Perez A., Burg M. B., Ferraris J. D. (2008) Activator protein-1 contributes to high NaCl-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J. Biol. Chem. 283, 2554– 2563 [DOI] [PubMed] [Google Scholar]
- 28.Ferraris J. D., Persaud P., Williams C. K., Chen Y., Burg M. B. (2002) cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/osmotic response element-binding protein. Proc. Natl. Acad. Sci. U. S. A. 99, 16800– 16805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye K., Hurt K. J., Wu F. Y., Fang M., Luo H. R., Hong J. J., Blackshaw S., Ferris C. D., Snyder S. H. (2000) Pike: a nuclear GTPase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 103, 919– 930 [DOI] [PubMed] [Google Scholar]
- 30.Ye K., Aghdasi B., Luo H. R., Moriarity J. L., Wu F. Y., Hong J. J., Hurt K. J., Bae S. S., Suh P. G., Snyder S. H. (2002) Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415, 541– 544 [DOI] [PubMed] [Google Scholar]
- 31.Plattner R., Irvin B. J., Guo S., Blackburn K., Kazlauskas A., Abraham R. T., York J. D., Pendergast A. M. (2003) A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-gamma1. Nat. Cell Biol. 5, 309– 319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.