The shed form of LRP1/CD91, which is generated at increased levels in inflammation, regulates cell-signaling and cytokine expression by macrophages.
Keywords: LDL receptor-related protein-1, LPS, IFN-γ, ADAM, MCP-1/CCL2, TNF-α, rheumatoid arthritis, systemic lupus erythematosus
Abstract
LRP1 is a type-1 transmembrane receptor that mediates the endocytosis of diverse ligands. LRP1 β-chain proteolysis results in release of sLRP1 that is present in human plasma. In this study, we show that LPS and IFN-γ induce shedding of LRP1 from RAW 264.7 cells and BMMs in vitro. ADAM17 was principally responsible for the increase in LRP1 shedding. sLRP1 was also increased in vivo in mouse plasma following injection of LPS and in plasma from human patients with RA or SLE. sLRP1, which was purified from human plasma, and full-length LRP1, purified from mouse liver, activated cell signaling when added to cultures of RAW 264.7 cells and BMMs. Robust activation of p38 MAPK and JNK was observed. The IKK-NF-κB pathway was transiently activated. Proteins that bind to the ligand-binding clusters in LRP1 failed to inhibit sLRP1-initiated cell signaling, however an antibody that targets the sLRP1 N terminus was effective. sLRP1 induced expression of regulatory cytokines by RAW 264.7 cells, including TNF-α, MCP-1/CCL2, and IL-10. These results demonstrate that sLRP1 is generated in inflammation and may regulate inflammation by its effects on macrophage physiology.
Introduction
Inflammation is a beneficial, adaptive response when triggered by infection or tissue injury [1]. However, dysregulated or excessive inflammation may contribute to disease pathogenesis in arthritis, cardiovascular disease, cancer, and a host of autoimmune conditions [2]. Identification of novel pro- and anti-inflammatory factors and pathways remains an important challenge for addressing these disease states.
LRP1 is a member of the LDLR gene family, which mediates the endocytosis of more than 40 structurally and functionally distinct ligands, including proteases and growth factors implicated in inflammation [3]. LRP1 also regulates the cell surface abundance of other membrane proteins, some of which have cell signaling activity [4, 5]. LRP1 is synthesized as a 600-kDa single-chain precursor and processed by a furin-like protease into the mature, 2-chain form [6]. The extracellular 515-kDa α-chain includes the binding sites for ligands [3]. The 85-kDa β-chain includes an ectodomain that associates with the α-chain, a single transmembrane domain, and the intracytoplasmic tail.
In addition to membrane-anchored LRP1, sLRP1 has been detected in human plasma, brain, and cerebral spinal fluid [7, 8]. Structurally, sLRP1 consists of the entire LRP1 α-chain and part of the β-chain ectodomain [8]. Shedding occurs near the plasma membrane, as the result of β-chain cleavage by the α-secretases, ADAM10 and ADAM17, or β-site amyloid precursor protein-cleaving enzyme 1/β-secretase [7, 9]. At least 1 activity attributed to sLRP1 is its ability to bind β-amyloid peptide and limit its transport into the brain [10]. sLRP1 also binds tPA and activated α2M, reflecting the intact, ligand-binding clusters in the LRP1 α-chain [11].
Membrane-anchored LRP1 has emerged as a suppressor of inflammation in a number of model systems. Overton et al. [12] demonstrated that in a mouse model of atherosclerosis, LRP1 is protective based on its ability to suppress expression of inflammatory mediators, including MCP-1/CCL2, TNF-α, and MMP-9 by macrophages in atheroma [12]. Decreased inflammatory mediator production may reflect the ability of membrane-anchored LRP1 to down-regulate cell surface TNFR1 and cell signaling through the IKK-NF-κB pathway [5]. A distinct pathway by which LRP1 suppresses inflammation requires ectodomain shedding, followed by γ-secretase cleavage and release of the LRP1 β-chain intracellular domaines, which is transported to the nucleus and promotes export of IFN regulatory factor-3 [13]. In the peripheral nervous system, sLRP1 binds directly to Schwann cell surfaces, inhibiting the cellular response to TNF-α [14].
In this study, we demonstrate that LRP1 is shed at increased levels by macrophages in vitro in response to inflammatory mediators. Increased levels of sLRP1 are also observed in mouse plasma after LPS treatment. Patients with RA or SLE have significantly increased levels of circulating sLRP1, suggesting that LRP1 shedding may be increased in inflammation in humans as well. ADAM17 was responsible for the generation of sLRP1 from macrophages in vitro in response to inflammatory mediators. Purified sLRP1 was biologically active, triggering cell signaling and inducing expression of regulatory cytokines by macrophages. Thus, sLRP1 may be generated in inflammation and functional as a regulator of inflammation.
MATERIALS AND METHODS
Reagents and proteins
α2M was purified from human plasma by the method of Imber and Pizzo [15]. Primers and probes for mouse TNF-α, IL-10, CCL2, and GAPDH were purchased from Applied Biosystems (Foster City, CA, USA). LPS from Escherichia coli 0111:B4, PMB, anti-LRP1 antibody, and antitubulin antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). mAb 11H4, which recognizes the 85-kDa LRP1 β-chain, was purified from hybridoma cells, available from ATCC (Manassas, VA, USA). mAb 8G1, which recognizes the 515-kDa human LRP1 α-chain [16], was purified from hybridoma cells, available from ATCC. Polyclonal LRP1 α-chain-specific antibody was from Sigma-Aldrich (Catalog #L2295). ON-TARGET plus SMART pool siRNAs targeting ADAM9, ADAM10, and ADAM17 were purchased from Thermo Scientific Dharmacon (Lafayette, CO, USA). JNK inhibitor II (420119) and p38 inhibitor III (506121) were from EMD Biosciences (San Diego, CA, USA). GST-RAP was prepared and purified as described previously [17]. GST-specific antibody coupled to HRP was from GE Healthcare (Waukesha, WI, USA). Anti-JNK, anti-p38, anti-phospho-p38, anti-phospho-JNK, and anti-IκBα were from Cell Signaling Technology (Beverly, MA, USA).
Purification of sLRP1 and mouse LRP1
sLRP1 was purified as described by Gaultier et al. [14]. In brief, fresh, frozen human plasma was supplemented with protease inhibitors, dialyzed against 50 mM Tris-HCl, 150 mM NaCl, pH 7.5, with 1 mM CaCl2 for 12 h at 4°C, and subjected to affinity chromatography on a matrix consisting of GST-RAP coupled to N-hydroxysuccinimide-activated Sepharose 4 Fast Flow (GE Healthcare). RAP-associated proteins were eluted in 0.1 M sodium acetate, 0.5 M NaCl, pH 4, and neutralized by rapid mixing with 50 mM Tris-HCl, pH 8. sLRP1 was purified further by molecular exclusion chromatography on Ultrogel AcA 34 (Sigma-Aldrich). Each sLRP1 preparation was assessed for integrity and purity by SDS-PAGE with Coomassie staining and by immunoblot analysis with antibody 8G1.
To purify intact LRP1, mouse liver was homogenized in 50-mM Tris-HCl, 150 mM NaCl, pH 7.5, with 1 mM CaCl2, Triton X-100 1%, and protease inhibitors. Homogenized liver was subjected to centrifugation at 13,000 g for 15 min at 4°C, filtered through a 0.22-μm filter, and then subjected to RAP-affinity chromatography, as described above. Purified preparations of mouse LRP1 were assessed by SDS-PAGE and Coomassie staining and by immunoblot analysis with antibody 11H4.
Cell culture
RAW 264.7 macrophage-like cells were cultured in RPMI 1640, supplemented with 10 mM L-glutamine, 10% FBS, and penicillin/streptomycin. Silencing of LRP1 in RAW 264.7 cells was accomplished using the pSUPER vector system (Oligoengine, Seattle, WA, USA), which expresses shRNA directed against LRP1 [18]. RAW 264.7 cells were transfected with this construct or with empty vector using the Nucleofector system from Amaxa (Lonza, Switzerland). Transfected cells were selected with puromycin (1 μg/mL). LRP1 gene-silenced cells were treated with Pseudomonas exotoxin A for 48 h (250 ng/ml) to eliminate any residual LRP1-positive cells [19].
BMMs were obtained from femurs of 12-week-old male C57BL/6 mice and from mice in which LRP1 was conditionally deleted in macrophages [12]. The cells were pelleted at 500 g and plated in DMEM/F12, 10 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 100 U/ml rM-CSF. The cultures were maintained in the same medium for 7 days before re-plating for experiments.
Patient plasma samples
Blood was obtained from patients with RA, OA, SLE, or FM at the University of California San Diego Rheumatology Clinic (San Diego, CA, USA). The protocol was approved by the Human Research Protection Program of University of California San Diego. Samples were processed to yield plasma and stored at –80°C until assayed. Median age and ranges for each of the diagnostic groups are as follows: FM, median 47.5, range 38–67 years; OA, median 69, range 43–90 years; RA, median 49.5, range 25–65 years; SLE, median 42, range 23–76 years.
ELISA-based detection of sLRP1 in human plasma
High-binding polystyrene 96-well plates (Corning Inc., Corning, NY, USA) were coated with 0.5 μg/well GST-RAP overnight. The efficiency of GST-RAP binding to the polystyrene and a saturation binding curve was predetermined. Nonspecific binding sites were blocked by subsequent coating with 5% BSA (w/v) for 1 h. A standard curve was generated by incubating different concentrations of sLRP1 (1–500 ng) in each well for 1 h at 4°C. Wells were washed with 20 mM Tris-HCl, 150 mM NaCl, pH 7.4, and 0.1% Tween-20 and incubated with LRP1-specific antibody 8G1 for 1 h at 4°C. Wells were washed again, incubated with secondary HRP-linked antibody for 1 h at 4°C, washed, and developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid). The lower limit of quantification was 20 ng sLRP-1/well. The standard curve was linear (r2>0.95) in the range 20–500 ng sLRP1. Human plasma samples (15 μl) were diluted into buffer for analysis by ELISA.
ELISA-based detection of CRP in human plasma
CRP was detected in human plasma samples using the CRP ELISA kit (Cat. #DCRP00; R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. Samples were subjected to serial dilution using the provided buffer.
qPCR
DNA-free total RNA was extracted from the RAW 264.7 cells using Trizol, as directed by the manufacturer (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). qPCR was performed using a System 7300 instrument (Applied Biosystems) and a 1-step program: 95°C, 10 min; 95°C, 30 s; 60°C, 1 min for 40 cycles. Hypoxanthine phosphoribosyl transferase gene expression was measured as a normalizer for each sample. Results were analyzed by the relative quantity (Ct) method. All experiments were performed in triplicate with internal triplicate determinations.
Immunoblot analysis and RAP ligand blotting
Cells were extracted in RIPA buffer, supplemented with protease inhibitors and protein phosphatase inhibitors. Equal amounts of cellular protein were subjected to SDS-PAGE and electrotransferred to PVDF membranes (Bio-Rad), which were blocked with 5% nonfat dry milk in Tris buffer (pH 7.4). Purified primary antibodies and HRP-conjugated secondary antibodies (GE Healthcare) were added in sequence. For RAP ligand-blotting studies, equal volumes of CM were subjected SDS-PAGE and electrotransferred to membranes. Blocked membranes were incubated with GST-RAP (0.1 μM) for 1 h at 20°C and then with GST-specific antibody coupled to HRP. Detection was performed using Western Lightning HRP chemiluminescence (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA) and Kodak Biomaxlight Films (Rochester, NY, USA).
Detection of sLRP1 in mouse plasma
All experiments were conducted according to guidelines approved by the University of California San Diego Institutional Animal Care and Usage Committee. LPS (10 mg/kg) or normal saline was injected i.p. in 12-week-old male C57BL/6 mice, which were observed closely for changes in neurological status, respiratory rate, or hemostasis abnormalities. According to our approved protocol, mice entering a moribund condition or demonstrating any of the conditions listed above were euthanized immediately; however, this was not necessary in this study. Blood samples were recovered by cardiac puncture under terminal anesthesia, 1, 3, and 6 h after LPS injection. Plasma was isolated and incubated with 1 μM GST-RAP overnight. The GST-RAP and associated proteins were recovered with glutathione-Sepharose and subjected to SDS-PAGE. RAP ligand blotting was performed. A single band at ∼500 kDa was observed together with mouse IgG. The sLRP1 band at 500 kDa was assessed by densitometry.
Determination of cell surface LRP1
Cell surface LRP1 was determined by measuring specific binding of the LRP1-specific ligand, α2M, which was converted into the LRP1-recognized conformation by reaction with 200 mM methylamine HCl [20]. Specific binding was measured as described previously [21]. In brief, the α2M was radioiodinated using Iodobeads (specific activity 2–3 μCi/μg). Methylamine-activated 125I-α2M was incubated in confluent cultures of RAW 264.7 cells in the presence and absence of a 100-fold molar excess of unlabeled, activated α2M. Specific radioligand binding was determined by the fraction that was displaced by nonlabeled α2M.
Statistical analysis
In cell culture experiments, replicates always refer to separate experiments typically performed with internal duplicates. Animal model experiments were performed using coded animal numbers to avoid observer bias. Data from qPCR studies and densitometry were subjected to one-way ANOVA. Tukey’s post hoc analysis was used to assess differences between treatment groups.
RESULTS
LPS and IFN-γ induce LRP1 shedding in macrophages
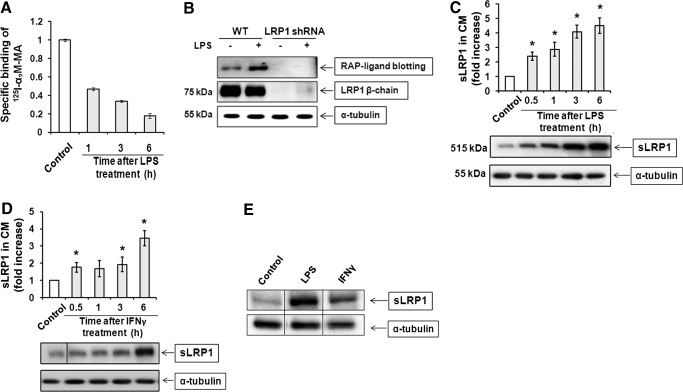
LPS and IFN-γ decrease the cell surface level of LRP1 [22]. Although this effect has been attributed to decreased LRP1 mRNA transcription [23], we hypothesized that LRP1 shedding may contribute as well. To test this hypothesis, RAW 264.7 macrophage-like cells were treated with LPS (10 ng/ml) for up to 6 h. Cell surface LRP1 was determined by measuring specific binding of the LRP1 ligand α2M at 4°C. The α2M was converted into the receptor-recognized conformation by reaction with methylamine [20]. Specific α2M binding decreased progressively with time and by 6 h after introducing LPS, was decreased by 82 ± 3% (Fig. 1A). Next, sLRP1 in CM was determined by RAP ligand blotting, as described previously [14]. RAP binds with high affinity to the ligand-binding clusters in the 515-kDa LRP1 α-chain, which are intact in sLRP1 [14]. As RAP may bind to related receptors in the LDLR family, as a control, we examined CM from RAW 264.7 cells in which LRP1 was constitutively silenced with shRNA. The cultures were treated with LPS or vehicle for 1 h. Fig. 1B shows that a band at ∼500 kDa was detected in CM from LRP1-expressing cells but not cells in which LRP1 was silenced. Cell extracts were subjected to immunoblotting with LRP1 β-chain-specific antibody 11H4 to confirm that LRP1 gene silencing was effective. We also probed for α-tubulin as a control for load. When RAW 264.7 cells were cultured in the absence of inflammatory mediators, sLRP1 in the CM remained low over a 6-h time course. By contrast, when cells were treated with LPS, sLRP1 accumulated progressively in CM (Fig. 1C). By 0.5 h, the sLRP1 level was already increased significantly compared with control cells, which were cultured in the absence of LPS for 6 h (P<0.05). Fig. 1D shows that sLRP1 was also increased in CM by IFN-γ. By 6 h, IFN-γ increased shedding 3.5 ± 0.5-fold.
Figure 1. LPS and IFN-γ promote LRP1 shedding.
(A) RAW 264.7 cells were cultured in SFM and then treated with 10 ng/ml LPS for 1–6 h. Cell surface LRP1 was determined by measuring specific binding of methylamine-activated α2M (α2M-MA) at 4°C. (B) RAW 264.7 cells (WT) and cells in which LRP1 was silenced with shRNA were treated with 10 ng/ml LPS in SFM for 1 h or with vehicle. CM was recovered and subjected to RAP ligand blotting to detect sLRP1. The absence of sLRP1 in CM from cells in which LRP1 was silenced confirmed that our RAP ligand-blotting method is detecting sLRP1 specifically. (C) RAW 264.7 cells were cultured in SFM for 1 h and then challenged with 10 ng/ml LPS in SFM for the indicated times. sLRP1 in the medium was determined by RAP ligand blotting (mean±sem; n=3). For each well, cell extracts were prepared and subjected to immunoblot analysis to detect α-tubulin as a control for the number of cells in each well. (D) RAW 264.7 cells were cultured in SFM for 1 h and then treated with IFN-γ (25 ng/ml) in SFM. sLRP1 levels were determined in CM by RAP ligand blotting. Cell extracts were immunoblotted to detect α-tubulin. (C and D) The relative levels of sLRP1 were determined by comparison with CM from control cells that were incubated in the absence of stimulant for 6 h. *P<0.05. (E) Primary cultures of BMMs were treated with LPS (10 ng/ ml) or IFN-γ (25 ng/ml) for 3 h. sLRP1 was determined in CM. α-Tubulin was determined in cell extracts. The presented study is representative of 3 separate replicates.
To confirm the ability of LPS and IFN-γ to promote LRP1 shedding from macrophage cell surfaces, we isolated BMMs from C57BL/6 mice. Cultured BMMs were treated with LPS, IFN-γ, or vehicle for 6 h. Fig. 1E shows that sLRP1 was increased in BMM CM by LPS and IFN-γ, as determined by RAP ligand blotting.
ADAM17 is responsible for LPS-induced LRP1 shedding in macrophages
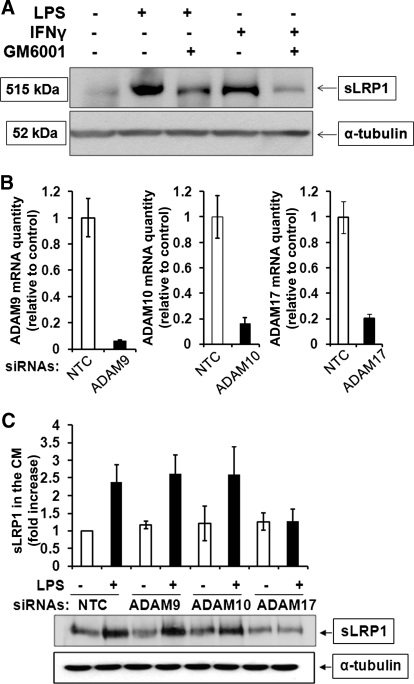
Members of the ADAM family, including ADAM9, ADAM10, and ADAM17, express α-secretase activity and promote shedding of membrane proteins [24, 25]. To test whether ADAMs are responsible for LRP1 shedding, first, we studied the effects of the broad-spectrum metalloprotease inhibitor, GM6001. In these experiments, sLRP1 was detected in CM using a polyclonal LRP1 α-chain-specific antibody. Fig. 2A shows that LPS and IFN-γ increased sLRP1 recovered in the medium, confirming the results of our RAP ligand-blotting experiments. GM6001 substantially decreased sLRP1, generated by LPS and IFN-γ.
Figure 2. ADAM17 is responsible for LPS-induced LRP1 shedding.
(A) RAW 264.7 cells were pretreated with GM6001 (50 μM) in 1% FBS for 12 h and then challenged with LPS (10 ng/ml) or IFN-γ (25 ng/ml) in SFM for 6 h. sLRP1 in the medium was determined by immunoblotting with LRP1 α-chain-specific polyclonal antibody. For each well, cell extracts were prepared and subjected to immunoblot analysis to detect α-tubulin, as a control for load. (B) ON-TARGET plus SMART pool siRNA was used to silence ADAM9, ADAM10, or ADAM17 in RAW 264.7 cells. Control cells were transfected with NTC siRNA. Expression of each mRNA was determined by qPCR 24 h later. (C) RAW 264.7 cells were transfected to silence ADAM9, ADAM10, or ADAM17 or with NTC siRNA. Each cell population was cultured in SFM for 1 h and then treated with LPS (10 ng/ml) for 3 h. sLRP1 in CM was determined by RAP ligand blotting. Cell extracts were subjected to immunoblot analysis for α-tubulin as a control for the number of cells present in each well. The bar graph shows the mean ± sem; n=3.
To determine which ADAMs are involved in LPS-induced LRP1 shedding from RAW 264.7 macrophages, we applied a gene-silencing approach, using specific siRNAs. Fig. 2B shows that the efficiency of gene silencing was 80% or better at the mRNA level for each targeted ADAM, as determined by qPCR. Gene silencing was also specific, as determined by measuring mRNA levels for nontargeted ADAMs (results not shown).
Cells in which 1 of the 3 ADAMs was silenced and control cells transfected with NTC siRNA were treated with LPS (10 ng/ml) or vehicle for 3 h. sLRP1 accumulation in CM was not affected by silencing of ADAM9 or ADAM10 (Fig. 2C). By contrast, ADAM17 gene silencing blocked LPS-induced LRP1 shedding completely. These results suggest that ADAM17 is selectively responsible for the increase in sLRP1 that occurs in inflammation.
sLRP1 levels are increased in mouse plasma by LPS and in human plasma in RA and SLE
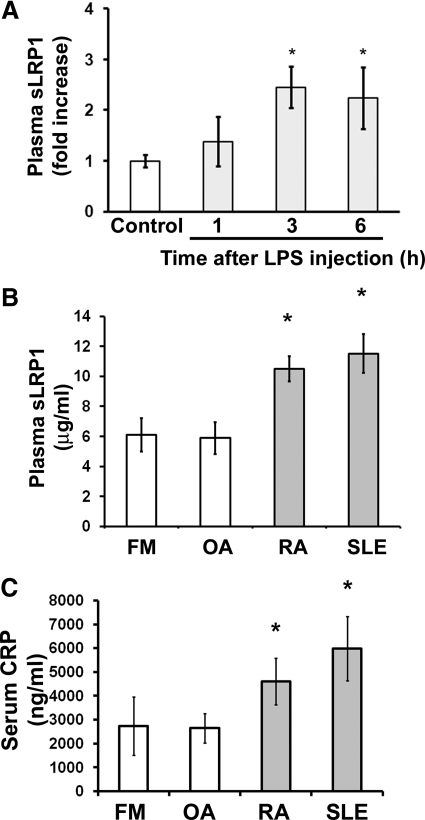
Next, we conducted experiments to test whether inflammation increases LRP1 shedding in vivo. Systemic, acute inflammation was induced in 12-week-old C57BL/6 male mice by i.p. injection of LPS (10 μg/g). sLRP1 in plasma samples was determined by RAP-affinity precipitation and RAP ligand blotting. Within 3 h of LPS injection, the level of sLRP1 in the plasma was increased significantly (P<0.05; Fig. 3A). The sLRP1 level remained elevated at 6 h.
Figure 3. sLRP1 levels in mouse and human plasma are increased by inflammation.
(A) LPS or normal saline was injected i.p. in mice. Blood samples were recovered by cardiac puncture. Plasma was isolated. sLRP1 levels were determined by RAP ligand blotting. The total number of animals studied in 3 independent experiments was control, n=13; LPS treatment for 1 h, n=7; LPS treatment for 3 h, n=9; LPS treatment for 6 h, n=7. Results were analyzed by densitometry (mean±sem; *P<0.05). (B) sLRP1 in human plasma samples was determined by ELISA. The patients were diagnosed with FM, n=9; OA, n=18; RA, n=20; or SLE, n=17 (mean±sem; *P<0.05). (C) CRP in human samples was determined by ELISA. Samples selected at random from those assayed in B included those from patients with FM, n=8; OA, n=11; RA, n=11; and SLE, n=10. One-way ANOVA was used to determine statistical significance. Tukey’s post hoc analysis was used to assess differences between treatment groups.
We also examined samples of human plasma from patients with RA (n=20) and SLE (n=17). These diseases are associated with systemic, chronic inflammation [26]. Our control populations included patients with OA (n=18) or FM (n=7), which are not associated with clinical laboratory signs of systemic inflammation [27]. Fig. 3B shows that the level of sLRP1 was increased in plasma samples from patients with SLE and RA. By one-way ANOVA and Tukey’s post-hoc test, the increase in sLRP1 in SLE plasma was significant compared with both control groups (P<0.01). The increase in sLRP1 in RA plasma was significant compared with OA plasma (P<0.05) or when patients in both control groups were combined (P<0.01). Our patient populations included adult males and females, ages 23–90. Although our populations were too small to stratify based on age or gender, we observed no trends to suggest that either factor influenced the results.
To confirm that our patient populations included individuals with systemic inflammation, the identical plasma samples were studied by ELISA to detect CRP. As anticipated, the level of CRP was elevated significantly in RA and SLE, compared with OA and FM (P<0.05).
sLRP1 initiates cell signaling in macrophages
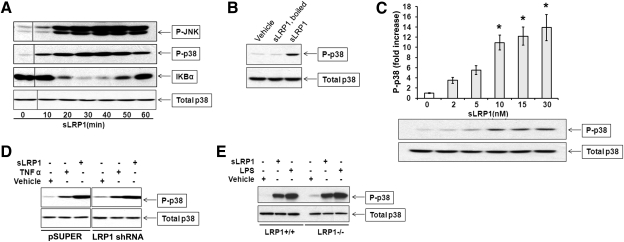
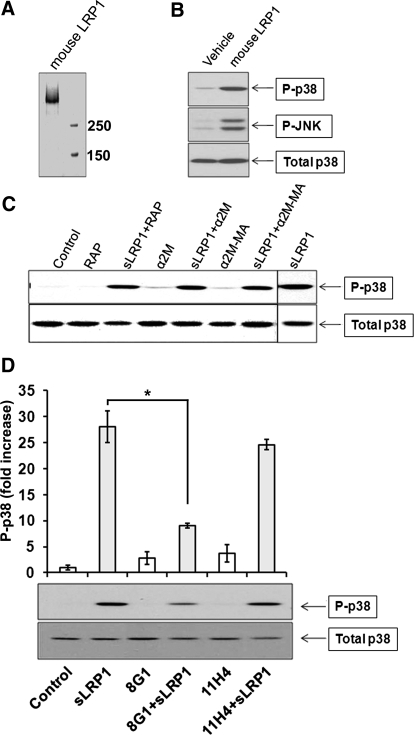
sLRP1 binds directly to Schwann cells without triggering a demonstrable cell signaling response; however, this interaction inhibits the subsequent response to TNF-α [14]. By contrast, in RAW 264.7 cells, sLRP1 activated p38 MAPK and JNK (Fig. 4A). sLRP1 also induced a transient but substantial decrease in IκB, indicating activation of the IKK-NF-κB pathway [28]. To prove that activation of p38 MAPK was not a result of small molecules carried in purified sLRP1 preparations, sLRP1 was heated at 100°C for 15 min, which eliminated activation of p38 MAPK entirely (Fig. 4B). Nondenatured sLRP1 induced a significant increase in p38 MAPK activation (P<0.05; n=3) even when added to cultures of RAW 264.7 cells at concentrations as low as 2 nM (Fig. 4C). As it has been proposed previously that membrane-anchored LRP1 initiates cell signaling by dimer formation [3, 29], we tested whether sLRP1-initiated cell signaling in RAW 264.7 cells is dependent on expression of membrane-anchored LRP1. RAW 264.7 cells, in which LRP1 was constitutively silenced, and control cells were treated with 50 nM sLRP1. As shown in Fig. 4D, p38 MAPK activation by sLRP1 was not inhibited in cells in which LRP1 was silenced. Similar experiments were performed using cultures of BMMs obtained from WT mice and C57BL/6 mice, in which LRP1 was conditionally deleted [12]. sLRP1 induced robust and equivalent activation of p38 MAPK in both BMM cultures (Fig. 4E). These results demonstrate that sLRP1 triggers cell signaling in RAW 264.7 cells and BMMs and that the response does not require membrane-anchored LRP1. To test whether cell signaling in response to sLRP1 requires exposure of sites resulting from proteolytic release from the cell surface, we purified intact LRP1 from mouse liver. When the intact form of LRP1 was eluted from the affinity resin, it remained partially soluble in the absence of detergents. The preparation is shown in Fig. 5A. Purified mouse LRP1 activated p38 MAPK in RAW 264.7 cells (Fig. 5B). Next, we tested whether the ligand-binding repeats in sLRP1 are required for cell signaling. In these studies, sLRP1 was preincubated with RAP or activated α2M. The native form of α2M, which does not bind to LRP1, was also preincubated with sLRP1 as a control. Although activated α2M induces robust cell signaling in Schwann cells and PC12 cells [30], activated α2M failed to activate p38 MAPK in RAW 264.7 cells (Fig. 5C). Native α2M and RAP also failed to activate cell signaling as anticipated. Cell signaling in response to sLRP1 was not inhibited by RAP, native α2M, or activated α2M. These results suggest that the ligand-binding repeats in sLRP1 are not responsible for its cell signaling activity [16, 31].
Figure 4. sLRP1 initiates cell signaling in macrophages.
(A) RAW 264.7 cells were cultured in SFM for 1 h and then challenged with 30 nM sLRP1 for the indicated times. Immunoblot analysis was performed to detect the indicated signaling proteins. P-, Phosphorylated. (B) RAW 264.7 cells were treated with sLRP1 (50 nM), boiled sLRP1 (50 nM), or vehicle for 20 min. Phosphorylated and total p38 MAPK were determined by immunoblot analysis. (C) RAW 264.7 cells were treated with increasing concentrations of sLRP1 for 20 min. Phosphorylated and total p38 MAPK were determined. Immunoblots were subjected to densitometry. The results of 3 experiments were averaged (mean±sem; *P<0.05). (D) RAW 264.7 cells in which LRP1 was stably silenced and control cells that were transfected with empty vector (pSUPER) were cultured in SFM for 1 h and then treated with sLRP1 (50 nM), TNF-α (10 μg/ml), or vehicle for 20 min. Phopho-p38 MAPK and total p38 MAPK were determined by immunoblot analysis. (E) Primary cultures of mouse BMMs were isolated from mice in which LRP1 was conditionally deleted in macrophages (LRP1−/−) and from WT mice in the same genetic background. These cells were treated with sLRP1 (50 nM), LPS (10 ng/ml), or vehicle for 20 min. Immunoblot analysis was performed to detect phospho-p38 and total p38.
Figure 5. Characterization of sLRP1-initiated cell signaling.
(A) Coomassie-stained gel showing purified mouse LRP1. (B) RAW 264.7 cells were cultured in SFM for 1 h and then treated with purified mouse LRP1 (50 nM), rat LRP1 (50 nM), or vehicle. Immunoblot analysis was performed to assess cell signaling. (C) RAW 264.7 cells were treated for 20 min with sLRP1 (50 nM), sLRP1 that was preincubated with RAP (250 nM), native α2M that does not bind to sLRP1 (100 nM), sLRP1 (50 nM) that was preincubated with native α2M (100 nM), methylamine-activated α2M (100 nM), and methylamine-activated α2M (100 nM) that was preincubated with sLRP1 (50 nM). Immunoblot analysis was performed to detect phospho-p38 MAPK and total p38 MAPK. (D) sLRP1 (50 nM) was preincubated with a 50-fold molar excess of antibody 8G1 or 11H4 or with vehicle. Cells were treated with the preincubated sLRP1 or with the antibodies alone. Immunoblot analysis was performed to detect phospho-p38 MAPK and total p38 MAPK. All experiments were performed in triplicate. The studies shown in D were analyzed by densitometry. The bar graph shows the mean ± sem; *P<0.05.
Antibody 11H4, which recognizes the C terminus of the LRP1 β-chain [14], did not inhibit p38 MAPK activation by sLRP1, as anticipated (Fig. 5D). However, mAb 8G1, which recognizes an epitope near the N terminus of the α-chain [16, 31], inhibited p38 MAPK activation by over 70%.
sLRP1 induces cytokine expression by macrophages
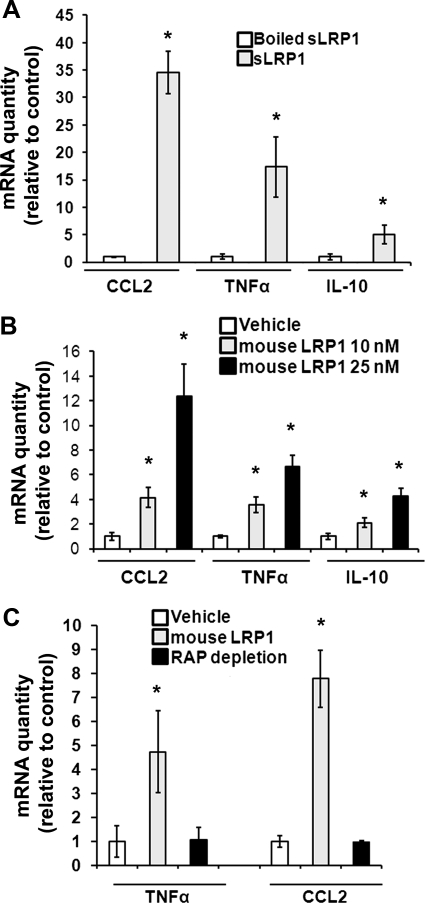
In inflammation, macrophages express regulatory cytokines under the control of cell signaling pathways [32, 33], which are activated by sLRP1. Fig. 6A shows that exposure of RAW 264.7 cells to sLRP1 (50 nM) for 5 h significantly increased expression of the mRNAs for CCL2, TNF-α, and IL-10. When sLRP1 was preincubated at 100°C, cytokine expression by sLRP1 was neutralized, arguing against the possibility that cytokine expression occurred secondary to LPS or other small molecules associated with the sLRP1 preparation. Purified mouse LRP1 also induced expression of CCL2, TNF-α, and IL-10 (Fig. 6B). When mouse LRP1 was pre-extracted with RAP-Sepharose, cytokine induction was neutralized (Fig. 6C), again arguing against contaminants as causative agents for cytokine induction.
Figure 6. sLRP1 induces cytokine expression.
(A) RAW 264.7 cells were cultured in SFM for 1 h and then stimulated with sLRP1 (50 nM), vehicle, or as a control, sLRP1 (50 nM), which had been boiled. Cytokine expression was determined by qPCR. Results are expressed as the fold increase relative to the vehicle control. (B) RAW 264.7 cells were treated with mouse LRP1 or vehicle. Cytokine expression was determined. (C) Mouse LRP1 was pre-extracted with RAP-Sepharose. The extracted preparation and LRP1, which was not pre-extracted, were added to cultures for 5 h. Cytokine expression was determined. All expression studies were completed by qPCR (mean±sem; n=3). Relative expression of each cytokine was determined by comparison with cells that were treated with vehicle (*P<0.05).
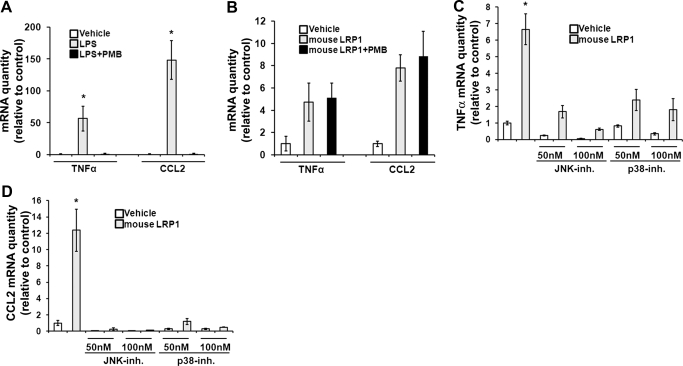
Given the sensitivity of macrophages and macrophage-like cell lines to low levels of LPS, we performed studies to confirm that LPS was not responsible for the induction of cytokine expression by our soluble LRP1 preparations. PMB is a peptide antibiotic that binds and neutralizes LPS [34, 35]. Fig. 7A shows that in control studies with RAW 264.7 cells, PMB (50 μg/ml) blocked cytokine induction completely by LPS. By contrast, the equivalent concentration of PMB had no effect on the ability of purified mouse LRP1 to induce cytokine expression (Fig. 7B).
Figure 7. LRP1-induced cytokine expression results from activation of cell signaling.
(A) RAW 264.7 cells were cultured in SFM for 1 h and then treated with LPS (10 ng/ml) or with LPS that had been pretreated with PMB (50 μg/ml) for 1 h. Cytokine expression was determined by qPCR. (B) RAW 264.7 cells were treated with mouse LRP1 (25 nM) or with mouse LRP1 that was pretreated with PMB (50 μg/ml) for 1 h. Cytokine expression was determined. (C) RAW 264.7 cells were treated with mouse LRP1 (25 nM; shaded bars) or with vehicle (open bars) for 5 h in the presence and absence of inhibitors (inh.) of p38 MAPK or JNK, as indicated. TNF-α mRNA levels were determined. (D) The effects of inhibitors on expression of CCL2 were determined. All results were analyzed by the relative quantity (Ct) method (mean±sem; n=3; *P<0.05).
Pharmacologic inhibitors of p38 MAPK (50 nM) and JNK (50 nM) independently blocked mouse LRP1-induced expression of TNF-α (Fig. 7C) and CCL2 (Fig. 7D). These results suggest that simultaneous activation of complementary cell signaling pathways may be necessary for sLRP1-induced cytokine expression. Overall, these studies demonstrate that sLRP1, isolated from human plasma, and the intact receptor, purified from mouse liver and added in soluble form, activate macrophage cell signaling and regulatory cytokine expression.
DISCUSSION
Although LRP1 gene deletion in mice is embryonic-lethal [36], conditional gene deletion in a variety of cell types, including vascular smooth muscle cells [37], neurons [38], and macrophages [12], has yielded robust phenotypes. The importance of LRP1 in diverse cells and pathophysiologic processes probably reflects the role of LRP1 as an endocytic receptor for over 40 ligands [3], as a regulator of other cell signaling receptors in the plasma membrane [3, 4], and at least in some cell types, as a direct cell signaling receptor [30, 39]. All of these activities are attributed to membrane-anchored LRP1, which localizes in clathrin-coated pits and undergoes constitutive endocytosis and efficient recycling to the cell surface [16, 31]. Most ligands are delivered to lysosomes, although LRP1-associated receptors such as uPAR may recycle to the cell surface with LRP1 [40]. In some cell types, LRP1 localizes transiently in lipid rafts, possibly explaining its cell signaling activity [41]. A role for membrane-anchored LRP1 in inflammation in vivo was identified by Overton et al. [12]. These investigators transplanted BM from mice, in which LRP1 was conditionally deleted in macrophages, into LDLR-deficient mice and demonstrated an increase in atherogenesis, which they attributed to increased production of TNF-α, CCL2, and MMP-9. A number of mechanisms have been described to explain why membrane-anchored LRP1 may be anti-inflammatory. We demonstrated that LRP1 decreases the cell surface abundance of TNFR1 and thereby, inhibits cell signaling through the IKK-NF-κB pathway [5]. A second pathway, which is not mutually exclusive, was described by Zurhove et al. [13]. Following cleavage of the LRP1 ectodomain, the residual transmembrane receptor is subject to a second intracellular, γ-secretase-induced proteolytic event, which releases a 25-kDa LRP1 β-chain fragment. This fragment may translocate to the nucleus, where it suppresses inflammatory gene expression.
The results presented here demonstrate that in vitro, LPS and IFN-γ promote shedding of LRP1 into the medium. In response to LPS, shedding is mediated by ADAM17. We also show for the first time that LPS promotes LRP1 shedding in vivo in rodents. In humans, sLRP1 plasma levels are increased significantly in RA and SLE, suggesting that a wide variety of inflammatory stimuli may be associated with increased LRP1 shedding. sLRP1 may represent a biomarker of inflammation.
Given the presence of sLRP1 in vivo and its increased production in inflammation, we conducted studies to evaluate further the biological activity of sLRP1. It was demonstrated previously that sLRP1 binds ligands, such as tPA, providing 1 mechanism by which sLRP1 may be biologically active [11]. In Schwann cell cultures, sLRP1 binds directly to the cell surface and inhibits the subsequent response to TNF-α [14]. By this pathway, sLRP1 may inhibit inflammation in an injured peripheral nerve.
To test whether sLRP1 regulates macrophage physiology, we designed in vitro experiments using sLRP1 at concentrations that were near the range detected in plasma from SLE and RA patients. It is quite possible that higher sLRP1 concentrations are achieved locally at sites of inflammation. In RAW 264.7 cells, 10 nM sLRP1 increased p38 MAPK phosphorylation significantly; 50 nM sLRP1 robustly activated cell signaling in RAW 264.7 cells and BMMs. As sLRP1 was purified from human plasma, and RAW 264.7 cells and BMMs are from mouse, we compared the activity of LRP1 purified from mouse liver and demonstrated equivalent cell signaling results. Unfortunately, the low level of sLRP1 in plasma precluded purification from mouse blood. Nevertheless, the activity of intact, soluble LRP1 from the mouse indicates that proteolysis of the β-chain is not necessary to observe the activities demonstrated by sLRP1.
The cell signaling events triggered by sLRP1 in RAW 264.7 cells resulted in increased expression of TNF-α, CCL2, and IL-10. Thus, sLRP1 may regulate macrophage activity at a site of inflammation. Given the likely function of sLRP1 as a carrier protein, we conducted a number of experiments to confirm that its activity in cell signaling and cytokine expression was not a result of associated small molecules. First, boiling, which would be expected to denature proteins but not affect small molecules, including LPS, blocked the activity of sLRP1. We further ruled out a role for LPS contamination in experiments with PMB. Finally, we demonstrated that pre-extraction with RAP neutralized the activity of mouse LRP1. Although it is possible that the ability of sLRP1 to initiate cell signaling and induce cytokine expression reflected proteins that bind to sLRP1 and are copurified from the plasma or liver, the concentrations of sLRP1, which demonstrated activity (low nanomolar range), argue against this possibility. Any protein carried by sLRP1 would be present at a trace concentration. Furthermore, the inability of activated α2M and RAP to inhibit sLRP1-induced cell signaling argues against a mechanism that requires the ligand-binding repeats in sLRP1. Overall, our results favor a model in which sLRP1 triggers cell signaling in macrophages by direct binding to the cells. The activity of antibody 8G1 suggests that the extreme N terminus of the 515-kDa LRP1 α-chain may be involved.
Although we have not yet identified the cell surface binding site for sLRP1, the activity of sLRP1 against cells, in which LRP1 was silenced, argues against dimerization with membrane-anchored LRP1 as a mechanism of activity. Identifying the cell surface receptor for sLRP1 is a goal for future investigation. Based on our results, LRP1 emerges as a gene product that may regulate cell physiology as a transmembrane receptor and as a soluble form. A precedent for such a paradigm is derived from known examples of receptors that are functional as membrane-anchored and soluble proteins, including, for example, uPAR [42], amyloid precursor protein [43], and the erythropoietin receptor [44]. The increase in sLRP1, observed in RA and OA patients, suggests that sLRP1 may represent a biomarker of inflammation. Furthermore, LRP1 shedding may contribute to the progression of diseases in humans in which inflammation plays an important role.
ACKNOWLEDGMENTS
This work was supported by grant HL-060551 to S.L.G. and NS-057456 to W.M.C. M.G. was supported by a fellowship from the National Institutes of Health (1F32HL097598). The human plasma repositories used in this study were developed with support from grants AR-047825, AI-070555, and AI-06775. We thank David Boyle for assistance with human plasma acquisition.
Footnotes
- α2M
- α2-macroglobulin
- ADAM17
- a disintegrin and metalloproteinase 17
- ATCC
- American Type Culture Collection
- BMM
- bone marrow-derived macrophages
- CM
- conditioned medium
- CRP
- C-reactive protein
- Ct
- comparative threshold
- FM
- fibromyalgia
- LRP1
- LDLR-related protein-1
- MMP-9
- matrix metalloprotease-9
- NTC
- nontargeting control
- OA
- osteoarthritis
- PMB
- polymixin B sufate
- qPCR
- quantitative PCR
- RA
- rheumatoid arthritis
- RAP
- receptor-associated protein
- SFM
- serum-free medium
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- SLE
- systemic lupus erythematosus
- sLRP1
- shed form of LDLR-related protein-1
- tPA
- tissue-type plasminogen activator
- uPAR
- urokinase-type plasminogen activator receptor
AUTHORSHIP
M.G.—conception, experimental design, data collection, data analysis, manuscript preparation; A.G.—conception, experimental design, data collection, data analysis; W.M.C.—conception, experimental design, data analysis, critical review; G.S.F.—conception, data analysis, critical review; S.L.G.—conception, experimental design, data analysis, critical review, manuscript preparation.
REFERENCES
- 1.Medzhitov R. (2008) Origin and physiological roles of inflammation. Nature 454, 428–435 [DOI] [PubMed] [Google Scholar]
- 2.Edwards D. R., Handsley M. M., Pennington C. J. (2008) The ADAM metalloproteinases. Mol. Aspects Med. 29, 258–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillis A. P., Van Duyn L. B., Murphy-Ullrich J. E., Strickland D. K. (2008) LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 88, 887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonias S. L., Wu L., Salicioni A. M. (2004) Low density lipoprotein receptor-related protein: regulation of the plasma membrane proteome. Thromb. Haemost. 91, 1056–1064 [DOI] [PubMed] [Google Scholar]
- 5.Gaultier A., Arandjelovic S., Niessen S., Overton C. D., Linton M. F., Fazio S., Campana W. M., Cravatt B. F., III, Gonias S. L. (2008) Regulation of tumor necrosis factor receptor-1 and the IKK-NF-κB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood 111, 5316–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willnow T. E., Moehring J. M., Inocencio N. M., Moehring T. J., Herz J. (1996) The low-density-lipoprotein receptor-related protein (LRP) is processed by furin in vivo and in vitro. Biochem. J. 313, 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q., Zhang J., Tran H., Verbeek M. M., Reiss K., Estus S., Bu G. (2009) LRP1 shedding in human brain: roles of ADAM10 and ADAM17. Mol. Neurodegener. 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn K. A., Pye V. J., Dai Y. P., Chesterman C. N., Owensby D. A. (1999) Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP). Exp. Cell Res. 251, 433–441 [DOI] [PubMed] [Google Scholar]
- 9.Von Arnim C. A., Kinoshita A., Peltan I. D., Tangredi M. M., Herl L., Lee B. M., Spoelgen R., Hshieh T. T., Ranganathan S., Battey F. D., Liu C. X., Bacskai B. J., Sever S., Irizarry M. C., Strickland D. K., Hyman B. T. (2005) The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J. Biol. Chem. 280, 17777–17785 [DOI] [PubMed] [Google Scholar]
- 10.Deane R., Sagare A., Zlokovic B. V. (2008) The role of the cell surface LRP and soluble LRP in blood-brain barrier Aβ clearance in Alzheimer′s disease. Curr. Pharm. Des. 14, 1601–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn K. A., Grimsley P. G., Dai Y. P., Tapner M., Chesterman C. N., Owensby D. A. (1997) Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J. Biol. Chem. 272, 23946–23951 [DOI] [PubMed] [Google Scholar]
- 12.Overton C. D., Yancey P. G., Major A. S., Linton M. F., Fazio S. (2007) Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ. Res. 100, 670–677 [DOI] [PubMed] [Google Scholar]
- 13.Zurhove K., Nakajima C., Herz J., Bock H. H., May P. (2008) γ-Secretase limits the inflammatory response through the processing of LRP1. Sci. Signal. 1, ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaultier A., Arandjelovic S., Li X., Janes J., Dragojlovic N., Zhou G. P., Dolkas J., Myers R. R., Gonias S. L., Campana W. M. (2008) A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J. Clin. Invest. 118, 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imber M. J., Pizzo S. V. (1981) Clearance and binding of two electrophoretic ″fast″ forms of human α 2-macroglobulin. J. Biol. Chem. 256, 8134–8139 [PubMed] [Google Scholar]
- 16.Strickland D. K., Gonias S. L., Argraves W. S. (2002) Diverse roles for the LDL receptor family. Trends Endocrinol. Metab. 13, 66–74 [DOI] [PubMed] [Google Scholar]
- 17.Herz J., Goldstein J. L., Strickland D. K., Ho Y. K., Brown M. S. (1991) 39-kDa Protein modulates binding of ligands to low density lipoprotein receptor-related protein/α 2-macroglobulin receptor. J. Biol. Chem. 266, 21232–21238 [PubMed] [Google Scholar]
- 18.Montel V., Gaultier A., Lester R. D., Campana W. M., Gonias S. L. (2007) The low-density lipoprotein receptor-related protein regulates cancer cell survival and metastasis development. Cancer Res. 67, 9817–9824 [DOI] [PubMed] [Google Scholar]
- 19.FitzGerald D. J., Fryling C. M., Zdanovsky A., Saelinger C. B., Kounnas M., Winkles J. A., Strickland D., Leppla S. (1995) Pseudomonas exotoxin-mediated selection yields cells with altered expression of low-density lipoprotein receptor-related protein. J. Cell Biol. 129, 1533–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonias S. L., Reynolds J. A., Pizzo S. V. (1982) Physical properties of human α 2-macroglobulin following reaction with methylamine and trypsin. Biochim. Biophys. Acta 705, 306–314 [DOI] [PubMed] [Google Scholar]
- 21.Hussaini I. M., Srikumar K., Quesenberry P. J., Gonias S. L. (1990) Colony-stimulating factor-1 modulates α 2-macroglobulin receptor expression in murine bone marrow macrophages. J. Biol. Chem. 265, 19441–19446 [PubMed] [Google Scholar]
- 22.LaMarre J., Wolf B. B., Kittler E. L., Quesenberry P. J., Gonias S. L. (1993) Regulation of macrophage α 2-macroglobulin receptor/low density lipoprotein receptor-related protein by lipopolysaccharide and interferon-γ. J. Clin. Invest. 91, 1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussaini I. M., LaMarre J., Lysiak J. J., Karns L. R., VandenBerg S. R., Gonias S. L. (1996) Transcriptional regulation of LDL receptor-related protein by IFN-γ and the antagonistic activity of TGF-β(1) in the RAW 264.7 macrophage-like cell line. J. Leukoc. Biol. 59, 733–739 [DOI] [PubMed] [Google Scholar]
- 24.Tanzi R. E., Bertram L. (2005) Twenty years of the Alzheimer′s disease amyloid hypothesis: a genetic perspective. Cell 120, 545–555 [DOI] [PubMed] [Google Scholar]
- 25.Walter J., Kaether C., Steiner H., Haass C. (2001) The cell biology of Alzheimer′s disease: uncovering the secrets of secretases. Curr. Opin. Neurobiol. 11, 585–590 [DOI] [PubMed] [Google Scholar]
- 26.Firestein G. S. (2003) Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 [DOI] [PubMed] [Google Scholar]
- 27.Wessely S. (1996) Chronic fatigue syndrome. Summary of a report of a joint committee of the Royal Colleges of Physicians, Psychiatrists and General Practitioners. J. R. Coll. Physicians Lond. 30, 497–504 [PMC free article] [PubMed] [Google Scholar]
- 28.Henkel T., Machleidt T., Alkalay I., Kronke M., Ben-Neriah Y., Baeuerle P. A. (1993) Rapid proteolysis of I κ B-α is necessary for activation of transcription factor NF-κ B. Nature 365, 182–185 [DOI] [PubMed] [Google Scholar]
- 29.May P., Woldt E., Matz R. L., Boucher P. (2007) The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann. Med. 39, 219–228 [DOI] [PubMed] [Google Scholar]
- 30.Mantuano E., Mukandala G., Li X., Campana W. M., Gonias S. L. (2008) Molecular dissection of the human α2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J. Biol. Chem. 283, 19904–19911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herz J., Strickland D. K. (2001) LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 108, 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 [DOI] [PubMed] [Google Scholar]
- 33.Raman M., Chen W., Cobb M. H. (2007) Differential regulation and properties of MAPKs. Oncogene 26, 3100–3112 [DOI] [PubMed] [Google Scholar]
- 34.Cornu J. (1980) Effects of sulfomethylation on the mechanism of action of colimycin towards ″Escherichia coli″ B (author′s transl.). Ann. Microbiol. (Paris) 131B, 121–129 [PubMed] [Google Scholar]
- 35.Storm D. R., Rosenthal K. S., Swanson P. E. (1977) Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 46, 723– 763 [DOI] [PubMed] [Google Scholar]
- 36.Herz J., Clouthier D. E., Hammer R. E. (1992) LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71, 411–421 [DOI] [PubMed] [Google Scholar]
- 37.Boucher P., Gotthardt M., Li W. P., Anderson R. G., Herz J. (2003) LRP: role in vascular wall integrity and protection from atherosclerosis. Science 300, 329–332 [DOI] [PubMed] [Google Scholar]
- 38.May P., Rohlmann A., Bock H. H., Zurhove K., Marth J. D., Schomburg E. D., Noebels J. L., Beffert U., Sweatt J. D., Weeber E. J., Herz J. (2004) Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell. Biol. 24, 8872–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y., Mantuano E., Inoue G., Campana W. M., Gonias S. L. (2009) Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci. Signal. 2, ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nykjaer A., Conese M., Christensen E. I., Olson D., Cremona O., Gliemann J., Blasi F. (1997) Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J. 16, 2610–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu L., Gonias S. L. (2005) The low-density lipoprotein receptor-related protein-1 associates transiently with lipid rafts. J. Cell. Biochem. 96, 1021–1033 [DOI] [PubMed] [Google Scholar]
- 42.Jo M., Thomas K. S., Wu L., Gonias S. L. (2003) Soluble urokinase-type plasminogen activator receptor inhibits cancer cell growth and invasion by direct urokinase-independent effects on cell signaling. J. Biol. Chem. 278, 46692–46698 [DOI] [PubMed] [Google Scholar]
- 43.Mattson M. P. (1997) Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77, 1081–1132 [DOI] [PubMed] [Google Scholar]
- 44.Baynes R. D., Reddy G. K., Shih Y. J., Skikne B. S., Cook J. D. (1993) Serum form of the erythropoietin receptor identified by a sequence-specific peptide antibody. Blood 82, 2088–2095 [PubMed] [Google Scholar]