Stimulation of TLR4 by LPS results in factor B synthesis by macrophages, while factor B production in response to poly I:C is independent of TRL3 and TRIF.
Keywords: Toll-like receptors, complement, inflammation
Abstract
TLRs and complement are critical to the host response in sepsis, trauma, and ischemia/reperfusion. We hypothesize that TLR stimulation leads to synthesis and release of complement components by macrophages, an important source of extrahepatic complement. RAW264.7 macrophages or peritoneal macrophages from WT and TLR4-, TLR3-, TRIF-, or MyD88-deficient mice were cultured under standard conditions. In some experiments, cells were pretreated with inhibitors of MAPKs or a NF-κB inhibitor. Cells were stimulated with TLR ligands at known stimulatory concentrations. Intratracheal and i.p. injections were also performed in mice. RT-PCR, Western blotting, and immunocytochemistry were used for analysis. Using a RT-PCR-based panel, we demonstrate that of 18 complement components tested, factor B of the alternative pathway is the most robustly up-regulated complement component in macrophages in response to LPS. This up-regulation results in release of factor B into the media. Up-regulation of factor B by LPS is dependent on TLR4, TRIF, JNK, and NF-κB. A screen of other TLR ligands demonstrated that stimulation with poly I:C (dsRNA analog) also results in up-regulation of factor B, which is dependent on JNK and NF-κB but independent of TLR3 and TRIF. Up-regulation of factor B is also observed after intratracheal and i.p. injection of LPS or poly I:C in vivo. PRR stimulation profoundly influences production and release of factor B by macrophages. Understanding the mechanisms of PRR-mediated complement production may lead to strategies aimed at preventing tissue damage in diverse settings, including sepsis, trauma, and ischemia/reperfusion.
Introduction
TLRs are a family of transmembrane receptors that play a critical role in innate immunity [1]. By recognizing conserved microbial motifs, they alert the host to the presence of microbial invaders and initiate an inflammatory response [2]. TLRs recognize a variety of microbial ligands. It has also been demonstrated that some TLRs can sense the presence of endogenous molecules released from stressed, damaged, or ischemic tissues [3]. All known TLRs, with the exception of TLR3, signal through the adaptor protein known as MyD88 [4]. TLR3 uses an alternative adaptor known as TRIF [5, 6]. TLR4 is unique in that it is able to use the MyD88- and TRIF-dependent pathways. Activation of TLR4 through the MyD88-dependent pathway ultimately results in NF-κB activation and release of proinflammatory cytokines, whereas the TRIF pathway results in activation of IFN regulatory factors 3 and 7 and type I IFN production, as well as delayed activation of NF-κB [7].
The complement system is an intricate, tightly regulated network of serum and membrane-bound proteins that also plays a critical role in the host response to microbial invasion. Complement is activated through one of three major pathways, including the classical, alternative, and lectin pathways. In the classical pathway, C1q binds to antigen-antibody complexes to trigger activation of the complement cascade. MBL and ficolins bind directly to microbial surfaces and activate the complement cascade through the lectin pathway. In the alternative pathway, spontaneously hydrolyzed C3 or C3b bound to microbial surfaces binds to factor B, which in turn, leads to activation of the alternative pathway of complement.
In addition to its role in host defense, complement can recognize damaged host cells and plays a major role in clearing cell debris and damaged or apoptotic cells [8]. Interestingly, more recent observations demonstrate that complement produced locally at sites of inflammation by cells outside of the liver contributes to the pathogenesis of organ injury in the setting of ischemia [9–11]. Macrophages are important sources of extrahepatic complement, and these cells have been shown to modulate the synthesis and release of complement components dynamically [12, 13].
The interface between TLR regulation of innate immune responses and the complement system is poorly understood. C5a has been shown to modulate the response to TLR4 in macrophages [14]. How TLR stimulation modifies or regulates the complement system is less clear. In this study, we undertook experiments to determine if TLR stimulation regulates the synthesis of key complement components in macrophages. We found that factor B synthesis and release by macrophages are markedly stimulated in response to the ligands LPS and poly I:C but not other TLR ligands. Although previous studies have also demonstrated up-regulation of factor B in response to these ligands, the underlying molecular mechanisms have not been defined [15–20]. We demonstrate here that TLR4 stimulation or activation of RNA-sensing mechanisms results in synthesis and release of factor B by macrophages through distinct but overlapping mechanisms.
MATERIALS AND METHODS
Reagents
All tissue-culture plates and flasks were purchased from Corning (Corning, NY, USA). Ultrapure LPS (Escherichia coli 0111:B4, TLR4 ligand) was purchased from List Biological Laboratories, Inc. (Vandell Way, CA, USA). Polymyxin B and NF-κB activation inhibitor were from Sigma Chemical Co. (St. Louis, MO, USA). Inhibitors of p38 (SB 203580), JNK (JNK inhibitor II in solution), and ERK (98059) were purchased from Calbiochem (San Diego, CA, USA). Pam3CSK4 (TLR2/1 ligand), purified LTA from Staphylococcus aureus (TLR2 ligand), poly I:C (TLR3 ligand), FSL-1 (TLR2/6 ligand), purified flagellin from Bacillus subtilis (TLR5 ligand), Imiquimod (R837), CpG oligonucleotide1826 (TLR9 ligand), and synthetic MPLA were all from Invivogen (San Diego, CA, USA). The poly I:C used in these experiments has been tested by Limulus amoebocyte lysate assay, and LPS was not detectable (minimum sensitivity of 0.125 EU/ml). Concentrations of ligands and inhibitors are indicated in the corresponding figure legends. Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). poly I:C was complexed with Lipofectamine 2000, according to the manufacturer's instructions. Lipofectamine 2000 (1 μL) was used for each 1 μg poly I:C.
Cells and cell culture
RAW 264.7 cells (American Type Culture Collection, Manassas, VA, USA) were maintained in DMEM containing 10% FBS, 2 mM L-glutamine, and 100 U/ml penicillin and streptomycin. HUVECs were cultured in Clonetics EGM-2 supplemented with EGM-2 BulletKit, per the manufacturer's instructions (Lonza, Basel, Switzerland). Cultures were maintained 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Thioglycollate-elicited peritoneal macrophages were collected 6 days after i.p. injection of 1 ml 3% Brewer thioglycollate (Sigma Chemical Co.) by peritoneal washout with 5 ml PBS. Cells were subsequently pelleted, resuspended, and plated in RPMI 1640 (Invitrogen) containing 10% FBS, 2 mM L-glutamine, and 100 U/ml penicillin and streptomycin. The cell populations obtained contained >96% macrophages. All cell culture experiments were performed in triplicate.
Western blot analysis
Samples were separated by SDS 10% PAGE and transferred onto a nitrocellulose membrane, which was blocked for 1 h in 5% milk in TBST, followed by immunostaining with optimized dilutions of primary antibody in 1% milk in TBST overnight at 4°C. Factor B antibody (1:5000) was obtained from Quidel Corp. (San Diego, CA, USA). Anti-β-actin mAb was obtained from Novus Biologicals (Littleton, CO, USA). Membranes were washed three times for 10 min in TBST, and antibody binding was detected with HRP-conjugated secondary antibodies in a standard ECL reaction, according to the manufacturer's instructions (Pierce, Rockford, IL, USA) and exposed to Kodak X-Omat film. β-Actin was used as a loading control in experiments involving cell lysates, and in experiments involving cell culture supernatants, equal volumes of supernatant were loaded into each well.
Immunocytochemistry
RAW264.7 cells on coverslips were fixed with 4% paraformaldehyde (Canemco-Marivac, Quebec, Canada) for 15 min, permeabilized with 0.1% Triton X-100 in PBS for 10 min, and blocked with 2% BSA for 1 h. Cells were then washed five times with 0.2% BSA, incubated with antibodies against factor B (1:100, Quidel Corp.), and counterstained with a high-affinity probe for F-actin rhodamine phalloidin from Invitrogen (1:250) and DRAQ5 (1 μM, Alexis Corp., Lausen, Switzerland). After washing, cells were then incubated with Alexa 488-conjugated secondary antibodies from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Comparative RT-PCR
RNA from cultured cells was isolated using a silica gel-based method (RNEasy Miniprep kits, Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. Reverse transcription reactions were performed using 1 ug RNA and Omniscript RT (Qiagen). Prevalidated primers for C1q β, C1r, C1 s, C2, C3, C4 α, C4 β, factor B, factor D, properdin, C5, C6, C7, C8 α, C8 β, C8 γ, C9, MBL, and β-actin were obtained from Qiagen. Comparative PCR was performed using Brilliant SYBR Green QPCR Master Mix kits obtained from Stratagene (La Jolla, CA, USA). All samples were assayed in duplicate. Mx3000P instrument and software were used for analysis with results normalized to actin levels and compared with baseline-untreated cells.
ELISAs
Levels of TNF-α in cell culture supernatants were measured 6 h after stimulation with PRR ligands using Quantikine immunoassay systems (R&D Systems, Minneapolis, MN, USA), according to the manufac-turer's instructions. Levels of IFN-β in cell culture supernatants were measured 6 h after stimulation with PRR ligands using the mouse IFN-β ELISA kit (PBL Biomedical Laboratories, Piscataway, NJ, USA), according to the manufacturer's instructions.
Mice
Male WT (C57/BL6) mice (8–12 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). MyD88 KO mice were provided by Dr. Ruslan Medzhitov (Howard Hughes Medical Institute, Yale University, New Haven, CT, USA). TRIF-deficient (TrifLPS2/LPS2) mice were provided by Dr. Bruce Beutler (Scripps Institute, La Jolla, CA, USA). Male C57Bl/10ScNJ-Tlr4 (TLR4 KO) and C57Bl/10J controls (WT) were purchased from the Jackson Laboratory. Mice that are homozygous for a targeted mutation in TLR3 (B6;129S1-Tlr3tm1Flv/J) and their WT counterparts (B6129SF2/J) were also purchased from the Jackson Laboratory. Animals used in all experiments were maintained in laminar flow cages in a specific pathogen-free atmosphere at the University of Pittsburgh (Pittsburgh, PA, USA). A standard diet and water were provided ad libitum. MyD88-deficient mice were provided water, supplemented with trimethoprim (4 mg/ml) and sulfamethoxazole (40 mg/ml) until 8 weeks of age. Antibiotics were stopped for at least 2 weeks prior to mice being used in experiments. Experimental protocols were approved by the Animal Care and Use Committee of the University of Pittsburgh, and all experiments were performed in adherence to the National Institutes of Health Guidelines for the use of laboratory animals.
In vivo LPS/poly I:C injections
Intratracheal injection of LPS and poly I:C
Mice were anesthetized with 50 mg/kg ketamine and 5 mg/kg xylazine, administered via i.p. injection. A tracheotomy was performed with a 20-gauge catheter, and LPS (30 ng/kg in 200 μl saline; n=4), poly I:C (30 ug/kg in 200 μl of saline; n=4), or saline (200 μl; n=4) alone was administered intratracheally. Alveolar macrophages were harvested by BAL, as described previously [21]. An immunomagnetic separation system (BD Biosciences PharMingen, San Diego, CA, USA) was used to isolate alveolar macrophages from BAL fluid. Magnetic nanoparticle-conjugated antibodies (anti-mouse Gr-1, anti-CD4, anti-CD8, and anti-CD45R/B220 antibodies; BD Biosciences PharMingen) were chosen to label and remove polymorphonuclear leukocytes and lymphocytes. The resulting cells consisted of >98% macrophages, and cell viability was >95%.
i.p. injection of LPS and poly I:C
Brewer thioglycollate (1 ml 3%; Sigma Chemical Co.) was injected into the peritoneal cavity of each mouse. After 6 days, 1 ml PBS (n=4), 10 ng LPS in 1 ml PBS (n=4), or 10 μg poly I:C in 1 ml PBS (n=4) was injected into the peritoneal cavity. After 6 h, peritoneal washout was performed using 5 ml ice-cold PBS to collect cells. The cell populations obtained contained >96% macrophages. Cells were subsequently pelleted, and RNA was harvested for RT-PCR as described above.
Statistical analysis
Data are presented as mean ± sd. Experimental results are analyzed for their significance by Student's t-test or ANOVA using SigmaStat (SPSS, Chicago, IL, USA). Significance was established at the 95% confidence level (P <0.05).
RESULTS
Activation of TLR4 signaling by LPS results in synthesis and release of complement factor B by macrophages
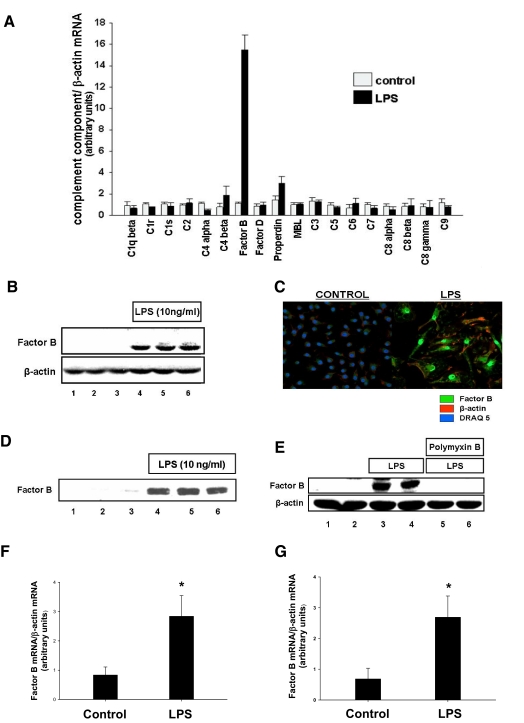
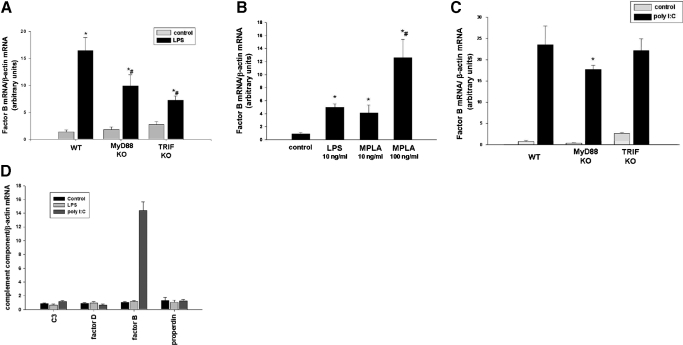
We hypothesized that stimulation of TLR4 on macrophages with LPS would modulate synthesis and release of complement components by the macrophage. To test this hypothesis, RAW264.7 macrophages in culture were stimulated with LPS. Comparative RT-PCR with primers specific for 18 components of the complement cascade was used to evaluate changes in mRNA transcript levels. After stimulation with LPS, we observed robust (15-fold; P<0.001) up-regulation of factor B mRNA (Fig. 1A). Modest up-regulation of properdin mRNA was also observed (2.1-fold; P<0.01). Because of the substantial increase in factor B mRNA, we chose to study this further. To confirm that factor B protein was also up-regulated after stimulation of RAW264.7 cells by LPS, we performed Western blotting and immunohistochemistry with antibodies specific for factor B. As shown in Figure 1, B and C, factor B protein was detected in the cells after stimulation with LPS. Release of factor B into the media was observed (Fig. 1D). Polymyxin B binds to LPS and prevents it from activating TLR4 signaling. To confirm that LPS, rather than another microbial contaminant of the LPS preparation, is responsible for these results, we stimulated RAW264.7 macrophages again with LPS and assayed for factor B. Addition of polymyxin B into cell culture media prevented increased synthesis of factor B protein, suggesting that LPS, rather than a contaminant, is responsible for these observations (Fig. 1E).
Figure 1. LPS stimulates complement factor B synthesis and release by macrophages.
(A) RAW 264.7 macrophages were treated with LPS (10 ng/ml) for 6 h. Comparative RT-PCR with primers specific for complement components was performed. (B) RAW 264.7 macrophages were treated with LPS (10 ng/ml) for 24 h. Cell lysates were harvested, and factor B protein production was confirmed by Western blot. β-Actin was used as a loading control. (C) RAW 246.7 macrophages were treated with LPS (10 ng/ml) for 24 h. Factor B protein production was confirmed by immunocytochemistry. (D) RAW 264.7 macrophages were treated with LPS (10 ng/ml) for 24 h. Cell culture supernatants were analyzed for factor B by Western blot. Equal volumes of cell culture supernatants were loaded into each well. (E) RAW 264.7 macrophages were treated with LPS (10 ng/ml) for 24 h in the presence or absence of polymyxin B. Cell lysates were harvested, and factor B protein production was confirmed by Western blot. β-Actin was used as a loading control. (F) Intratracheal injections of LPS or carrier were performed in mice. After 6 h, alveolar macrophages were harvested, and comparative RT-PCR for factor B was performed. *, P < 0.001, versus control. (G) Macrophages were elicited into the peritoneal cavities of mice using thioglycollate. After 6 days, i.p. injections of LPS or carrier were performed. At 6 h after injection of LPS, macrophages were harvested, and comparative RT-PCR for factor B was performed. *, P < 0.001, versus control.
We next hypothesized that LPS would stimulate up-regulation of factor B in macrophages in vivo. To test this hypothesis, we performed intratracheal injections of LPS or carrier, harvested alveolar macrophages, and measured levels of factor B mRNA by comparative RT-PCR. We found that factor B mRNA was significantly (2.8-fold; P<0.001) up-regulated in alveolar macrophages after LPS injection (Fig. 1F). In separate experiments, macrophages were elicited into the peritoneal cavities of mice using thioglycollate injections. LPS or carrier was then injected into the peritoneal cavity, peritoneal macrophages were harvested, and comparative RT-PCR was performed. Similarly, increased synthesis of factor B mRNA (2.7-fold increase; P<0.001) was also observed in peritoneal macrophages treated with LPS compared with controls in these experiments (Fig. 1G).
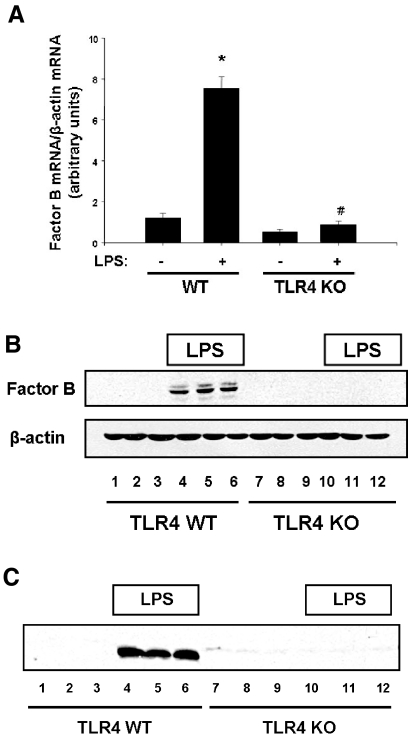
To confirm that TLR4 signaling is responsible for up-regulation of factor B mRNA and protein, peritoneal macrophages were harvested from TLR4 WT mice and mice deficient in TLR4 and stimulated with LPS. Robust up-regulation of factor B mRNA (7.5-fold compared with unstimulated cells; P<0.001) and protein was observed after stimulation of WT cells with LPS. However, this response was completely absent in TLR4-deficient cells (Fig. 2, A and B). In addition, there was an increase in factor B levels in cell culture supernatants after stimulation of WT cells with LPS compared with untreated WT controls. However, there was no appreciable increase in factor B levels in cell culture supernatants from TLR4-deficient macrophages compared with untreated controls (Fig. 2C). Together, these observations demonstrate that upon stimulation by LPS, TLR4 signaling results in robust up-regulation of factor B in the macrophage.
Figure 2. Stimulation of TLR4 by LPS results in synthesis and release of complement factor B by macrophages.
Thioglycollate-elicited peritoneal macrophages were elicited into the peritoneal cavities of WT and TLR4 KO mice by i.p. injection of thioglycollate. Macrophages were harvested after 6 days. (A) Peritoneal macrophages from WT and TLR4 KO mice were stimulated with LPS (10 ng/ml) for 6 h, RNA was harvested, and comparative RT-PCR was performed. *, P < 0.001, versus WT control; #, P < 0.001, versus WT stimulated with LPS. (B) Peritoneal macrophages from WT and TLR4 KO mice were stimulated with LPS (10 ng/ml) for 24 h, cell lysates were harvested, and Western blotting for factor B was performed. β-Actin was used as a loading control. (C) Peritoneal macrophages from WT and TLR4 KO mice were stimulated with LPS (10 ng/ml) for 24 h. Cell culture supernatants were analyzed for factor B by Western blot. Equal volumes of cell culture supernatants were loaded into each well.
poly I:C stimulates synthesis and release of complement factor B by macrophages
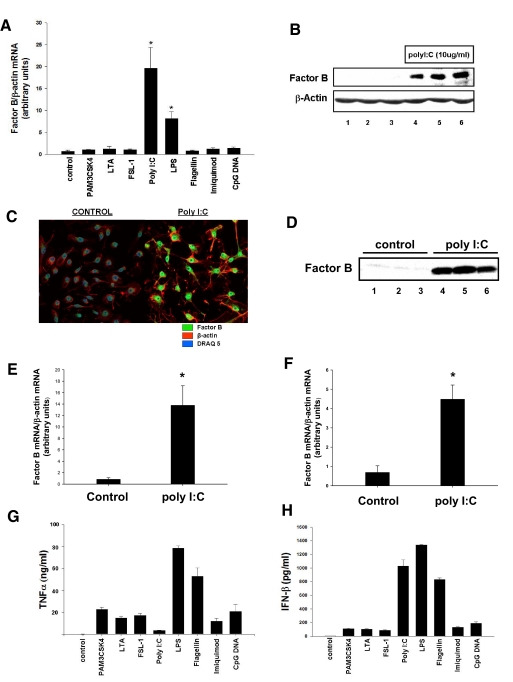
Having established that TLR4 stimulation leads to a selective and robust increase in factor B expression, we sought to determine if ligands that trigger signaling through other TLR family members regulate factor B expression in macrophages, which were stimulated with a variety of TLR ligands at known stimulatory concentrations. Of the ligands tested, only poly I:C, a synthetic analog of dsRNA, which is known to stimulate TLR3, resulted in significant up-regulation (19.6-fold compared with unstimulated cells; P<0.001) of factor B mRNA 6 h after addition to RAW264.7 cell cultures (Fig. 3A). We next confirmed that stimulation of RAW264.7 macrophages with poly I:C resulted in up-regulation of factor B protein by Western blot (Fig. 3B) and by immunocytochemistry (Fig. 3C). In a separate set of experiments, up-regulation of factor B mRNA and protein after treatment of macrophages with poly I:C was not abrograted by addition of polymyxin B in the cell culture media, indicating that the effects of poly I:C are not attributable to LPS contamination (data not shown).
Figure 3. poly I:C stimulates synthesis and release of complement factor B by macrophages.
(A) RAW 264.7 macrophages were treated for 6 h with PAM3CSK4 (1 μg/ml), LTA (2 μg/ml), FSL-1 (1 μg/ml), poly I:C (10 μg/ml), LPS (10 ng/ml), flagellin (10 μg/ml), Imiquimod (10 μg/ml), and CpG DNA (5 μM). RT-PCR was used to measure relative levels of factor B mRNA expression. *, P < 0.001, versus control. (B) RAW 264.7 macrophages were treated with poly I:C (10 μg/ml) for 24 h. Cell lysates were harvested, and factor B protein production was confirmed by Western blot. β-Actin was used as a loading control. (C) RAW 264.7 macrophages were treated with poly I:C (10 μg/ml) for 24 h. Factor B protein production was confirmed by immunocytochemistry. (D) RAW 264.7 macrophages were treated with poly I:C (10 μg/ml) for 24 h. Cell culture supernatants were analyzed for factor B by Western blot. Equal volumes of cell culture supernatants were loaded into each well. (E) Intratracheal injections of poly I:C or carrier were performed in mice. After 6 h, alveolar macrophages were harvested, and comparative RT-PCR for factor B was performed.*, P < 0.001, versus control. (F) Macrophages were elicited into the peritoneal cavities of mice using thioglycollate. After 6 days, i.p. injections of poly I:C or carrier were performed. At 6 h after injection of poly I:C, macrophages were harvested, and comparative RT-PCR for factor B was performed. *, P < 0.001, versus control. (G) Levels of TNF-α in cell culture supernatants were measured by ELISA 6 h after stimulation with PRR ligands. (H) Levels of IFN-β in cell culture supernatants were measured by ELISA 6 h after stimulation with PRR ligands.
We next tested whether factor B is released into the extracellular environment after stimulation by poly I:C by harvesting media from cells treated with poly I:C or carrier and performing Western blots with antibodies specific for factor B. The results of these experiments demonstrate that factor B is released into the cell culture media after stimulation with poly I:C (Fig. 3D). To evaluate whether poly I:C would result in up-regulation of factor B in vivo, intratracheal and i.p. injections of poly I:C or carrier were performed (Fig. 3, E and F). Significant (P<0.001) up-regulation of factor B mRNA was observed in macrophages stimulated with poly I:C administered by intratracheal (13.8-fold) and i.p. (4.5-fold) injection.
Factor B synthesis was not observed after stimulation with TLR ligands other than LPS and poly I:C (Fig. 3A). To confirm that TLR activation was achieved when cells were stimulated with TLR ligands used in this experiment, the levels of TNF-α and IFN-β in the cell culture supernatant were measured. Release of TNF-α and/or IFN-β was observed after treatment of RAW264.7 with TLR ligands used here, indicating that cell activation was achieved (Fig. 3, G and H).
Cytoplasmic dsRNA sensors, rather than TLR3, mediate up-regulation of factor B after stimulation with poly I:C
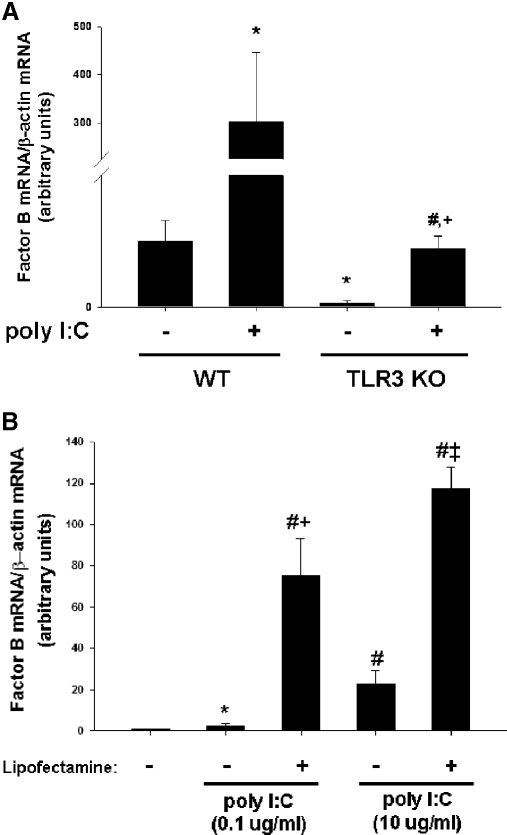
The presence of dsRNA can be detected by the innate immune system by TLR3 or by cytoplasmic sensors, including RIG-like helicases, such as RIG-I or MDA-5 [22, 23]. TLR3, which is located in the endosomal compartment, permits detection of dsRNA phagocytosed from the extracellular space. In contrast, RIG-like helicases are located in the cytosol and detect the presence of cytosolic dsRNA. To test the hypothesis that TLR3 mediates up-regulation of factor B after stimulation of macrophages with poly I:C, peritoneal macrophages were harvested from mice that are homozygous for a targeted mutation in TLR3 or their WT counterparts. Upon stimulation of WT macrophages with poly I:C, robust up-regulation of factor B mRNA was observed (12.2-fold compared with unstimulated WT control; P<0.05; Fig. 4A). Although baseline levels of factor B mRNA were significantly lower in macrophages harvested from TLR3 mutant mice, macrophages from TLR3 mutant mice were also able to up-regulate factor B mRNA (14.0-fold compared with unstimulated mutant control; P<0.005; Fig. 4A). This observation suggests that TLR3 is not required for dynamic modulation of factor B synthesis after stimulation of macrophages with poly I:C.
Figure 4. Cytoplasmic RNA sensors, rather than TLR3, mediate up-regulation of factor B after stimulation with poly I:C.
(A) Thioglycollate-elicited peritoneal macrophages were elicited into the peritoneal cavities of WT and TLR3-deficient (TLR3 KO) mice by i.p. injection of thioglycollate. Macrophages were harvested after 6 days and placed in culture. Peritoneal macrophages from WT and TLR3-deficient mice were stimulated with poly I:C (10 μg/ml) for 6 h, RNA was harvested, and comparative RT-PCR was performed. *, P < 0.05, versus WT unstimulated control; #, P < 0.005, versus TLR3-deficient unstimulated control; †, P < 0.05, versus WT treated with poly I:C. (B) RAW 264.7 macrophages were treated for 6 h with poly I:C (0.1 μg/ml or 10 μg/ml), alone or complexed with Lipofectamine 2000. RT-PCR was used to measure relative levels of factor B mRNA expression. *, P < 0.05, versus untreated control; #, P < 0.001, versus untreated control; †, P < 0.01, versus poly I:C 0.1 μg/ml; ‡, P < 0.001, versus poly I:C 10 μg/ml.
If cytosolic RNA sensors are predominantly responsible for mediating synthesis of factor B after stimulation of macrophages with poly I:C, then facilitating entry of poly I:C into the cytoplasm by lipofection should result in marked up-regulation of factor B. Stimulation of RAW264.7 macrophages with low concentrations of poly I:C (0.1 ug/ml) alone resulted in little up-regulation of factor B mRNA (2.7-fold compared with unstimulated cells; P<0.05; Fig. 4B). However, stimulation of macrophages with poly I:C at the same concentration when complexed with a lipofection reagent resulted in a significantly greater increase in factor B mRNA (75-fold compared with unstimulated cells; P<0.001). As in previous experiments, significant up-regulation (22-fold compared with unstimulated cells; P<0.001) of factor B mRNA was observed when a higher concentration of poly I:C (10 ug/ml) alone was used to stimulate RAW264.7 macrophages. A more pronounced increase in factor B mRNA (117-fold compared with unstimulated cells; P<0.001) was also observed when poly I:C at 10 ug/ml was complexed with a lipofection reagent prior to stimulating RAW264.7 cells in culture (Fig. 4B). These results demonstrate that facilitating entry of poly I:C into the cytosol results in marked up-regulation of factor B mRNA.
TRIF mediates up-regulation of factor B after stimulation with LPS, but up-regulation of factor B after stimulation with poly I:C is independent of TRIF
The intracellular adaptor protein MyD88 is used as an intracellular signaling adaptor protein by all known mammalian TLRs, with the exception of TLR3. Instead, intracellular signaling by TLR3 is mediated through the adaptor TRIF. TLR4 is unique among TLRs, as it uses MyD88 and TRIF as intracellular signaling adaptors. As TLR3 and TLR4 use TRIF as an adaptor, and we observed that stimulation of macrophages with LPS, which stimulates TLR4, and poly I:C, a TLR3 ligand, results in synthesis and release of factor B, we hypothesized that up-regulation of complement factor B occurs through TRIF-mediated signaling.
To test this hypothesis, we first harvested peritoneal macrophages from mice deficient in MyD88, mice deficient in TRIF, and WT mice. After stimulation with LPS, robust up-regulation of factor B mRNA was observed in cells from WT mice (16.4-fold; P<0.001). Up-regulation of factor B was abrogated partially in cells deficient in MyD88 (9.8-fold up-regulation) and in cells from mice deficient in TRIF (7.2-fold up-regulation; Fig. 5A). MPLA is a synthetic analog of LPS that stimulates TLR4 signaling preferentially through the TRIF-dependent pathway [24]. If LPS stimulates factor B production through the TRIF-dependent pathway, then treatment of macrophages with MPLA should result in factor B synthesis. Upon stimulation of macrophages with MPLA at 10 ng/ml or 100 ng/ml for 6 h, significantly increased factor B mRNA production (4.1-fold, 12.6-fold increase, respectively, P<0.001 vs. unstimulated cells) was observed compared with untreated cells, suggesting that signaling through the TRIF-dependent pathway is sufficient for up-regulation of factor B (Fig. 5B).
Figure 5. TRIF mediates up-regulation of factor B after stimulation with LPS, but up-regulation of factor B after stimulation with poly I:C is independent of TRIF.
(A) Peritoneal macrophages were harvested from WT, MyD88 KO, or TRIF KO mice and placed in culture. Cells were treated with LPS (10 ng/ml) or carrier, and comparative RT-PCR for factor B mRNA expression was performed. *, P < 0.001, versus WT:control; #, P < 0.001, versus WT:LPS. (B) RAW264.7 macrophages were stimulated with LPS (10 ng/ml) or MPLA (10 ng/ml or 100 ng/ml) for 6 h, and comparative RT-PCR for factor B mRNA expression was performed. *, P < 0.001, versus control; #, P < 0.001, versus MPLA-10 ng/ml. (C) Peritoneal macrophages were harvested from WT, MyD88 KO, or TRIF KO and placed in culture. Cells were treated with poly I:C (10 μg/ml) or carrier, and comparative RT-PCR for factor B mRNA was performed. *, P < 0.05, versus WT:poly I:C-stimulated. (D) HUVEC cells were stimulated with LPS (10 ng/ml) or poly I:C (10 μg/ml) for 6 h. Comparative RT-PCR for alternative pathway complement components was performed.
To evaluate the hypothesis that up-regulation of factor B by macrophages after stimulation with poly I:C is also dependent on TRIF, macrophages from mice deficient in MyD88, mice deficient in TRIF, and WT mice were again harvested and placed in culture. Upon stimulation with poly I:C, robust up-regulation of factor B mRNA was observed in WT macrophages (23.5-fold) and also macrophages deficient in TRIF (22.1-fold; Fig. 5C). This observation suggests that unlike LPS, poly I:C does not stimulate factor B synthesis through TRIF. As TLR3 is thought to signal through the adaptor TRIF, this result is consistent with our previous observation that up-regulation of factor B after stimulation of macrophages with poly I:C is independent of TLR3. There was a small, but statistically significant, difference in the up-regulation of factor B by MyD88-deficient cells compared with WT cells (Fig. 5C).
It has been demonstrated previously that endothelial cells, like macrophages, can also dynamically modulate synthesis of complement components, including factor B [25]. Interestingly, endothelial cells lack the adaptor protein TRAM, which is required for TRIF-dependent signaling [26]. The results of our previous experiments suggest that stimulation of macrophages with LPS results in synthesis of factor B, largely through TRIF-mediated signaling, but synthesis of factor B after stimulation with poly I:C is independent of TRIF. Based on these observations, we hypothesized that endothelial cells, which lack the adaptor TRAM, would up-regulate factor B mRNA in response to poly I:C, but not LPS. Upon treatment of HUVEC cells in culture with LPS or poly I:C, dramatic up-regulation of factor B mRNA was observed after stimulation with poly I:C (14.4-fold; P<0.001 vs. unstimulated cells), but this up-regulation was completely absent upon stimulation with LPS (Fig. 5D). The results of this experiment support the conclusion that LPS stimulates factor B expression through TRIF, but poly I:C does not. Also, this experiment shows that up-regulation of factor B in response to poly I:C occurs in another cell type important to host immune responses.
p38, JNK, and NF-κB mediate factor B production in the macrophage
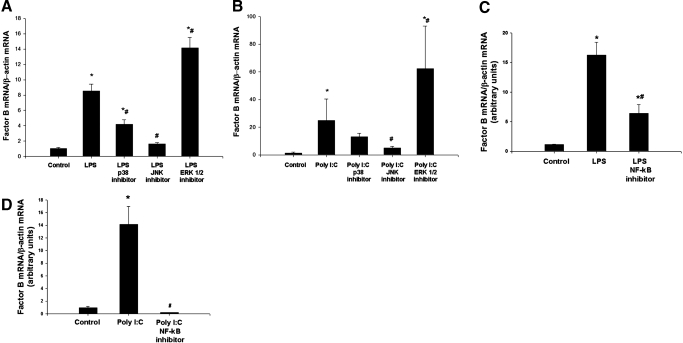
MAPK and NF-κB are signaling components activated downstream of TLRs. We therefore next hypothesized that MAPK and NF-κB mediate downstream signaling that results in up-regulation of factor B after stimulation of macrophages with LPS or poly I:C. To test this hypothesis, RAW264.7 macrophages were pretreated with small molecule inhibitors of p38, JNK, and ERK1/2 or carrier prior to stimulation with LPS. As in previous experiments, up-regulation of factor B mRNA was observed after stimulation of cells with LPS (8.5-fold). Pretreatment of cells with an inhibitor of p38 partially prevented up-regulation of factor B mRNA (4.2-fold), and pretreatment of cells with an inhibitor of JNK largely prevented up-regulation (1.2-fold) of factor B mRNA. Conversely, inhibition of ERK1/2 enhanced synthesis of factor B mRNA (14.2-fold increase) after stimulation with LPS (Fig. 6A). Similarly, when cells were stimulated with poly I:C, inhibition of p38 partially prevented up-regulation (13.0-fold) of factor B mRNA, and pretreatment of cells with an inhibitor of JNK largely prevented up-regulation (4.9-fold) of factor B mRNA compared with cells that were stimulated with poly I:C alone (24.9-fold). Inhibition of ERK1/2 enhanced synthesis of factor B mRNA after stimulation with poly I:C (62.4-fold; Fig. 6B). Further, inhibition of NF-κB partially prevented up-regulation of factor B mRNA after stimulation of cells with LPS and completely prevented up-regulation of factor B mRNA after stimulation of cells with poly I:C (Fig. 6, C and D).
Figure 6. p38, JNK, and NF-κB mediate factor B production in the macrophage.
(A) RAW 264.7 macrophages were pretreated overnight with p38 (SB 203580, 20 μM), JNK (JNK inhibitor II in solution, 20 μM), and ERK (98059, 20 μM) inhibitor and then treated with LPS (10 ng/ml), 6 h. Comparative RT-PCR for factor B mRNA expression was performed. *, P < 0.001, versus control; #, P < 0.001, versus LPS. (B) RAW 264.7 macrophages were pretreated overnight with p38 (SB 203580, 20 μM), JNK (JNK inhibitor II in solution, 20 μM), and ERK (98059, 20 μM) inhibitor and then treated with poly I:C (10 μg/ml), 6 h. Comparative RT-PCR for factor B mRNA expression was performed. *, P < 0.001, versus control; #, P < 0.001, versus poly I:C. (C) RAW 264.7 macrophages were pretreated with NF-κB activation inhibitor (10 μM) overnight and then treated with LPS (10 ng/ml) for 6 h. Comparative RT-PCR for factor B mRNA expression was performed. *, P < 0.001, versus control; #, P < 0.001, versus LPS. (D) RAW 264.7 macrophages were pretreated with NF-κB activation inhibitor (10 μM) overnight and then treated with poly I:C (10 μg/ml), 6 h. Comparative RT-PCR for factor B mRNA expression was performed. *, P < 0.001, versus control; #, P < 0.001, versus poly I:C.
DISCUSSION
PRRs and complement play a critical role in host defense. Recent data demonstrate that complement breakdown products can influence PRR signaling [14]. The data presented here demonstrate that cells dynamically produce complement components in response to PRR ligands in a tightly regulated manner. Specifically, factor B of the alternative pathway of complement is modulated in response to LPS and poly I:C. The observation that a nucleic acid can stimulate macrophages to produce complement components is particularly intriguing.
In this manuscript, we demonstrate that stimulation of TLR4 by LPS results in factor B synthesis and release by the macrophage. Although the MyD88-dependent pathway may play some role, this response appears to be mediated predominantly by TRIF. The central role of TRIF is demonstrated by the observation that synthesis of factor B after stimulation with LPS is largely impaired in macrophages isolated from TRIF-deficient mice. As MPLA results in preferential stimulation of the TRIF-dependent pathway of TLR4 signaling [24], the observation that stimulation of macrophages with MPLA results in factor B synthesis also supports a role for TRIF and suggests that signaling through the TRIF-dependent pathway is sufficient for up-regulation of factor B. Lastly, endothelial cells lack the adaptor protein TRAM, which is required for TRIF-dependent signaling [26]. Our data demonstrate that although endothelial cells can also dynamically modulate factor B synthesis, LPS does not stimulate factor B production in endothelial cells.
Although the adaptor TRIF plays a critical role in factor B synthesis and release by the macrophage in response to LPS, our results demonstrate that synthesis of factor B after stimulation of cells with poly I:C is independent of TRIF. As TLR3 is known to signal exclusively through TRIF [6, 27], this observation suggests that TLR3 is not the receptor that is responsible for initiating factor B production in response to poly I:C. Further, as we have observed that macrophages from TLR3-deficient mice are able to up-regulate factor B in response to poly I:C, another class of receptors must be responsible for synthesis of factor B in response to poly I:C.
Cytosolic sensors, including MDA-5 and RIG-I, detect and respond to dsRNA [22, 23]. Our results demonstrate that facilitating entry of poly I:C into the cytoplasm of cells using a lipofection reagent greatly increases factor B production. In conjunction with our observation that TLR3-deficient cells are able to up-regulate factor B in response to poly I:C, this data suggest that cytosolic sensors of dsRNA are predominantly responsible for factor B synthesis in response to poly I:C.
Previous work has demonstrated that cytokines, including TNF-α and IFN-γ, play an important role in regulating factor B synthesis in the macrophage [28]. Stimulation of RAW264.7 macrophages in culture with various TLR ligands led to release of significant amounts of TNF-α, but levels of IFN-γ were unchanged in our experiments (data not shown). Although our data do not exclude a role for cytokine-mediated up-regulation of factor B, synthesis of factor B did not necessarily correlate with cytokine release. For example, stimulation of cells with flagellin resulted in release of significant amounts of TNF-α and IFN-β. However, no increase in factor B mRNA levels was observed after stimulation of cells with flagellin.
We demonstrate here that TLR4 stimulation or activation of RNA-sensing mechanisms by microbial ligands results in synthesis and release of factor B by macrophages through distinct but overlapping mechanisms involving NF-κB and the MAPKs p38 and JNK. Extrahepatic complement production, specifically, synthesis of factor B, appears to play an important role in the pathogenesis of ischemic tissue injury [9, 11]. Furthermore, factor B appears to play a critical role in a number of inflammatory disease processes, including autoimmune glomerulonephritis, vasculitis, lupus, and arthritis [29–33]. Although our current study provides insight into the mechanisms by which microbial ligands stimulate factor B synthesis and release, the nature of the molecules that stimulate factor B synthesis in the setting of endogenous tissue injury has not been defined and is an area of active investigation in our laboratory. A better understanding of the underlying mechanisms of PRR-mediated complement production and release may lead to the development of strategies aimed at preventing tissue damage in diverse settings, including sepsis, autoimmunity, trauma, and ischemia/reperfusion.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant P50-GM-53789 (T.R.B. and J.F.), National Institutes of Health grant R01-HL-079669 (J. F.), and VA Merit Award (J.F.). D.J.K. and R.D.E. are each recipients of American College of Surgeons Resident Research Scholarships. We thank Meihua Bo, Hong Liao, and Danielle Reiser for technical assistance.
Footnotes
- BAL
- bronchoalveolar lavage
- dsRNA
- double-stranded RNA
- EGM-2
- endothelial growth media 2
- FSL-1
- fibroblast-stimulating lipoprotein-1
- KO
- knockout
- LTA
- lipoteichoic acid
- MBL
- mannose-binding lectin
- MDA-5
- melanoma differentiation-associated gene-5
- MPLA
- monophosphoryl lipid A
- Pam3CSK4
- palmitoyl-3-cysteine-serine-lysine-4
- poly I:C
- polyinosine-polyctyidylic acid
- PRR
- pattern recognition receptor
- RIG-I
- retinoic acid-induced protein I
- TRAM
- Toll/IL-1R-domain-containing adapter-inducing IFN-β-related adaptor molecule
- TRIF
- Toll/IL-1R-domain-containing adapter-inducing IFN-β
- WT
- wild-type
AUTHORSHIP
David J. Kaczorowski: study conception, experimental design, experiment performance, and manuscript preparation. Amin Afrazi: experimental design and experiment performance. Melanie J. Scott: experimental design and manuscript preparation. Joan H. Kwak: experimental design and experiment performance. Roop Gill: experimental design and experiment performance. Rebecca D. Edmonds: experimental design and experiment performance. Yujian Liu: experimental design and experiment performance. Jie Fan: experimental design, experiment performance, and manuscript preparation. Timothy R. Billiar: study conception, experimental design, and manuscript preparation.
REFERENCES
- 1.Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 2.Kapetanovic R., Cavaillon J. M. (2007) Early events in innate immunity in the recognition of microbial pathogens. Expert Opin. Biol. Ther. 7, 907–918 [DOI] [PubMed] [Google Scholar]
- 3.Kaczorowski D. J., Mollen K. P., Edmonds R., Billiar T. R. (2008) Early events in the recognition of danger signals after tissue injury. J. Leukoc. Biol. 83, 546–552 [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A., Jr. (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2, 253–258 [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., Akira S. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. (2003) Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301, 640–643 [DOI] [PubMed] [Google Scholar]
- 7.Takeda K., Akira S. (2005) Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 [DOI] [PubMed] [Google Scholar]
- 8.Trouw L. A., Blom A. M., Gasque P. (2008) Role of complement and complement regulators in the removal of apoptotic cells. Mol. Immunol. 45, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 9.Singh M. V., Kapoun A., Higgins L., Kutschke W., Thurman J. M., Zhang R., Singh M., Yang J., Guan X., Lowe J. S., Weiss R. M., Zimmermann K., Yull F. E., Blackwell T. S., Mohler P. J., Anderson M. E. (2009) Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J. Clin. Invest. 119, 986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W., Medof M. E., Heeger P. S., Sacks S. (2007) Graft-derived complement as a mediator of transplant injury. Curr. Opin. Immunol. 19, 569–576 [DOI] [PubMed] [Google Scholar]
- 11.Farrar C. A., Zhou W., Lin T., Sacks S. H. (2006) Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 20, 217–226 [DOI] [PubMed] [Google Scholar]
- 12.Huang Y., Krein P. M., Winston B. W. (2001) Characterization of IFN-γ regulation of the complement factor B gene in macrophages. Eur. J. Immunol. 31, 3676–3686 [DOI] [PubMed] [Google Scholar]
- 13.Laszlo D. J., Henson P. M., Remigio L. K., Weinstein L., Sable C., Noble P. W., Riches D. W. (1993) Development of functional diversity in mouse macrophages. Mutual exclusion of two phenotypic states. Am. J. Pathol. 143, 587–597 [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Kimura Y., Fang C., Zhou L., Sfyroera G., Lambris J. D., Wetsel R. A., Miwa T., Song W. C. (2007) Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 110, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogasen A. K., Hestdal K., Abrahamsen T. G. (1993) Granulocyte-macrophage colony-stimulating factor, but not macrophage colony-stimulating factor, suppresses basal and lipopolysaccharide-stimulated complement factor production in human monocytes. J. Immunol. 151, 3215–3224 [PubMed] [Google Scholar]
- 16.Miyama A., Kawamoto Y., Ichikawa H., Okamoto K., Hara S., Inoue T. (1980) Complement proteins and macrophages. II. The secretion of factor B by lipopolysaccharide-stimulated macrophages. Microbiol. Immunol. 24, 1223–1232 [DOI] [PubMed] [Google Scholar]
- 17.Riches D. W., Henson P. M., Remigio L. K., Catterall J. F., Strunk R. C. (1988) Differential regulation of gene expression during macrophage activation with a polyribonucleotide. The role of endogenously derived IFN. J. Immunol. 141, 180–188 [PubMed] [Google Scholar]
- 18.Strunk R. C., Fleischer J. A., Katz Y., Cole F. S. (1994) Developmentally regulated effects of lipopolysaccharide on biosynthesis of the third component of complement and factor B in human fibroblasts and monocytes. Immunology 82, 314–320 [PMC free article] [PubMed] [Google Scholar]
- 19.Sundsmo J. S., Chin J. R., Papin R. A., Fair D. S., Werb Z. (1985) Factor B, the complement alternative pathway serine proteinase, is a major constitutive protein synthesized and secreted by resident and elicited mouse macrophages. J. Exp. Med. 161, 306–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton M. B., Strunk R. C., Cole F. S. (1986) Regulation of the synthesis of the third component of complement and factor B in cord blood monocytes by lipopolysaccharide. J. Immunol. 136, 1366–1372 [PubMed] [Google Scholar]
- 21.Fan J., Marshall J. C., Jimenez M., Shek P. N., Zagorski J., Rotstein O. D. (1998) Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J. Immunol. 161, 440–447 [PubMed] [Google Scholar]
- 22.Kawai T., Akira S. (2007) Antiviral signaling through pattern recognition receptors. J. Biochem. 141, 137–145 [DOI] [PubMed] [Google Scholar]
- 23.Meylan E., Tschopp J. (2006) Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell 22, 561–569 [DOI] [PubMed] [Google Scholar]
- 24.Mata-Haro V., Cekic C., Martin M., Chilton P. M., Casella C. R., Mitchell T. C. (2007) The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316, 1628–1632 [DOI] [PubMed] [Google Scholar]
- 25.Kawakami Y., Watanabe Y., Yamaguchi M., Sakaguchi H., Kono I., Ueki A. (1997) TNF-α stimulates the biosynthesis of complement C3 and factor B by human umbilical cord vein endothelial cells. Cancer Lett. 116, 21–26 [DOI] [PubMed] [Google Scholar]
- 26.Harari O. A., Alcaide P., Ahl D., Luscinskas F. W., Liao J. K. (2006) Absence of TRAM restricts Toll-like receptor 4 signaling in vascular endothelial cells to the MyD88 pathway. Circ. Res. 98, 1134–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoebe K., Du X., Georgel P., Janssen E., Tabeta K., Kim S. O., Goode J., Lin P., Mann N., Mudd S., Crozat K., Sovath S., Han J., Beutler B. (2003) Identification of Lps2 as a key transducer of MyD88-independent TIR signaling. Nature 424, 743–748 [DOI] [PubMed] [Google Scholar]
- 28.Huang Y., Krein P. M., Muruve D. A., Winston B. W. (2002) Complement factor B gene regulation: synergistic effects of TNF-α and IFN-γ in macrophages. J. Immunol. 169, 2627–2635 [DOI] [PubMed] [Google Scholar]
- 29.Ji H., Ohmura K., Mahmood U., Lee D. M., Hofhuis F. M., Boackle S. A., Takahashi K., Holers V. M., Walport M., Gerard C., Ezekowitz A., Carroll M. C., Brenner M., Weissleder R., Verbeek J. S., Duchatelle V., Degott C., Benoist C., Mathis D. (2002) Arthritis critically dependent on innate immune system players. Immunity 16, 157–168 [DOI] [PubMed] [Google Scholar]
- 30.Taube C., Thurman J. M., Takeda K., Joetham A., Miyahara N., Carroll M. C., Dakhama A., Giclas P. C., Holers V. M., Gelfand E. W. (2006) Factor B of the alternative complement pathway regulates development of airway hyperresponsiveness and inflammation. Proc. Natl. Acad. Sci. USA 103, 8084–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hietala M. A., Jonsson I. M., Tarkowski A., Kleinau S., Pekna M. (2002) Complement deficiency ameliorates collagen-induced arthritis in mice. J. Immunol. 169, 454–459 [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H., Garnier G., Circolo A., Wetsel R. A., Ruiz P., Holers V. M., Boackle S. A., Colten H. R., Gilkeson G. S. (2000) Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J. Immunol. 164, 786–794 [DOI] [PubMed] [Google Scholar]
- 33.Alexander J. J., Jacob A., Vezina P., Sekine H., Gilkeson G. S., Quigg R. J. (2007) Absence of functional alternative complement pathway alleviates lupus cerebritis. Eur. J. Immunol. 37, 1691–1701 [DOI] [PubMed] [Google Scholar]